Abstract
Microglial activation has been implicated in many astrogliosis-related pathological conditions including astroglioma; however, the detailed mechanism is not clear. In this study, we used primary enriched microglia and astrocytes cultures to determine the role of microglial prostaglandin E2 (PGE2) in the proliferation of astrocytes. The proliferation of astrocytes was measured by BrdU incorporation. The level of PGE2 was measured by ELISA method. Pharmacological inhibition or genetic ablation of COX-2 in microglia were also applied in this study. We found that proliferation of astrocytes increased following lipopolysaccharide (LPS) treatment in the presence of microglia. Furthermore, increased proliferation of astrocytes was observed in the presence of conditioned media from LPS-treated microglia. The potential involvement of microglial PGE2 in enhanced astrocyte proliferation was suggested by the findings that PGE2 production and COX-2 expression in microglia were increased by LPS treatment. In addition, activated microglia-induced increases in astrocyte proliferation were blocked by the PGE2 antagonist AH6809, COX-2 selective inhibitor DuP-697 or by genetic knockout of microglial COX-2. These findings were further supported by the finding that addition of PGE2 to the media significantly induced astrocyte proliferation. These results indicate that microglial PGE2 plays an important role in astrocyte proliferation, identifying PGE2 as a key neuroinflammatory molecule that triggers the pathological response related to uncontrollable astrocyte proliferation. These findings are important in elucidating the role of activated microglia and PGE2 in astrocyte proliferation and in suggesting a potential avenue in the use of anti-inflammatory agents for the therapy of astroglioma.
Keywords: Cyclooxygenase-2, Prostaglandin E2, Microglia, Astrocyte, LPS
Introduction
Reactive astrogliosis is a phenomenon characterized by increased astroglial proliferation, cellular hypertrophy and elongation of astrocytic processes. These factors can be detected using the typical astroglial marker, glial fibrillary acidic protein (GFAP) (Neary et al., 1996; Ridet et al., 1997). Signs of reactive astrogliosis are detected in mammalian brains following various types of traumatic or hypoxic insults, as well as in chronic demyelination and neurodegenerative disorders characterized by marked inflammatory responses (Oksenberg et al., 1996; Douhou et al., 2003; Hirsch et al., 2003). Uncontrolled astrogliosis is also the main component of most gliomas. Recently, increased attention has been focused on the cellular and molecular mechanisms underlying astrogliosis; however, these issues are still not completely understood. Nevertheless, recent studies indicate the potent influence of activated microglia on astrogliosis (Penkowa et al., 2002; Rohl et al., 2007; Tilleux et al., 2007) and many environmental toxins and other environmental pathomechanisms (pharmacologic, infectious, and traumatic) have been implicated.
Microglia activation/proliferation and reactive astrogliosis are commonly observed and have been considered to be a closely related pathological process during neuropathological changes of the central nervous system (CNS). In general, activation of microglia precedes or accompanies astrogliosis (Iravani et al., 2005; Herber et al., 2006). Several in vivo studies report that reduction of microglial activation is associated with the reduction of astrogliosis (Cernak et al., 2005; Gunther et al., 2005; Miller and McAllister, 2007), therefore, it has been proposed that microgliosis may be involved in the onset and maintenance of astrogliosis (Balasingam and Yong, 1996; Haga et al., 2002). This hypothesis was further supported by the fact that microglia are the major sources in the brain producing pro-inflammatory factors, which in turn could act as triggers and modulators of astrogliosis.
The purpose of this study was to determine the mechanism underlying the regulation of astrocyte proliferation by microglia. Here, we report that presence of activated microglia is essential in eliciting astrocyte proliferation. Increased Cyclooxygenase (COX-2) expression in activated microglia and the subsequent enhancement of PGE2 released from activated microglia were found to be associated with enhanced astrocyte proliferation in vitro.
Materials and Methods
Reagents
Lipopolysaccharide (LPS) (Escherichia coli strain O111:B4) was purchased from Calbiochem (La Jolla, CA). 96 insert-well plates and 6 insert-well plates were purchased from Millipore Company (Bedford, MA). Polyclonal antibody against COX-2 was purchased from Caymen (Ann Arbor, MI). Rabbit anti-GAPDH was obtained from Abcam (Cambridge, MA). The Vectastain avidin-biotin complex (ABC) kit and biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). Tissue culture media, supplements, fetal bovine serum and horse serum were obtained from Invitrogen (San Diego, CA). The endotoxin level in the serum is less than 0.3 unit/ml. Dup-697 and AH6809 were purchased from Cayman Chemical Company (Ann Arbor, MI).
Animals
Timed-pregnant Fisher F344 rats were obtained from Charles River Laboratories (Raleigh, NC). Rat cultures were used in Figure 1–4. Timed-pregnant C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). The COX-2−/− mice on a C57BL/6-129Ola background have been maintained by heterozygous × heterozygous for more than 50 generations (Langenbach et al., 1999). The COX-2−/− mice used in the present study were produced by breeding COX-2+/− male and female mice so that COX-2+/+, COX-2+/− and COX-2−/− pups were produced in each litter. Mouse cultures were used in Figure 5. The breeding of the mice was carried out to achieve timed pregnancy with an accuracy of ±0.5 days. Animals were treated humanely and with regard for alleviating suffering. Housing and breeding of animals were done in accordance with National Institutes of Health guidelines (Office of Laboratory Animal Welfare 2002). Animals were maintained on a 12:12 h light: dark cycle and fed ad libitum. Housing and breeding of the animals were performed in accordance with the National Institutes of Health Guidelines strictly.
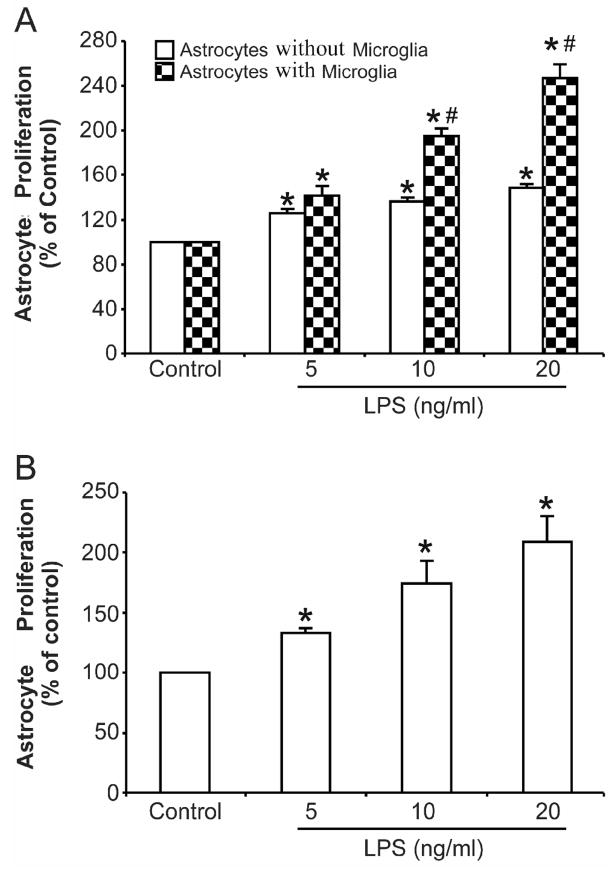
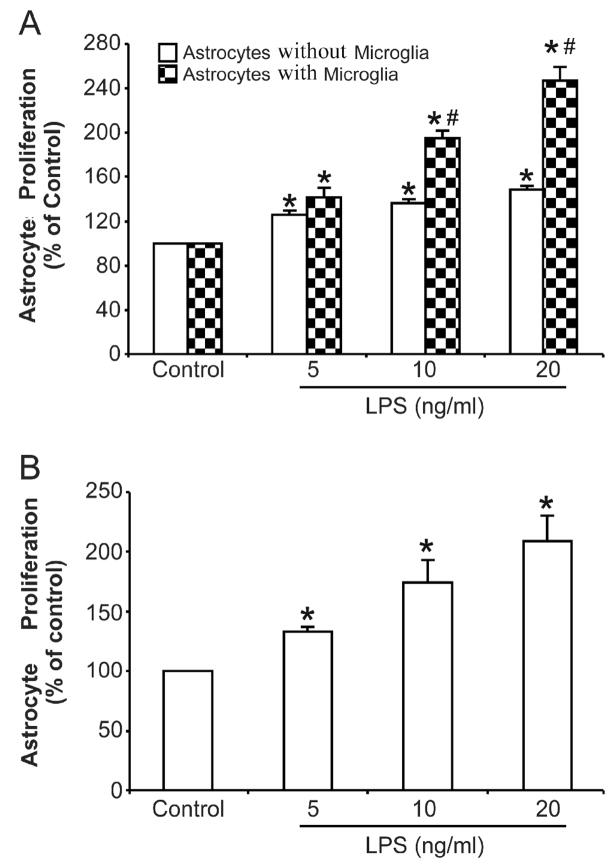
Figure 1. Activated microglia contribute to astrocyte proliferation.
(A) Primary enriched astrocyte cultures with or without microglia within insert wells were treated with various concentrations of LPS in a final volume of 200 μl/well for 72 h. (B) Primary enriched microglial cultures were treated with different concentrations of LPS for 72 h, then the conditioned media was collected and added to primary enriched astrocyte cultures. Incubation was lasted for an additional 72 h. Cell proliferation was assayed using a BrdU ELISA kit as described in Material and Methods. Results are mean ± S.E.M of three experiments performed in triplicate. * p <0.05, compared with corresponding control cultures; # p <0.05, compared with astrocytes alone after same treatment.
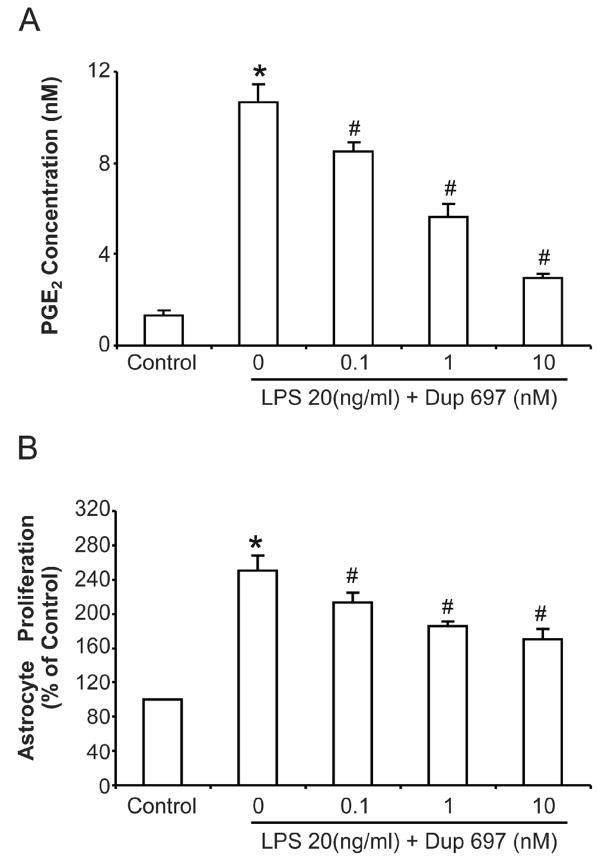
Figure 4. COX-2 specific inhibitor DuP697 attenuated LPS-induced astrocyte proliferation.
COX-2 specific inhibitor DuP697 was applied 1 h prior to LPS treatment in primary enriched astrocyte cultures together with insert wells with microglia. (A) The supernatant were collected 72 h after treatment and PGE2 concentration was assayed using an EIA kit. (B) Cell proliferation was assayed at 72 h after treatment using a BrdU ELISA kit. Results are mean ± S.E.M of three experiments performed in triplicate. * p <0.05, compared with control cultures; # p <0.05, compared with LPS treatment group.
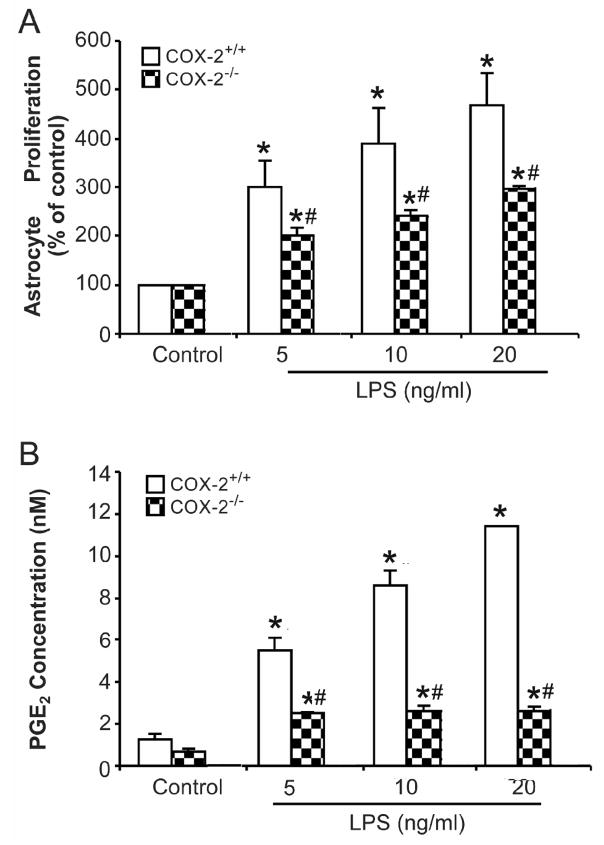
Figure 5. Microglia from COX-2−/− mice showed impaired effect in mediating astrocyte activation.
Primary enriched astrocyte cultures from COX-2+/+ mice along with insert wells with microglia from COX-2+/+ and COX-2−/− mice were treated with different concentrations of LPS for 72 h. (A) Cell proliferation was assayed using a BrdU ELISA kit. (B) The supernatant were collected 72 h after treatment and PGE2 concentration was assayed using an EIA kit. Results are mean ± S.E.M of three experiments performed in triplicate. * p <0.05, compared with corresponding control cultures; # p <0.05, compared with insert wells plus COX-2+/+ microglial cultures.
Cell culture
Primary microglia-enriched cultures and astrocyte-enriched cultures were prepared from the whole brains of 1 or 2-day-old mice, as described previously (Liu et al., 2003). Briefly, brain tissues, devoid of meninges and blood vessels, were dissociated by a mild mechanical trituration. The isolated cells (5 × 107) were seeded in 150 cm2 culture flasks in DMEM/F12 containing 10% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/ml penicillin and 50 μg/ml streptomycin. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The complete medium was changed 4 d later. When reaching confluence (12–14 d), microglia were separated from astrocytes by shaking the flasks at 180 rpm for 5 h. The astrocytes were maintained for one to two more weeks; during the time 0.1 mM L-leucine methyl ester was added to avoid the residue microglia. Astrocytes were then collected with trypsin/EDTA solution. After determination the concentration, astrocytes at 1 × 104/well were seeded to 96-well plates. The purity of the microglia-enriched cultures was >98% and the contamination of microglia in the astrocyte-enriched culture was less than 0.1% as determined by immunocytochemical staining (Iba-1 and GFAP were used as staining marker for microglia and astrocyte, respectively).
Transwell coculture
Astrocytes at 1 × 104/well were seeded to 96-well plates and microglia at 3 × 103/well were plated onto the top side of the transwell inserts. The astrocytes and microglial cells share the same medium but no direct cell–cell interactions are possible due to the physical separation of the cells by a polycarbonate membrane. The pore size of the transwell (0.4 μm) allows no cell migration through the membrane. At the time of seeding, microglia were 2 weeks old and astrocytes were 3–4 weeks old. Viability of the astrocytes on the 96-well plate and microglia on the transwells prior to treatment was verified using trypan blue staining.
Cell proliferation assay
Cell proliferation was detected with a cell proliferation ELISA kit from Roche (Indianapolis, IN), based on the measurement of BrdU incorporation during DNA synthesis. Assays were performed according to the protocol provided by the manufacturer. Briefly, primary enriched astrocyte cultures were treated with 5–20 ng/ml of LPS (15–60 EU/ml) in 96-well plates in a final volume of 100 μl/well for 72 h. This concentration range of LPS has been routinely used in our laboratory to generate moderate level of inflammatory response in microglia. Additionally, the PGE2 level stimulated by 5–20 ng/ml LPS was comparable to the PGE2 level detected in the brain (Loh et al., 2002; Yang et al., 2008; Zhang et al., 2008). The inhibitors used in the present study were applied to the cell culture 1 h prior to LPS challenge and the incubation lasted for 72 h. Subsequently, 10 μl/well BrdU was added to the cells (final concentration: 10 μM) and the cells were reincubated for 4 h. During this labeling period, the pyrimidine analogue BrdU was incorporated in place of thymidine into the DNA of proliferating cells. After removing the culture medium, cells were fixed and the DNA was denatured by adding 200 μl/well FixDenat to the cells and incubation for 30 min at room temperature. After removing the FixDenat solution, a 100 μl/well anti-BrdU-POD working solution was added for another 90 min at 15–25°C. The antibody conjugate was removed by flicking off and rinsing wells three times with 300 μl/well washing solution. Cells were then incubated with 100 μl/well substrate solutions for 15 min. The absorbance at 370 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). The developed color, and thereby the absorbance values, directly correlates with the amount of DNA synthesis and thus with the number of proliferating cells in the respective microcultures.
PGE2 production assay
Cultures were treated with LPS at given doses. After 72 h, supernatants were collected and PGE2 concentration in the supernatant was determined using a PGE2 Enzyme Immunometric Assay (EIA) kit from Cayman, according to the manufacturer’s instructions.
Protein extraction and Western blot analysis
Astrocyte-enriched cultures and microglia-enriched cultures treated with 20 ng/ml LPS for 72 h were detached by scraping in sample buffer (pH 7.9) containing 10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 1.5 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol. Isolated proteins were adjusted to equal concentrations and amounts. The loaded protein amount for microglia is 20 μg, for astrocyte is 60 μg. Proteins were separated using 4 to 12% Bis-Tris ν-polyacrylamide gel electrophoresis gel and then transferred to polyvinylidene difluoride membranes (Novex, San Diego, CA). Membranes were blocked with 5% nonfat milk and incubated with rabbit anti-COX-2 antibody (1: 2000) or rabbit anti-GAPDH (1:2000) overnight at 4°C. Membranes were placed in horseradish peroxidase-linked anti-rabbit IgG (1:3000) for 1 h at 25°C and ECL+Plus reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK) were used as a detection system. Densitometric analysis for each sample was performed (ImageMaster RVDS; GE Healthcare) using three measurements on three separate bolts.
Real-time RT-PCR analysis
Expression levels of selected genes were quantified using real-time RT-PCR analysis. Briefly, total RNA was reverse transcribed using MuLV reverse transcriptase and oligo-dT primers. The forward and reverse primers for select and the sequences were designed using the ABI Primer Express software (Foster City, CA) as follows. COX-2, Forward: GCTGGCCTGGTACTCAGTAGGTT; Reverse: CGAGGCCACTGATACCTATTGC. GAPDH, Forward: CCTGGAGAAACCTGCCAAGTAT; Reverse: AGCCCAGGATGCCCTTTAGT. SYBR green PCR master mix (Applied Biosystems, Cheshire, UK) was used for real-time PCR analysis. Cycle time values of the genes were first normalized with GAPDH of the same sample, and then the relative differences between control and treatment groups were calculated and expressed as relative increases, setting the control as 100%.
Statistical Analysis
Data are expressed as mean ± S.E.M. The level of significance was set of p < 0.05 in both ANOVA and post-hoc Bonferroni’s t-test statistics using the StatView program (Abacus Concepts, Berkeley, CA). A value of p < 0.05 was considered statistically significant.
Results
Microglia enhance LPS-induced astrocyte proliferation
In order to study the role of microglia in astrocyte activation, LPS, a widely used endotoxin, was used to stimulate the activation of microglia and induce astrocyte proliferation. Primary enriched astrocyte cultures with or without microglia in the insert wells of a transwell culture apparatus were treated with different concentrations of LPS (5, 10, 20 ng/ml) in a final volume of 200 μl/well for 72 h. Cell proliferation was then detected using a BrdU incorporation kit. As shown in Fig. 1A, BrdU incorporation was greatly potentiated to roughly 140%, 200% and 250% in astrocyte-microglia co-cultures following 5, 10, 20 ng/ml LPS, respectively. LPS treatment also increased BrdU incorporation in primary enriched astrocyte cultures but to a much less extent (30 to 50%). These results indicated that the existence of activated microglia contributed to astrocyte proliferation. Since there were no direct cell-cell contacts between microglia and astrocytes in this experiment, it is likely that pro-inflammatory factors released by activated microglia are involved in the astrocyte responses. To confirm this speculation, primary enriched microglial cultures were treated with equal concentrations of LPS for 72 h, and the conditioned media was collected without being concentrated and added to primary enriched astrocyte cultures as 100%. Incubation with conditioned media lasted for 72 h and then BrdU incorporation was measured to evaluate astrocyte proliferation. As showed in Fig. 1B, conditioned media from activated microglia induced significant astrocyte proliferation in a LPS dose-dependent manner. These results further suggest that the pro-inflammatory factors released by activated microglia are associated with astrocyte proliferation.
LPS treatment induced COX-2 gene and protein expression in primary enriched microglial and astrocyte cultures
To determine whether prostaglandins are involved in microglia-astrocyte communication, the expression of COX-2, an inducible enzyme expressed in inflamed tissues and its product PGE2 in both microglia and astrocytes were measured. Enriched microglial and astrocyte cultures were treated with the same range of concentrations of LPS (5–20 ng/ml). COX-2 gene expression was measured using RT-PCR at 6 h after LPS treatment and COX-2 protein expression was measured by immunoblotting 24 h following of LPS treatment. As shown in Fig. 2A, the basal expression of COX-2 gene was low in non-treated microglial and astrocytes cultures. LPS treatment significantly enhanced the expression level of microglial COX-2 gene in a dose-dependent manner. In contrast, the levels of COX-2 mRNA in astrocytes were more subdued compared to microglia. Consistent with the gene expression change, similar changes were observed in the levels of COX-2 protein after LPS treatment (Fig. 2B and 2C).
Figure 2. LPS treatment induced COX-2 gene and protein expression in primary enriched microglial and astrocyte cultures.
Enriched microglial and astrocyte cultures were treated with different concentrations of LPS. Then COX-2 gene expression was assayed using RT-PCR (A) at 6 h after treatment and COX-2 protein expression was assayed using Western blot (B) at 24 h post treatment. Quantitative analysis of COX-2 protein expression was shown in C. Data have been normalized to GAPDH and are mean ± S.E.M of three experiments performed in triplicate. * p <0.05, compared with corresponding control cultures; # p <0.05, compared with astrocytes after same treatment.
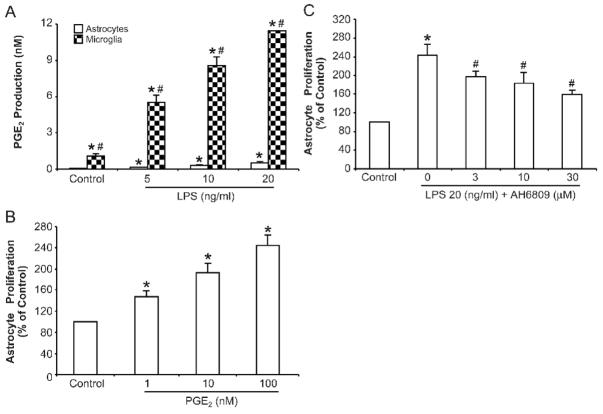
PGE2 is involved in LPS-induced astrocyte proliferation
We then measured the ability of microglia and astrocytes to produce PGE2 after LPS treatment. Primary enriched microglial and astrocyte cultures were treated with various LPS concentrations. Supernatants were collected 72 h after treatment and levels of PGE2 were determined by ELISA. As shown in Fig. 3A, LPS treatment significantly induced PGE2 production from enriched microglial cultures in a dose-dependent manner. When treated with 20 ng/ml LPS, PGE2 levels produced by activated microglia were roughly 20 times higher than that produced by activated astrocytes. This observation indicates that microglia are a major source of PGE2 production.
Figure 3. PGE2 is involved in astrocyte proliferation.
(A) Primary enriched astrocyte and microglial cultures were treated with LPS at given doses. The supernatant were collected 72 h after treatment and PGE2 concentrations were assayed using an ELA kit as described in Material and Methods. * p <0.05, compared with corresponding control cultures; # p <0.05, compared with astrocytes after same treatment. (B) Different concentrations of PGE2 were added to purified astrocyte cultures and the incubated for 72 h. Cell proliferation was assayed using a BrdU ELISA kit. * p <0.05, compared to corresponding control cultures. (C) Astrocytes and microglia co-cultures in transwell were pre-treated with the non-selective PGE2 receptor antagonist AH6809 for 1 h then challenged with 20 ng/ml LPS for 72 h. Astrocyte proliferation was then measured. Results are mean ± S.E.M of three experiments performed in triplicate. * p <0.05, compared with control cultures; # p <0.05, compared with LPS treatment group.
To ascertain that PGE2 released by activated microglia plays a role in mediating astrocyte proliferation, exogenous PGE2 in the concentration range produced by microglia after LPS treatment (Fig. 3A) was added to enriched astrocytes culture and incubated for 72 h. As shown in Fig. 3B, PGE2 significantly increased the incorporation of BrdU in astrocytes in a dose-dependent fashion.
To further confirm the essential role of microglial PGE2 in mediating astrocyte proliferation, we pretreated microglia-astrocyte co-cultures with a non-selective PGE2 receptor antagonist AH6809 for 1 h and then challenged the cells with 20 ng/ml LPS. As shown in Fig 3C, AH6809 significantly inhibited LPS induced astrocyte proliferation in a dose-dependent manner. However, AH6809 at high concentrations failed to abolish astrocyte proliferation. Although this might because of the failure to block all the receptors of PGE2 by AH6809, it is likely that pro-inflammatory factors other than PGE2, released by activated microglia also contribute to the enhanced astrocyte proliferation.
COX-2 specific inhibitor DuP697 attenuated LPS-induced PGE2 production and astrocyte proliferation
The effect of microglial PGE2 in astrocyte proliferation was further confirmed using a COX-2 specific inhibitor DuP697. Similar to Fig 1, transwell astrocyte-microglia co-cultures were used for this experiment. Different concentrations of COX-2 specific inhibitor DuP697 were applied 1 h prior to LPS treatment. Dup-697 effectively inhibited the PGE2 level in the medium (Fig. 4A). The same pretreatment also significantly attenuated LPS-induced BrdU incorporation in enriched astrocyte cultures in a dose-dependent manner (Fig. 4B). However, high concentrations of DuP697 failed to abolish the astrocyte proliferation induced by LPS. Thus, these two independent experiments both indicated that pro-inflammatory factors other than PGE2 released by activated microglia participated in the LPS-induced increase of astrocyte proliferation.
Microglia from COX-2−/− mice showed impaired effect in mediating astrocyte activation
To further confirm that microglial PGE2 is involved in mediating astrocyte proliferation, primary enriched microglia prepared from COX-2+/+ and COX-2−/− mice were seeded in insert wells, then loaded on top of enriched COX-2+/+ astrocyte cultures pre-seeded in 96 wells. Cultures were then treated with different concentrations of LPS for 72 h and astrocyte proliferation was determined. As shown in Fig. 5A, LPS treatment significantly increased BrdU incorporation in astrocyte cultures when microglia from COX-2+/+ mice were present in the insert wells. In contrast, BrdU incorporation in astrocytes was much lower when microglia from COX-2−/− mice were used. This result was consistent with the finding that LPS-induced increase levels of PGE2 was significantly abolished in COX-2−/− microglia cultures (Fig. 5B).
Discussion
In this study we provided strong evidence indicating that activation of microglia by LPS promoted an increase in astrocyte proliferation. We further demonstrated that activated microglia enhanced astrocyte proliferation through the up-regulation of COX-2 and subsequent release of PGE2. Pharmacological inhibition or genetic ablation of COX-2 in microglia reduced the release of PGE2 and greatly diminished its ability to promote astrocyte proliferation. Furthermore, a PGE2 receptor antagonist, AH6809, was shown to attenuate microglia-mediated increase in astrocyte proliferation.
Astrocytes can be activated in response to many brain injuries, such as stroke, trauma, tumor, or neurodegenerative diseases, as well as multiple endogenous or exogenous stimuli including fibroblast growth factor or cannabinoids released by damaged neurons (Barbeito et al., 2004; O’Callaghan and Sriram, 2005; Pehar et al., 2005; Widmer et al., 2008). Neuronal death is capable of inducing astrocyte proliferation even without the involvement of microglia. For example, Aicha Douhou reported in the substantia nigra of weaver knowout mice, the onset of dopaminergic cell loss was correlated with a large and permanent increase in the number of astrocyte while only a transient activation of microglial cells was found (Douhou et al., 2003). Besides neuronal death induced astrocyte proliferation, there are also numerous studies describe intimate interaction between microglia and astrocytes. There have been several references reporting the effect of microglia on astrocyte proliferation in vivo. It is reported that early onset microglial activation/proliferation can significantly influence the subsequent development of reactive astrogliosis and glial scar formation in spinal cord injury animal model (Tian et al., 2007). Besides, intra-gliomal density of amoeboid microglia is reported to be higher than in normal brain and correlates with the grade of malignancy (Wierzba-Bobrowicz et al., 1994; Graeber et al., 2002). The presence of microglia in and around gliomas reflects the immune response or microglial recruitment by gliomas and may play a functional role in glioma survival. It is also documented that many substances produced by microglia can influence glioma proliferation and migration (Watters et al., 2005). Concerning the astroglial cell number and cell trophy (GFAP expression), it’s reported that activated microglia enhance the astrocyte growth but do not have a hypertrophic effect on astrocyte (Rohl et al., 2007). Besids, proinflammatory cytokines like IL-1 and TNFα are reported to produce a decrease of GFAP expression in astrocyte culture (Selmaj et al., 1991; Oh et al., 1993; Lee et al., 1995; Murphy et al., 1995). It’s speculated that activated microglia might be to a great extent involved in the onset of astrogliosis but other or additional cell interaction might be responsible for the typical increase of GFAP in astrogliosis. As an important feature of astrogliosis, astrocyte proliferation has been more and more used in evaluation the extent of astrogliosis. So, in the present study, we use astrocyte proliferation as a parameter to evaluate the effect of activated microglia. By using transwell cultures of purified astrocytes and microglia, we provided strong evidence that microglia mediate LPS-induced astrocyte proliferation. In the presence of microglia, LPS greatly enhanced the proliferation of astrocytes. In contrast, LPS-induced increase in BrdU incorporation was minimal in enriched astrocyte cultures. These results clearly point out the essential role of microglia in astrocyte proliferation.
We further examined the possible factors released from activated microglia which may contribute to LPS-elicited astrocyte proliferation. LPS has been widely used as a means to stimulate the release of inflammatory mediators from activated microglia. Among the pro-inflammatory factors released by microglia, PGE2 plays a crucial role in various biological events such as neuronal function, female reproduction, vascular hypertension, tumorigenesis, kidney function and inflammation (Hori et al., 1998; Zacharieva et al., 2004; Glodny and Pauli, 2006). Two enzymes responsible for the production of PGE2 are designated as COX-1 (constitutive) and COX-2 (inducible) (Terlain et al., 1995; Griswold and Adams, 1996; Park et al., 2006). It is generally believed that constitutive COX-1 is involved in normal physiological processes and inducible COX-2 is primarily responsible for the production of PGs in inflamed tissues and associated with inflammatory diseases. Accumulating evidence indicates that increased PGE2 levels resul from COX-2 over expression are important in cell proliferation, apoptosis and migration (Murakami and Kudo, 2004). Under pathological conditions such as tumor, especially those associated with a glial response, increased COX-2 expression and elevated levels of PGE2 have been found in the brain (Hata and Breyer, 2004; Takemiya et al., 2007). In the present study, the direct effect of exogenous PGE2 on astrocyte activation and the inhibitory effect of AH6809, a non-selective PGE2 receptor inhibitor, suggested the involvement of PGE2 in microglia-enhanced astrocyte proliferation. Further evidence showed that although LPS treatment increased COX-2 expression and PGE2 production in both microglia and astrocytes, PGE2 produced by microglia is roughly 20 fold more than that produced by an equal number of actrocytes. Thus, these observations demonstrate that microglia are the major sources for PGE2 production. These results are consistent with findings that LPS induces minimal increases in astrocyte proliferation in the absence of microglia.
Microglia are the major sources in the brain producing pro-inflammatory factors, which in turn could act as triggers and modulators of astrocyte proliferation. The effect of other pro-inflammatory factors on the induction of astrocyte proliferation has been examined in several studies. Pro-inflammatory factors such as IL-1, IL-6, PGD2 and TNFα have been shown to induce, enhance or accompany astrocyte proliferation (Barna et al., 1990; Selmaj et al., 1991; Balasingam et al., 1994; Herx and Yong, 2001; Cardenas and Bolin, 2003; Mohri et al., 2006). However, the concentrations of these proinflammatory factors used in previous studies were 2 to 3 orders of magnitude higher than the levels detected in microglia conditioned media or in the brain. In contrast, the concentration of PGE2 added to the cultures to promote astrocyte proliferation in this study was in the range of that produced by LPS-activated microglia and in the range of PGE2 levels that detected in brain (Loh et al., 2002). This is of critical importance that inhibition of PGE2 synthesis or antagonism of PGE2 receptors might be useful in the prevention and/or treatment of diseases related to un-controlled astrocyte proliferation. It is worth pointing out that the PGE2 receptor inhibitor used in the present study is reported to bind to PGD2 receptor, too (Kiriyama et al., 1997). PGD2 signal pathway is reported to be involved in astrocyte proliferation (Mohri et al., 2006). So, the inhibitory effect of AH6809 we showed in Figure 3C is possible part of the effect on PGD2 pathway besides the PGE2 pathway.
The role of microglial COX-2 and PGE2 in astrocyte proliferation was further confirmed by using a COX-2 selective inhibitor and COX-2 deficient microglia. Dup-697, a selective COX-2 inhibitor, significantly and dose-dependently inhibited LPS-induced increases in production of PGE2 and astrocyte proliferation. Results from pharmacological manipulation were further substantiated by using glial cultures prepared from COX-2-deficient mice. COX-2-deficient microglial cultures produced significant less PGE2 and elicited a much lower rate of astrocyte proliferation than microglial cultures from wild type control mice. Taken together, these findings indicate that PGE2 is a potent mediator for microglia-induced astrocyte activation and suggest therapeutic possibilities that either inhibition of PGE2 synthesis or treatment with antagonists of PGE2 receptors might be useful in the prevention and/or treatment of glioma.
The findings in this study are of particular clinical significance for the mechanistic investigation and treatment of gliomas, which develop mainly from astrocytes and are the most common primary brain tumors and one of the leading causes of cancer-related deaths in young children and adults (Noble and Mayer-Proschel, 1997; Shapiro, 2001). All current treatment options have limitations. Radiation therapy may be associated with neurotoxicity in adjacent normal tissues. Chemotherapy is constrained by the blood-brain barrier. Surgical resection is limited by non-circumscribed borders of most gliomas. The newest generation of treatments includes more effective cytotoxic agents, so-called targeted compounds, and biologics/immunotherapeutics. To date, none of these has proven highly effective across the wide spectrum of tumors. Such limitations explain the reasons why survival rates have improved so little over the past few decades of research regarding treatment of glioma. Thus, identifying and ultimately targeting the molecules that lead to glioma progression will have a strong impact on future treatment strategies (Salgaller and Liau, 2006; Lukas et al., 2007).
In summary, this study supports the view that activated microglia may be critically involved in the onset and maintenance of astrocyte proliferation. Moreover, our data provide evidence that astrocyte activation is likely mediated in part through the up-regulation of COX-2 expression in microglia and subsequent PGE2 production. Though detailed molecular characterization of this process awaits further investigations, the present study demonstrates that interruption of PGE2 effect inhibits proliferative in astrocytes. In conjugation with previous studies indicating COX-2 inhibitors are effective in the treatment of glioma (Joki et al., 2000; Giglio and Levin, 2004; New, 2004), PGE2 might be an important potential target for therapeutic interventions in the management of glioma.
Acknowledgments
Acknowledgments/grant support
We thank F. Gordon for helpful comments and suggestions. We also thank Anthony Lockhart for assistance with animal colony management and maintenance of the timed pregnant mice.
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences.
Footnotes
Disclaimers/Competing Interests Declaration
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasingam V, Yong VW. Attenuation of astroglial reactivity by interleukin-10. J Neurosci. 1996;16:2945–2955. doi: 10.1523/JNEUROSCI.16-09-02945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Barna BP, Estes ML, Jacobs BS, Hudson S, Ransohoff RM. Human astrocytes proliferate in response to tumor necrosis factor alpha. J Neuroimmunol. 1990;30:239–243. doi: 10.1016/0165-5728(90)90108-y. [DOI] [PubMed] [Google Scholar]
- Cardenas H, Bolin LM. Compromised reactive microgliosis in MPTP-lesioned IL-6 KO mice. Brain Res. 2003;985:89–97. doi: 10.1016/s0006-8993(03)03172-x. [DOI] [PubMed] [Google Scholar]
- Cernak I, Stoica B, Byrnes KR, Di Giovanni S, Faden AI. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle. 2005;4:1286–1293. doi: 10.4161/cc.4.9.1996. [DOI] [PubMed] [Google Scholar]
- Douhou A, Debeir T, Michel PP, Stankovski L, Oueghlani-Bouslama L, Verney C, Raisman-Vozari R. Differential activation of astrocytes and microglia during post-natal development of dopaminergic neuronal death in the weaver mouse. Brain Res Dev Brain Res. 2003;145:9–17. doi: 10.1016/s0165-3806(03)00190-1. [DOI] [PubMed] [Google Scholar]
- Giglio P, Levin V. Cyclooxygenase-2 inhibitors in glioma therapy. Am J Ther. 2004;11:141–143. doi: 10.1097/00045391-200403000-00009. [DOI] [PubMed] [Google Scholar]
- Glodny B, Pauli GF. The vasodepressor function of the kidney: prostaglandin E2 is not the principal vasodepressor lipid of the renal medulla. Acta Physiol (Oxf) 2006;187:419–430. doi: 10.1111/j.1748-1716.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40:252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev. 1996;16:181–206. doi: 10.1002/(SICI)1098-1128(199603)16:2<181::AID-MED3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Gunther A, Kuppers-Tiedt L, Schneider PM, Kunert I, Berrouschot J, Schneider D, Rossner S. Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischaemia. Eur J Neurosci. 2005;21:3189–3194. doi: 10.1111/j.1460-9568.2005.04151.x. [DOI] [PubMed] [Google Scholar]
- Haga S, Haga C, Aizawa T, Ikeda K. Neuronal degeneration and glial cell-responses following trimethyltin intoxication in the rat. Acta Neuropathol. 2002;103:575–582. doi: 10.1007/s00401-001-0505-5. [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Herber DL, Maloney JL, Roth LM, Freeman MJ, Morgan D, Gordon MN. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia. 2006;53:382–391. doi: 10.1002/glia.20272. [DOI] [PubMed] [Google Scholar]
- Herx LM, Yong VW. Interleukin-1 beta is required for the early evolution of reactive astrogliosis following CNS lesion. J Neuropathol Exp Neurol. 2001;60:961–971. doi: 10.1093/jnen/60.10.961. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Hori T, Oka T, Hosoi M, Aou S. Pain modulatory actions of cytokines and prostaglandin E2 in the brain. Ann N Y Acad Sci. 1998;840:269–281. doi: 10.1111/j.1749-6632.1998.tb09567.x. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Leung CC, Sadeghian M, Haddon CO, Rose S, Jenner P. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur J Neurosci. 2005;22:317–330. doi: 10.1111/j.1460-9568.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, Seyfried NT, Abe T, Chen LB, Carroll RS, Black PM. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60:4926–4931. [PubMed] [Google Scholar]
- Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Brosnan CF. Interleukin-1, nitric oxide and reactive astrocytes. Brain Behav Immun. 1995;9:345–354. doi: 10.1006/brbi.1995.1032. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Li G, Zhang W, An L, Liu B, Hong JS. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther. 2003;305:212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- Loh JK, Hwang SL, Lieu AS, Huang TY, Howng SL. The alteration of prostaglandin E2 levels in patients with brain tumors before and after tumor removal. J Neurooncol. 2002;57:147–150. doi: 10.1023/a:1015782809966. [DOI] [PubMed] [Google Scholar]
- Lukas RV, Boire A, Nicholas MK. Emerging therapies for malignant glioma. Expert Rev Anticancer Ther. 2007;7:S29–36. doi: 10.1586/14737140.7.12s.S29. [DOI] [PubMed] [Google Scholar]
- Miller JM, McAllister JP., 2nd Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5. doi: 10.1186/1743-8454-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, Fukumoto N, Eguchi N, Kushi A, Sasai H, Kanaoka Y, Ozono K, Narumiya S, Suzuki K, Urade Y. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci. 2006;26:4383–4393. doi: 10.1523/JNEUROSCI.4531-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr, Lee YL, Jia XC, Yu AC, Majewska A, Song Y, Schmidt K, Eng LF. Tumor necrosis factor-alpha and basic fibroblast growth factor decrease glial fibrillary acidic protein and its encoding mRNA in astrocyte cultures and glioblastoma cells. J Neurochem. 1995;65:2716–2724. doi: 10.1046/j.1471-4159.1995.65062716.x. [DOI] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- New P. Cyclooxygenase in the treatment of glioma: its complex role in signal transduction. Cancer Control. 2004;11:152–164. doi: 10.1177/107327480401100303. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M. Growth factors, glia and gliomas. J Neurooncol. 1997;35:193–209. doi: 10.1023/a:1005898228116. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- Office of Laboratory Animal Welfare. Public Health Services Policy on Humane Care and Use of Laboratory Animals. Bethesda, MD: Office of Laboratory Animal Welfare; 2002. [Google Scholar]
- Oh YJ, Markelonis GJ, Oh TH. Effects of interleukin-1 beta and tumor necrosis factor-alpha on the expression of glial fibrillary acidic protein and transferrin in cultured astrocytes. Glia. 1993;8:77–86. doi: 10.1002/glia.440080203. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Seboun E, Hauser SL. Genetics of demyelinating diseases. Brain Pathol. 1996;6:289–302. doi: 10.1111/j.1750-3639.1996.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Cassina P, Barbeito AG, Beckman JS, Barbeito L. Complexity of astrocyte-motor neuron interactions in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:139–146. doi: 10.1159/000089619. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Poulsen C, Carrasco J, Hidalgo J. M-CSF deficiency leads to reduced metallothioneins I and II expression and increased tissue damage in the brain stem after 6-aminonicotinamide treatment. Exp Neurol. 2002;176:308–321. doi: 10.1006/exnr.2002.7968. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rohl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129:43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Salgaller ML, Liau LM. Current status of clinical trials for glioblastoma. Rev Recent Clin Trials. 2006;1:265–281. doi: 10.2174/157488706778250140. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Shafit-Zagardo B, Aquino DA, Farooq M, Raine CS, Norton WT, Brosnan CF. Tumor necrosis factor-induced proliferation of astrocytes from mature brain is associated with down-regulation of glial fibrillary acidic protein mRNA. J Neurochem. 1991;57:823–830. doi: 10.1111/j.1471-4159.1991.tb08225.x. [DOI] [PubMed] [Google Scholar]
- Shapiro JR. Genetics of brain neoplasms. Curr Neurol Neurosci Rep. 2001;1:217–224. doi: 10.1007/s11910-001-0021-y. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Matsumura K, Yamagata K. Roles of prostaglandin synthesis in excitotoxic brain diseases. Neurochem Int. 2007;51:112–120. doi: 10.1016/j.neuint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Terlain B, Jouzeau JY, Gillet P, Lecompte T, Netter P. Inducible cyclooxygenase. New relationships between non-steroidal anti-inflammatory agents and inhibition of synthesis of prostaglandins. Presse Med. 1995;24:491–496. [PubMed] [Google Scholar]
- Tian DS, Dong Q, Pan DJ, He Y, Yu ZY, Xie MJ, Wang W. Attenuation of astrogliosis by suppressing of microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury model. Brain Res. 2007;1154:206–214. doi: 10.1016/j.brainres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Berger J, Hermans E. Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol. 2007;189:23–30. doi: 10.1016/j.jneuroim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- Widmer M, Hanemann CO, Zajicek J. High concentrations of cannabinoids activate apoptosis in human U373MG glioma cells. J Neurosci Res. 2008 doi: 10.1002/jnr.21757. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Kuchna I, Matyja E. Reaction of microglial cells in human astrocytomas (preliminary report) Folia Neuropathol. 1994;32:251–252. [PubMed] [Google Scholar]
- Yang S, Zhang D, Yang Z, Hu X, Qian S, Liu J, Wilson B, Block M, Hong JS. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem Res. 2008;33:2044–2053. doi: 10.1007/s11064-008-9675-z. [DOI] [PubMed] [Google Scholar]
- Zacharieva S, Atanassova I, Orbetzova M, Kirilov G, Nachev E, Kalinov K, Shigarminova R. Vascular endothelial growth factor (VEGF), prostaglandin E2(PGE2) and active renin in hypertension of adrenal origin. J Endocrinol Invest. 2004;27:742–746. doi: 10.1007/BF03347516. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hu X, Wei SJ, Liu J, Gao H, Qian L, Wilson B, Liu G, Hong JS. Squamosamide derivative FLZ protects dopaminergic neurons against inflammation-mediated neurodegeneration through the inhibition of NADPH oxidase activity. J Neuroinflammation. 2008;5:21. doi: 10.1186/1742-2094-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]