Abstract
Purpose
Gemcitabine-radiotherapy is a standard treatment for locally advanced pancreatic cancer. Clinical data have demonstrated that gemcitabine plus erlotinib is superior to gemcitabine alone for advanced pancreatic cancer. Therefore, we investigated the effects of the combination of EGFR inhibitors with gemcitabine and radiation on a pancreatic cancer model.
Experimental Design
EGFR signaling was analyzed by measuring phosphorylated EGFR (pEGFR(Y845), (Y1173)) and AKT (pAKT(S473)) protein levels in pancreatic cancer cell lines and tumors. The effects of scheduling on gemcitabine-mediated cytotoxicity and radiosensitization combined with erlotinib were determined by clonogenic survival. In vivo, the effects of cetuximab or erlotinib in combination with gemcitabine-radiation on the growth of BxPC-3 tumor xenografts were measured.
Results
We found in vitro that gemcitabine induced phosphorylation of EGFR at Y845 and Y1173 that was blocked by erlotinib. Treatment of BxPC-3 cells with gemcitabine prior to erlotinib enhanced gemcitabine-mediated cytotoxicity without abrogating radiosensitization. In vivo, cetuximab or erlotinib in combination with gemcitabine-radiation inhibited growth compared to gemcitabine-radiation (time to tumor doubling: Gem+RT 19±3 days, Cet+Gem+RT 30±3 days; p<0.05, Erl+Gem+RT 28±3 days; p<0.1. Cetuximab or erlotinib in combination with gemcitabine-radiation resulted in significant inhibition of pEGFR(Y1173) and pAKT(S473) early in treatment, and pEGFR(Y845), pEGFR(Y1173), and pAKT(S473) by the end of treatment. This study demonstrates a novel difference pEGFR(Y845) and pEGFR(Y1173) in response to EGFR inhibition.
Conclusions
These results demonstrate that the EGFR inhibitors cetuximab and erlotinib increase the efficacy of gemcitabine-radiation. This work supports the integration of EGFR inhibitors with gemcitabine-radiation in clinical trials for pancreatic cancer.
Keywords: cetuximab, erlotinib, gemcitabine, radiation, pancreatic cancer
Introduction
Pancreatic cancer is the 4th leading cause of cancer related deaths in the U.S. and has one and five-year survival rates of 26% and 5%, respectively (1). In the past 10 years, gemcitabine has replaced 5-fluorouracil as the standard therapy for metastatic pancreatic cancer. The combination of highly conformal radiation with gemcitabine improves median survival in patients with locally advanced disease (12-13 months) (2) compared to historical controls who received gemcitabine alone (7-9 months) (3, 4). Although local tumor control is an important issue, the majority of treatment failures are due to systemic disease progression (5). Therefore, our approach in designing clinical trials for pancreatic cancer has emphasized improving systemic disease control while maintaining local tumor control.
A number of studies have been conducted in an effort to improve gemcitabineradiotherapy by adding other chemotherapeutic agents. Based on clinical data suggesting that cisplatin combined with gemcitabine produced a survival benefit in patients with metastatic pancreatic cancer (6), we designed a preclinical study combining cisplatin with gemcitabine-radiation (7). The finding that cisplatin and gemcitabine produced synergistic cytotoxicity without abrogating gemcitabine-mediated radiosensitization led us to investigate the combination in a Phase I/II clinical trial. This study suggested that full systemic doses of gemcitabine and cisplatin could be administered in combination with conformal tumor radiation, and demonstrated a promising median survival of 13 months (8).
More recently, our focus has shifted to the combination of molecularly targeted therapies with gemcitabine-radiation. EGFR is a member of the ErbB family of receptor tyrosine kinases. In response to stimulation by ligands such as EGF or TGFα, the receptor homoor heterodimerizes (with other ErbB family members) and undergoes autophosphorylation at a number of tyrosine residues, including 974, 992, 1045, 1068, 1086, 1148, and 1173 (9). Alternatively, the Src non-receptor kinase can phosphorylate EGFR at tyrosine residues 845 and 1101 (10, 11). EGFR phosphorylation produces activation of downstream signaling pathways involving STAT, AKT, ERK, and PKC, which induce cellular responses such as survival, oncogenesis, angiogenesis, cell cycle progression, and transformation. EGFR-targeting monoclonal antibodies, as well as small molecule inhibitors, have been approved by FDA for use in several tumor sites (12, 13), alone and in combination with gemcitabine for pancreatic cancer (14). Although EGFR mutation is rare in pancreatic cancer (2-4%) (15, 16), overexpression of EGFR occurs in at least one-half of all pancreatic cancers (17, 18), and correlates with a poor prognosis (19, 20). A recent Phase III clinical trial comparing patients with advanced pancreatic cancer randomized to receive gemcitabine plus the EGFR inhibitor, erlotinib, or gemcitabine alone, demonstrated a significant improvement in median survival of 6.2 versus 5.9 months as well as 1 year survival of 23 % versus 17 %, respectively (21).
Therefore, we designed a study to determine whether the addition of an EGFR antagonist to gemcitabine and radiation could produce radiosensitization greater than or equal to that produced by gemcitabine and radiation. This approach is consistent with our clinical goal of improving systemic therapy while maintaining or improving local control (radiosensitization). We hypothesized that the schedule of gemcitabine, radiation, and EGFR inhibitor as well as the EGFR phosphorylation status and the choice of EGFR antagonist would influence pancreatic cancer cell survival and tumor growth. We first identified a schedule in vitro that enhanced cytotoxicity, and radiosensitized at least as much as gemcitabine alone. We then adapted this schedule to a murine pancreatic tumor xenograft model, where we assessed the effect of adding either erlotinib or cetuximab to gemcitabine-radiotherapy on tumor growth and EGFR signaling.
Methods and Materials
Cell lines and drug solutions
The human pancreatic adenocarcinoma cell lines BxPC-3, Panc-1, and MPanc-96 were obtained from American Type Culture Collection (ATCC; Manassas, VA) and maintained in RPMI 1640 (BxPC-3 and MPanc-96) or DMEM mediums, with 10% fetal bovine serum and antibiotics at 37°C in 5% CO2. Gemcitabine (supplied by Eli Lilly, Indianapolis, IN) was dissolved in phosphate-buffered saline and stored at -20°C. Erlotinib was provided by OSI Pharmaceuticals (Melville, NY) and dissolved in DMSO. Cetuximab was provided by ImClone (New York, NY) as a 2 mg/ml aqueous solution. Cells were routinely screened for Mycoplasma contamination.
Clonogenic cell survival assay
Clonogenic assays were performed using standard techniques as described previously(22). Drug cytotoxicity was calculated as the ratio of surviving drug-treated cells relative to untreated controls. Radiation survival data from drug-treated cells were corrected for plating efficiency using an unirradiated plate treated with drug under the same conditions. Cell survival curves were fitted using the linear-quadratic equation, and the mean inactivation dose calculated according to the method of Fertil and colleagues(23). The cell survival enhancement ratio was calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose after drug exposure. A value significantly greater than 1 indicates radiosensitization.
Tumor growth studies
BxPC-3 cells (5×106) were transplanted subcutaneously into the flank of athymic Nude-Foxn1nu mice (Harlan, Indianapolis, IN). Treatment was started once a tumor reached 100 mm3. Animals were given gemcitabine on day 0 and 7, erlotinib on days 1-5 and 8-12, cetuximab on days 1 and 8, radiation on days 1-5 and 8-12 (4 hours post erlotinib or cetuximab), and no treatment on days 6 and 12. For immunoblot studies, treatment was ended and tumors harvested on day 2. Body weight and tumor size were measured 3 times/week. Tumor volume (TV) was calculated according to the equation for a prolate spheroid, TV = π / 6 (ab2), where a and b are the longer and shorter dimensions of the tumor, respectively. Measurements were made until day 90 or until the tumor volume increased by approximately a factor of ten, at which point the animals were sacrificed to avoid potential discomfort. Animals were handled according to the established procedures of the University of Michigan Laboratory Animals Maintenance Manual.
Irradiation
Irradiations were carried out using a Pantak Therapax DXT 300 Model X-ray unit (PANTAK, East Haven, CT) at a dose rate of approximately 3 Gy/min. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration. For tumor irradiation, animals were anesthetized with ketamine/xylazine and positioned such that the apex of each flank tumor was at the center of a 2.4 cm aperture in the secondary collimator and irradiated, with the rest of the mouse being shielded from radiation.
Immunoblotting
Cell pellets or pulverized frozen tumors were prepared in buffer containing 10 mmol/L Tris (pH 7.4), 2% sodium dodecyl sulfate, 1X Complete Protease Inhibitor Cocktail (Roche, Mannheim, Germany), 1 mmol/L sodium fluoride, 2 mmol/L sodium orthovanadate, 1 mmol/L sodium pyrophosphate, and phosphatase inhibitor cocktails 1 and 2 (according to the manufacturer's instructions; Sigma Chemical, St. Louis, MO). Protein concentration was determined with the BCA Protein Assay Reagent (Pierce, Rockford, IL). Samples were diluted in loading buffer (0.32 mol/L Tris-HCl, 10% glycerol, 2% sodium dodecyl sulfate, 0.2% bromophenol blue, 4% 2-mercaptoethanol, pH 6.8) and resolved on 4-12 % gradient Bis-Tris gels (Invitrogen, Carlsbad, CA). Separated proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA) and hybridized overnight at 4°C with antibodies recognizing pEGFR(Y845), pEGFR(Y1173), pAKT(S473), AKT (Cell Signaling Technology, Beverly, MA), EGFR, or GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then probed with secondary antibodies, incubated with ECL Plus reagent (Amersham Biosciences, Little Chalfont, Buckinghamshire, England), and exposed to film. The ImageJ program (National Institutes of Health) was used for quantification of the specific protein bands on film.
Statistics
All statistical analyses were performed using SAS v9.1 (SAS Institute, Cary, NC). The time to doubling was determined for each xenograft by identifying the earliest day on which it was at least twice as large as on the first day of treatment, and then estimating the exact time of doubling by linear interpolation with the previous measurement. Since most animals had two implants, the differential effect of treatments on time to doubling was analyzed by a random effects ANOVA, which accounts for correlation between tumors within the same animal. Estimates of means, differences between means, their standard errors and statistical significance are all derived from the ANOVA models. Phosphorylation data were analyzed using a standard ANOVA model.
Results
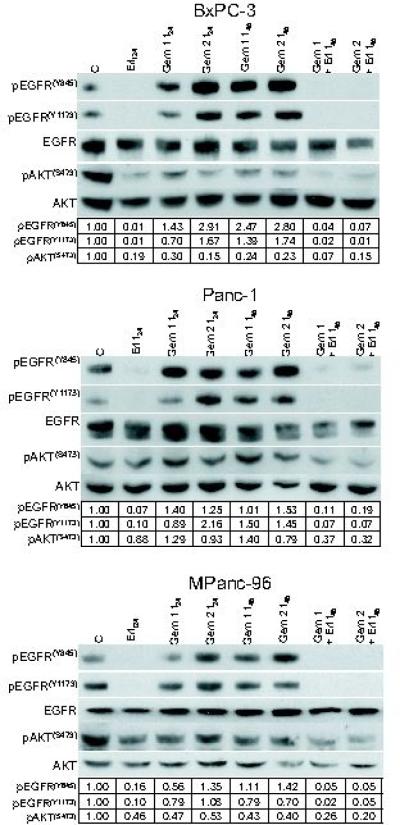
We began this study by examining the effect of gemcitabine and erlotinib on EGFR signaling in several pancreatic cancer cell lines. Since our previous work in head and neck cancer cells demonstrated that gemcitabine induced phosphorylation of EGFR at the Src-dependent phosphorylation site, Y845, we investigated this site as well as the autophosphorylation site, Y1173. We found in the BxPC-3, Panc-1, and MPanc-96 cell lines, treated for 2 hours with approximately IC30 (G1) or IC70 (G2) concentrations of gemcitabine that phosphorylation of EGFR was induced at Y845 by the 48 hours post-treatment time point (Figure 1). Phosphorylation of EGFR at Y1173 occurred in BxPC-3 and Panc-1 cells in response to gemcitabine. Furthermore, we found that a 24-hour exposure to 3uM erlotinib dramatically reduced the basal levels of pEGFR(Y845) and pEGFR(Y1173), as well as blocked the induction of pEGFR in response to gemcitabine. We also examined the levels of phosphorylated AKT (pAKT(S473)). In BxPC-3 cells, treatment with erlotinib, gemcitabine, or the combination of both resulted in reduced levels of pAKT(S473) (Figure 1). In contrast, in Panc-1 and MPanc-96 cells, pAKT(S473) levels were resistant to erlotinib or gemcitabine alone and were only reduced in response to the combination.
Figure 1.
The effects of gemcitabine and erlotinib on EGFR in pancreatic cancer cells. BxPc-3, Panc-1, or MPanc-96 cells were treated for 2 hours with 100nM (G1), 300nM (G2) (BxPC-3), 1uM (G1) or 3uM (G2) (Panc-1 and MPanc-96) gemcitabine. Cells were treated for 24 hours with 3uM erlotinib beginning 24 hours after gemcitabine exposure. Cells were harvested for immunoblotting at 24 and 48 hours post-gemcitabine (t24, 48) or immediately after erlotinib (t24, 48). The amounts of the indicated proteins are shown from a single experiment for each cell line that is representative of at least 3 independent experiments.
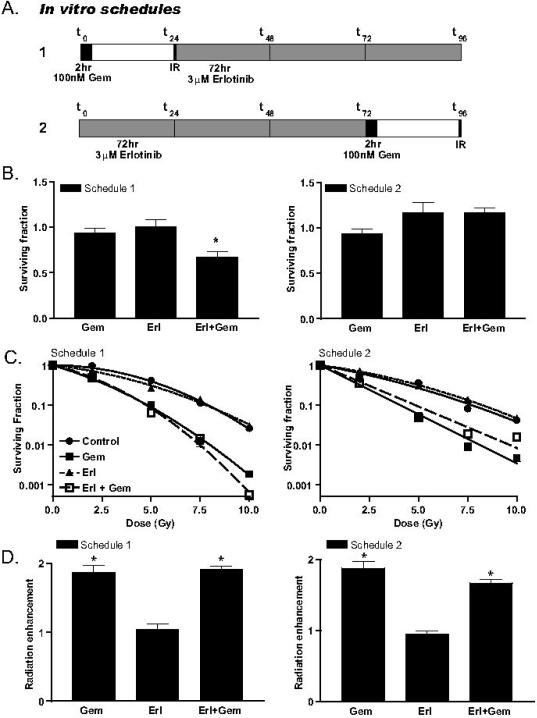
To understand the influence of EGFR inhibitors on gemcitabine-mediated radiosensitization, we first examined cytotoxicity in response to two different schedules of gemcitabine and erlotinib. In the first schedule, a 2-hour gemcitabine treatment was followed 24 hours later by a 72-hour erlotinib exposure (Figure 2A). Treatment under this condition resulted in an enhancement of gemcitabine-mediated cytotoxicity by erlotinib (Figure 2B). In the second schedule, erlotinib was given for 72 hours and followed by a 2-hour gemcitabine exposure and a 22-hour gap before plating for clonogenic survival. In contrast to the first schedule, the second schedule produced no enhanced gemcitabinecytotoxicity by erlotinib. To further understand the importance of scheduling EGFR inhibitors with gemcitabine and radiation, we performed radiosensitization experiments under these two treatment conditions, in which irradiation was performed 24 hours after gemcitabine treatment. For both schedules 1 and 2, gemcitabine alone produced significant radiosensitization (RER 1.9 ± 0.10) that was unaffected by the addition of erlotinib (RER 1.9 ± 0.04 and 1.7 ± 0.05, respectively) (Figure 2C-D). Furthermore, erlotinib alone did not produce radiosensitization under either schedule. While it would have been optimal if erlotinib had enhanced gemcitabine-mediated radiosenstiziation, the finding that erlotinib did not abrogate gemcitabine-mediated radiosensitization was an acceptable outcome, since our clinical goal for pancreatic cancer is to improve systemic therapy while maintaining or improving local radiosensitization.
Figure 2.
The effects of erlotinib on cytotoxicity and radiosensitization in response to gemcitabine. BxPC-3 cells were treated for 2 hours with 100nM gemcitabine and/or for 72 hours with 3uM erlotinib according to schedules 1 or 2 as illustrated (A). Cytotoxicity was calculated as the fraction of surviving colonies in treated cells relative to the number of colonies in the untreated control cells (surviving fraction = 1) (B). The radiation survival curves in response to gemcitabine and/or erlotinib (C) were used to calculate the mean inactivation dose (MID). Radiation enhancement (ER) was calculated as the ratio of the MID for drug treated cells to non-drug treated cells (ER = 1). Data are from the mean of 3 independent experiments ± standard error (B, D) or a single experiment (C). Statistically significant differences between gemcitabine versus erlotinib plus gemcitabine (B) or radiation versus radiation plus gemcitabine and/or erlotinib (D) are shown (*P<0.05).
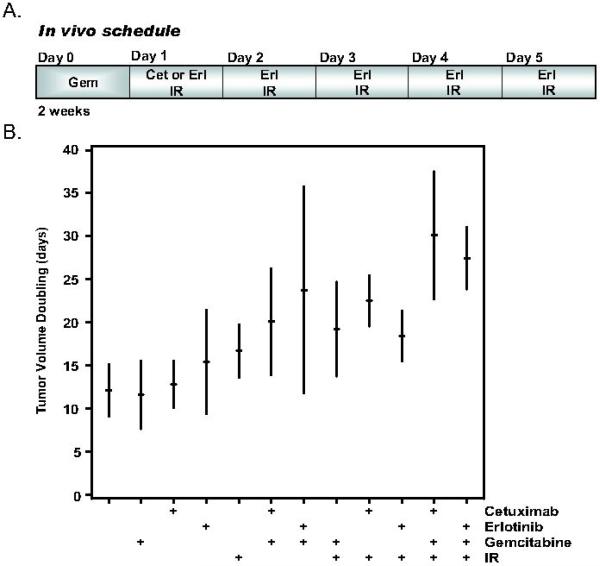
EGFR inhibitors have been shown to inhibit angiogenesis, invasion, and proliferation in addition to promote apoptosis (24). Therefore, we wanted to test the effects of EGFR inhibition, gemcitabine, and radiation in a murine pancreatic tumor xenograft model. Since previous data showed that cetuximab could enhance the inhibition of pancreatic tumor growth by gemcitabine-radiation (25), we wished to compare the efficacy of cetuximab and erlotinib. We selected relatively low doses of gemcitabine (120mg/kg) and radiation (1Gy X 10) for these studies, which would permit us to detect further inhibition of tumor growth by the addition of cetuximab or erlotinib if it occurred. We administered cetuximab once per week or erlotinib daily (each for 2 cycles and 4 hours post irradiation; Figure 3A) based on the substantially longer half-life of cetuximab (95 hours) (26) relative to erlotinib (36 hours) (27). Treatment with gemcitabine, cetuximab, erlotinib, or radiation alone did not produce any effect on tumor growth (Figure 3B, Table 1). As anticipated, the addition of gemcitabine to radiation extended the time required for tumor volume doubling, although it did not reach statistical significance (p=0.12). Cetuximab in combination with radiation produced a significant delay in the time to tumor volume doubling compared to either untreated tumors (Δ=10.4±4.5 days, p<0.03) or tumors treated with cetuximab alone (Δ=9.7±4.5 days, p<0.04). The time to tumor volume doubling was also significantly longer after treatment with gemcitabine and erlotinib compared to control (Δ=11.7±4.5 days, p<0.02) or gemcitabine alone (Δ=12.2±4.5 days, p<0.01). The most effective regimen for inhibition of tumor growth as evidenced by the significantly increased time to tumor volume doubling was the triple combination of either cetuximab or erlotinib plus gemcitabine-radiation (30.1±3.3 days or 27.5±3.3 days, respectively). The addition of cetuximab or erlotinib to gemcitabine and radiation produced minimal weight loss (Table 2) suggesting that these combinations did not produce marked normal tissue toxicity. Taken together, these results demonstrate that EGFR antagonists such as cetuximab or erlotinib can improve the anti-tumor efficacy of gemcitabine-radiation (without additional normal tissue toxicity) in a pancreatic cancer model.
Figure 3.
The effects of cetuximab or erlotinib on gemcitabine-mediated radiosensitization in vivo. Athymic nude mice bearing subcutaneous BxPC-3 xenografts were treated with the indicated combinations of gemcitabine (120mg/kg), cetuximab (50mg/kg), erlotinib (100mg/kg), or radiation (1 Gy × 10) as illustrated (A). Data are expressed as the time to tumor volume doubling in response to treatments that began on day 0 (B). The tic marks represent the mean time to tumor volume doubling and the vertical bars represent the 95% confidence intervals. Data are from 2 tumors/mouse with 6-7 mice per treatment group. Statistically significant differences are indicated in Table 1.
Table 1.
Tumor volume doubling (days)
| Control: | 12.1 ± 3.1 |
| Gemcitabine: | 11.6 ± 3.1 |
| Cetuximab: | 12.8 ± 3.1 |
| Erlotinib: | 15.4 ± 3.3 |
| IR: | 16.7 ± 3.1 |
| Gem+IR: | 19.2 ± 3.3 |
| Cet+Gem: | 20.1 ± 3.1 |
| Erl+Gem: | 23.8 ± 3.3#* |
| Cet+IR: | 22.5 ± 3.3#ŧ |
| Erl+IR: | 18.4 ± 3.1 |
| Cet+Gem+IR: | 30.1 ± 3.3#Ŧ‡ |
| Erl+Gem+IR: | 27.5 ± 3.3# |
P<0.05 versus Control
Gemcitabine
Cetuximab
Gem+IR
Cet+Gem
Table 2.
Relative* animal weight during and after therapy.
| Treatment | Day7 | Day15 | Day21 |
|---|---|---|---|
| Control: | 1.11 ± 0.01 | 1.22 ± 0.02 | 1.25 ± 0.01 |
| Gemcitabine: | 1.03 ± 0.02 | 1.16 ± 0.01 | 1.20 ± 0.01 |
| Cetuximab: | 1.06 ± 0.02 | 1.15 ± 0.02 | 1.15 ± 0.02 |
| Erlotinib: | 1.08 ± 0.01 | 1.17 ± 0.01 | 1.24 ± 0.01 |
| IR: | 1.02 ± 0.02 | 1.09 ± 0.03 | 1.16 ± 0.02 |
| Gem+IR: | 0.99 ± 0.01 | 1.08 ± 0.02 | 1.17 ± 0.01 |
| Cet+Gem: | 1.07 ± 0.02 | 1.17 ± 0.02 | 1.19 ± 0.01 |
| Erl+Gem: | 1.05 ± 0.02 | 1.15 ± 0.02 | 1.20 ± 0.02 |
| Cet+IR: | 1.06 ± 0.03 | 1.11 ± 0.02 | 1.15 ± 0.01 |
| Erl+IR: | 1.00 ± 0.03 | 1.11 ± 0.03 | 1.23 ± 0.02 |
| Cet+Gem+IR: | 1.02 ± 0.01 | 1.16 ± 0.02 | 1.24 ± 0.01 |
| Erl+Gem+IR: | 0.94 ± 0.01 | 1.04 ± 0.02 | 1.09 ± 0.02 |
Weights are relative to the first day of therapy (day 0).
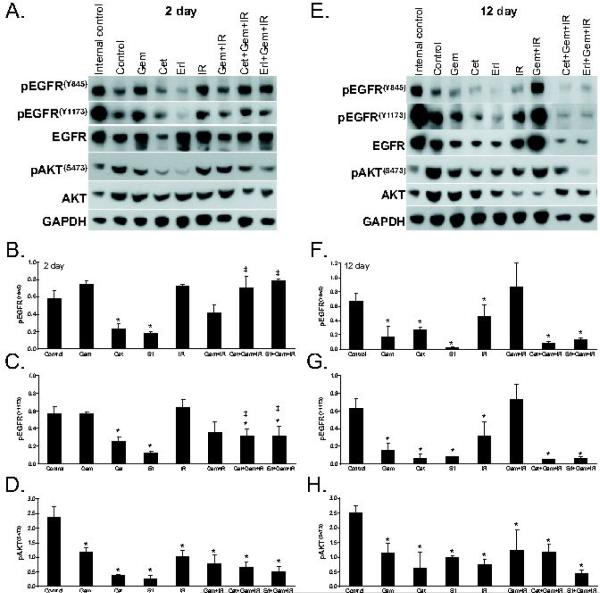
To characterize the molecular phenotype of tumors treated with cetuximab or erlotinib and gemcitabine-radiation, we analyzed EGFR signaling in BxPC-3 tumor xenografts both at the beginning (day 2; Figures 4A-D)) and end (day 12; Figures 4E-H) of the 2 week treatment cycle. Consistent with our in vitro findings, we found that phosphorylation of EGFR at Y845 was significantly reduced by treatment with cetuximab or erlotinib by treatment day 2 (both p<0.01), and we observed a trend for gemcitabine (p=0.11) or radiation (p=0.20) alone to increase EGFR(Y845) although the combination of gemcitabine and radiation did not (p=0.18). Interestingly, at this early time point, neither cetuximab nor erlotinib inhibited EGFR(Y845) in the presence of gemcitabine-radiation, despite the result that these treatments produced the greatest tumor growth inhibition. We therefore investigated EGFR(Y1173) and found that cetuximab or erlotinib alone significantly reduced EGFR(Y1173) relative to control (both p<0.01). In addition, the combination of either cetuximab or erlotinib with gemcitabine-radiation significantly reduced EGFR(Y1173) at the 2 day time point relative to control (p<0.03 and p<0.02, respectively). There were significant differences between EGFR(Y845) and EGFR(Y1173) phosphorylation in tumors treated with the combination of either cetuximab (p<0.02) or erlotinib (p<0.010) with gemcitabine-radiation; both cetuximab and erlotinib in combination with gemcitabine-radiation produced significant inhibition of EGFR(Y1173) but not EGFR(Y845), suggesting that these two phosphorylation sites respond differently to these combinations. When we assessed tumors at the end of the treatment cycle (day 12), we found significant inhibition of both EGFR(Y845) and EGFR(Y1173) in response to the single agents, gemcitabine, cetuximab, erlotinib, or radiation (all p<0.01). More importantly, the combination of cetuximab or erlotinib with gemcitabine-radiation resulted in significant inhibition of EGFR(Y845) and EGFR(Y1173) (relative to control; both p<0.01) demonstrating efficient inhibition of EGFR phosphorylation under the conditions which produced the greatest reduction in tumor growth. Together, these results suggest that inhibition of pEGFR(Y1173) in early treatment or EGFR(Y845) and pEGFR(Y1173) in late treatment are qualitatively (but not quantitatively) associated with tumor growth inhibition
Figure 4.
The effects of cetuximab or erlotinib, gemcitabine, and radiation on EGFR signaling in vivo. Mice were treated as described in Figure 3. Tumors were harvested on day 2 (A-D) or 12 (E-H) of treatment for immunoblotting. Data are from a single experiment (A, E) or the mean of 2-5 tumors ± standard error (B-D, F-H). Statistically significant differences of control versus treated* or EGFR(Y845) versus EGFR(Y1173)‡ are indicated (P<0.05).
We also examined the effects of cetuximab or erlotinib with gemcitabine and radiation on a pro-survival molecule downstream of EGFR, pAKT(S473). We found that the amount of pAKT(S473) in tumors following treatment with gemcitabine, cetuximab, erlotinib, radiation, or gemcitabine plus radiation was significantly reduced by the second day of treatment (all p<0.01). Furthermore, in response to either cetuximab or erlotinib in combination with gemcitabine-radiation, pAKT(S473) was also significantly reduced (relative to control; both p<0.01). The levels of pAKT(S473) remained repressed through out the duration of the treatment as evidenced by repression of pAKT(S473) compared to control pAKT(S473) levels at day 12 (p<0.002). Together these data demonstrate that cetuximab or erlotinib, as well as gemcitabine and radiation inhibit pAKT(S473).
Discussion
In this study we have shown in vitro that the EGFR-antagonist erlotinib can enhance gemcitabine's cytotoxicity in a schedule-dependent manner without abrogating gemcitabine-mediated radiosensitization. In mouse BxPC-3 pancreatic tumor xenografts, cetuximab or erlotinib in combination with gemcitabine-radiation produced significant tumor growth inhibition relative to untreated tumors. Most importantly, EGFR inhibition enhanced the growth delay produced by combination gemcitabine-radiation. Cetuximab or erlotinib alone, or in combination with gemcitabine-radiation, resulted in significant inhibition of the autophosphorylation site at Y1173 early in treatment, and both the Y845 and Y1173 EGFR phosphorylation sites by the end of treatment. This work supports the clinical use of EGFR-antagonists, such as cetuximab or erlotinib, in combination with gemcitabine-radiotherapy for the treatment of pancreatic cancer. Furthermore, this work suggests that pEGFR(Y1173) and pAKT(S473) might be useful qualitative markers for predicting response early in treatment.
A variety of preclinical studies have demonstrated that EGFR inhibitors can increase radiation sensitivity in both in vitro and in vivo model systems(28-32). While the majority of studies have reported additive effects resulting from the combination of EGFR antagonists and radiotherapy in vitro, the same combinations produce synergistic effects in xenograft models (25, 28, 29, 33-35). This might be because EGFR inhibitors and radiation impact several downstream signaling pathways(36). These include pathways regulating cellular proliferation and apoptosis, which would be evident in both in vitro and in vivo, and pathways regulating angiogenesis (28, 35) and tumor invasion (37), which might be detectable only in tumor xenograft models. Although we have not addressed the effects of EGFR inhibitors on angiogenesis and invasion, it is possible that the effect of erlotinib on gemcitabine-radiosensitization in vivo may be attributable to effects on angiogenesis and/or invasion.
Our finding that the addition of EGFR inhibition produces only a modest improvement over gemcitabine (alone or with concurrent radiation) is consistent with the recent clinical results obtained by the addition of erlotinib to gemcitabine therapy in the treatment of pancreatic cancer (21). The relative insensitivity of pancreatic cancers to EGFR inhibitors as well as the lack of correlation between tumor growth and pEGFR inhibition observed in this study could be explained by a number of factors. Mutant ras is detected in more than 85 % of pancreatic cancers(38), which makes cells resistant to EGFR inhibition (39-42). While this is not the case in BxPC-3, which bear wild type ras, BxPC-3 cells have been reported to express constitutively active Ras (43) that is also insensitive to cetuximab treatment. Furthermore, BxPC-3 cells have been shown to express elevated levels of ErbB3 (relative to other pancreatic cancer cells) and constitutively phosphorylated ErbB3 (44). It has been suggested that this ErbB3 expression results in heterodimerization of EGFR with ErbB3 and recycling of the heterodimer complex back to the cell surface, which may explain resistance to cetuximab. Thus, it is clear that constitutive expression of EGFR and its inhibition by an antibody or a small molecule may not always be sufficient to produce a response in the presence of other activated pathways.
Phosphorylation of EGFR at Y845 in response to gemcitabine has previously been demonstrated in head and neck cancer cells (45). Src-dependent phosphorylation of EGFR has also been demonstrated to occur in response to cellular stress by cisplatin (46), H2O2 (10), and UV (47). In addition phosphorylation of EGFR has been observed in response to a variety of chemotherapeutic agents including gemcitabine (45), cisplatin (46), oxaliplatin, 5-fluorouracil (48), paclitaxel (49), doxorubicin (50), and irinotecan (51). EGFR(Y845) as well as EGFR(992) were shown to be similarly inhibited by cetuximab or erlotinib (52). In this study, we found that cetuximab or erlotinib combined with gemcitabine-radiation inhibited EGFR(Y1173) but not EGFR(Y845) at the beginning of the treatment cycle. This study is the first to our knowledge to demonstrate a fundamental difference between two EGFR phosphorylation sites in response to EGFR inhibition. The finding that cetuximab or erlotinib combined with gemcitabine-radiation inhibited EGFR(Y1173) but not EGFR(Y845) suggests that differing mechanisms underlie phosphorylation of these two sites. While this difference was not observed at the end of the treatment cycle, it suggested that EGFR(Y845) and EGFR(Y1173) may have different functions and furthermore that multiple phosphorylation sites are required in order to fully assess EGFR signaling.
In this study, we were interested in the effects that cetuximab or erlotinib would have on tumor growth in response to gemcitabine-radiation. Thus, a limitation of this study is that we used low doses of gemcitabine (120mg/kg) and radiation (1Gyx10) that would permit us to observe a further delay of tumor growth by cetuximab or erlotinib. It is conceivable that the results of this study might have differed if we had used the maximally tolerated doses of gemcitabine and radiation, as would be the case in a clinical study. In fact, we attribute the difference between the gemcitabine-mediated induction of pEGFR observed in vitro (Figure 1) and the lack of significant pEGFR induction in vivo, to the low relatively non-cytotoxic dose of gemcitabine we chose for the in vivo studies. We designed the scheduling of EGFR inhibitors, gemcitabine, and radiation for the in vivo tumor growth experiments based upon scheduling experiments performed in vitro. We have not extensively investigated other schedules in vivo. We found this approach to be necessary for the efficient comparison of the number of variables involved when combining 4 agents, Furthermore, the schedule of EGFR inhibitor with gemcitabine-radiation selected in this study is amenable to integration with the current clinical regimen of once weekly gemcitabine with daily fractioned radiation-therapy.
The finding that cetuximab or erlotinib in combination with gemcitabine-radiation produced a modest delay in tumor growth is consistent with the clinical data combining EGFR inhibitors with gemcitabine (21). Although this clinical trial produced only a modest improvement in survival (0.3 months) compared to gemcitabine alone, it is exceptional in that no previous trials combining agents such as oxaliplatin, cisplatin, irinotecan, 5-fluorouracil, marimastat (matrix metalloproteinase inhibitor), and tipifarnib (farnesyltransferase inhibitor) with gemcitabine produced significant survival improvements over gemcitabine alone (53). It will be important in future studies to investigate molecular agents targeted to other pathways activated in pancreatic cancer with gemcitabine and radiation as well as to explore the combination of EGFR inhibitors with other chemoradiotherapy regimens, such as 5-fluorouracil and gemcitabineoxaliplatin based chemoradiation.
Acknowledgments
This work was supported by 2RO-1 CA078554 and Cancer Center Core Grant 5 P30 CA46592.
Footnotes
Conflict of Interest Statement: No conflicts of interest exist.
References
- 1.American Cancer Society. 2007. Cancer Facts and Figures. [Google Scholar]
- 2.McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–8. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer. 1999;85:1261–8. [PubMed] [Google Scholar]
- 5.Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. International journal of radiation oncology, biology, physics. 2007;68:801–8. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Philip PA, Zalupski MM, Vaitkevicius VK, et al. Phase II study of gemcitabine and cisplatin in the treatment of patients with advanced pancreatic carcinoma. Cancer. 2001;92:569–77. doi: 10.1002/1097-0142(20010801)92:3<569::aid-cncr1356>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Symon Z, Davis M, McGinn CJ, Zalupski MM, Lawrence TS. Concurrent chemoradiotherapy with gemcitabine and cisplatin for pancreatic cancer: from the laboratory to the clinic. International journal of radiation oncology, biology, physics. 2002;53:140–5. doi: 10.1016/s0360-3016(01)02790-0. [DOI] [PubMed] [Google Scholar]
- 8.Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22:238–43. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 9.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nature reviews. 2006;6:876–85. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 10.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1415–20. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Nagao T, Iwasaki T, Nishihira Y, Fukami Y. Src-dependent phosphorylation of the EGF receptor Tyr-845 mediates Stat-p21waf1 pathway in A431 cells. Genes Cells. 2003;8:995–1003. doi: 10.1046/j.1356-9597.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RM. Cetuximab. Nat Rev Drug Discov. 2005;Suppl:S10–1. doi: 10.1038/nrd1728. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R. FDA drug approval summary: erlotinib (Tarceva) tablets. The oncologist. 2005;10:461–6. doi: 10.1634/theoncologist.10-7-461. [DOI] [PubMed] [Google Scholar]
- 14.Moore MJ. Brief communication: a new combination in the treatment of advanced pancreatic cancer. Seminars in oncology. 2005;32(6 Suppl 8):5–6. doi: 10.1053/j.seminoncol.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Jang KT, Ki CS, et al. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109:1561–9. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 16.Kwak EL, Jankowski J, Thayer SP, et al. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin Cancer Res. 2006;12(14 Pt 1):4283–7. doi: 10.1158/1078-0432.CCR-06-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncology reports. 2007;18:151–5. [PubMed] [Google Scholar]
- 18.Tsiambas E, Karameris A, Lazaris AC, et al. EGFR alterations in pancreatic ductal adenocarcinoma: a chromogenic in situ hybridization analysis based on tissue microarrays. Hepato-gastroenterology. 2006;53:452–7. [PubMed] [Google Scholar]
- 19.Smeenk HG, Erdmann J, van Dekken H, et al. Long-term survival after radical resection for pancreatic head and ampullary cancer: a potential role for the EGF-R. Digestive surgery. 2007;24:38–45. doi: 10.1159/000100917. [DOI] [PubMed] [Google Scholar]
- 20.Ueda S, Ogata S, Tsuda H, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 21.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. International journal of radiation oncology, biology, physics. 1988;15:953–8. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 23.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 24.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 25.Buchsbaum DJ, Bonner JA, Grizzle WE, et al. Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. International journal of radiation oncology, biology, physics. 2002;54:1180–93. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- 26.Robert F, Ezekiel MP, Spencer SA, et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–43. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 27.Lu JF, Eppler SM, Wolf J, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clinical pharmacology and therapeutics. 2006;80:136–45. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Milas L, Mason K, Hunter N, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–8. [PubMed] [Google Scholar]
- 29.Nyati MK, Maheshwari D, Hanasoge S, et al. Radiosensitization by pan ErbB inhibitor CI-1033 in vitro and in vivo. Clin Cancer Res. 2004;10:691–700. doi: 10.1158/1078-0432.ccr-1041-03. [DOI] [PubMed] [Google Scholar]
- 30.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer research. 1999;59:1935–40. [PubMed] [Google Scholar]
- 31.Rao GS, Murray S, Ethier SP. Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. International journal of radiation oncology, biology, physics. 2000;48:1519–28. doi: 10.1016/s0360-3016(00)01358-4. [DOI] [PubMed] [Google Scholar]
- 32.Shintani S, Li C, Mihara M, et al. Enhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. International journal of cancer. 2003;107:1030–7. doi: 10.1002/ijc.11437. [DOI] [PubMed] [Google Scholar]
- 33.Solomon B, Hagekyriakou J, Trivett MK, Stacker SA, McArthur GA, Cullinane C. EGFR blockade with ZD1839 (“Iressa”) potentiates the antitumor effects of single and multiple fractions of ionizing radiation in human A431 squamous cell carcinoma. Epidermal growth factor receptor. International journal of radiation oncology, biology, physics. 2003;55:713–23. doi: 10.1016/s0360-3016(02)04357-2. [DOI] [PubMed] [Google Scholar]
- 34.Saleh MN, Raisch KP, Stackhouse MA, et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer biotherapy & radiopharmaceuticals. 1999;14:451–63. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 35.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–74. [PubMed] [Google Scholar]
- 36.Harari PM, Huang SM. Modulation of molecular targets to enhance radiation. Clin Cancer Res. 2000;6:323–5. [PubMed] [Google Scholar]
- 37.Li J, Lin ML, Wiepz GJ, Guadarrama AG, Bertics PJ. Integrin-mediated migration of murine B82L fibroblasts is dependent on the expression of an intact epidermal growth factor receptor. The Journal of biological chemistry. 1999;274:11209–19. doi: 10.1074/jbc.274.16.11209. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 39.Kita K, Saito S, Morioka CY, Watanabe A. Growth inhibition of human pancreatic cancer cell lines by anti-sense oligonucleotides specific to mutated K-ras genes. International journal of cancer. 1999;80:553–8. doi: 10.1002/(sici)1097-0215(19990209)80:4<553::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. International journal of cancer. 2006;118:209–14. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- 41.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer research. 2007;67:2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 42.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 43.Huang ZQ, Buchsbaum DJ, Raisch KP, Bonner JA, Bland KI, Vickers SM. Differential responses by pancreatic carcinoma cell lines to prolonged exposure to Erbitux (IMC-C225) anti-EGFR antibody. The Journal of surgical research. 2003;111:274–83. doi: 10.1016/s0022-4804(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 44.Arnoletti JP, Buchsbaum DJ, Huang ZQ, et al. Mechanisms of resistance to Erbitux (anti-epidermal growth factor receptor) combination therapy in pancreatic adenocarcinoma cells. J Gastrointest Surg. 2004;8:960–9. doi: 10.1016/j.gassur.2004.09.021. discussion 9-70. [DOI] [PubMed] [Google Scholar]
- 45.Chun PY, Feng FY, Scheurer AM, Davis MA, Lawrence TS, Nyati MK. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer research. 2006;66:981–8. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 46.Benhar M, Engelberg D, Levitzki A. Cisplatin-induced activation of the EGF receptor. Oncogene. 2002;21:8723–31. doi: 10.1038/sj.onc.1205980. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa D, Tanemura S, Ohata S, et al. Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. The Journal of biological chemistry. 2002;277:366–71. doi: 10.1074/jbc.M107110200. [DOI] [PubMed] [Google Scholar]
- 48.Van Schaeybroeck S, Karaiskou-McCaul A, Kelly D, et al. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin Cancer Res. 2005;11:7480–9. doi: 10.1158/1078-0432.CCR-05-0328. [DOI] [PubMed] [Google Scholar]
- 49.Sumitomo M, Asano T, Asakuma J, Asano T, Horiguchi A, Hayakawa M. ZD1839 modulates paclitaxel response in renal cancer by blocking paclitaxel-induced activation of the epidermal growth factor receptor-extracellular signal-regulated kinase pathway. Clin Cancer Res. 2004;10:794–801. doi: 10.1158/1078-0432.ccr-0948-03. [DOI] [PubMed] [Google Scholar]
- 50.Abdelmohsen K, von Montfort C, Stuhlmann D, et al. Doxorubicin induces EGF receptor-dependent downregulation of gap junctional intercellular communication in rat liver epithelial cells. Biological chemistry. 2005;386:217–23. doi: 10.1515/BC.2005.027. [DOI] [PubMed] [Google Scholar]
- 51.Azzariti A, Xu JM, Porcelli L, Paradiso A. The schedule-dependent enhanced cytotoxic activity of 7-ethyl-10-hydroxy-camptothecin (SN-38) in combination with Gefitinib (Iressa, ZD1839) Biochemical pharmacology. 2004;68:135–44. doi: 10.1016/j.bcp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Feng FY, Lopez CA, Normolle DP, et al. Effect of epidermal growth factor receptor inhibitor class in the treatment of head and neck cancer with concurrent radiochemotherapy in vivo. Clin Cancer Res. 2007;13:2512–8. doi: 10.1158/1078-0432.CCR-06-2582. [DOI] [PubMed] [Google Scholar]
- 53.Van Cutsem E, Verslype C, Grusenmeyer PA. Lessons learned in the management of advanced pancreatic cancer. J Clin Oncol. 2007;25:1949–52. doi: 10.1200/JCO.2006.09.4664. [DOI] [PubMed] [Google Scholar]