Abstract
Mutation of the DNA-binding region of the FOXP2 protein causes an inherited language disorder. A recent study provides the first data on mice with this mutation, which exhibit deficits in motor-skill learning and abnormal properties of neural circuits that contribute to these skills.
The renowned linguistics philosopher Noam Chomsky wrote: “When we study human language, we are approaching what some might call the ‘human essence’, the distinctive qualities of mind that are, so far as we know, unique to man” [1]. We are the only species on the planet which has the language phenotype. How this singularity evolved has captivated philosophers and scientists for centuries, and remains a puzzle. Enter the genomic era: now geneticists, in addition to neurologists, linguists and anthropologists, can take up the challenge. They sift the genome for complex multi-genetic components required for our language ability. In a revolutionary discovery reported in 2001 [2], the transcription factor known as FOXP2 was identified as the monogenetic locus of a mutation that causes an inherited speech and language disorder. In an instant, FOXP2 flashed as a key piece in the puzzle that connects with other molecules to pattern the brain for language. As reported recently in Current Biology, Groszer et al. [3] have now genetically engineered mice to show how human-like FOXP2 mutations alter learned motor behaviors.
How can studies in mice, which lack language, help us understand FOXP2’s function? It is important to recognize the distinction between language and speech. Language involves a cognitive system that conveys and processes linguistic information. A component of this system is speech, the vocal articulation of linguistic information. Speech involves learning precisely and rapidly to coordinate complex sequential movements of lingual, vocal and respiratory musculature in order to mimic or create new sounds. The evolution of speech is thus a necessary preadaptation for vocalized language.
The neural mechanisms for speech as a motor skill may have their evolutionary roots in more common ancient circuitries shared with non-speaking animals. Accordingly, several animal models, including vocal learners and non-learners, have been used to study neural expression patterns, function, and evolution of FoxP2 (by convention, human ‘FOXP2’ is capitalized, mouse ‘Foxp2’ is not, and ‘FoxP2’ denotes the molecule in mixed groups of animals). In human embryos, songbirds and rodents, FoxP2 is expressed in the cortex, striatum and thalamus in a conserved manner, suggestive of a role in the development of these structures [4–7]. In songbirds — the songs of which share key features with speech — expression persists into adulthood when FoxP2 mRNA is actively down-regulated in song control regions by singing. This implicates FoxP2 in the use, as well as the development, of such structures [8], a notion supported by the finding that targeted knock-down of FoxP2 in juvenile birds disrupts their adult songs. Interestingly, the acoustic abnormalities in knock-down birds were reported to resemble those exhibited by children with developmental verbal dyspraxia [9].
In general, FoxP2 is highly conserved, but it appears to have undergone accelerated evolution among echolocating bats and along the lineage between chimpanzees and humans [10–12]. Though the true cause of acceleration remains unclear, it is tempting to hypothesize that in certain phylogenetic lineages, the molecular sequence of FoxP2 is critical for specialized acoustic-motor function. (but see [13] with regard to birds). Connected with this idea, FOXP2 was recently sequenced from Neandertal bones, where the finding of the modern human form suggests a more sophisticated view of these archaic hominids [14].
FOXP2 was first identified as the genetic cause of a language impairment when a missense point mutation was found to cosegregate with a disorder that has been inherited through multiple generations of a British family, referred to as KE [2]. Just as the famous patient HM has been important for understanding hippocampal-based memory, the KE family is teaching us much about language. Subsequently, additional cases of verbal dyspraxia linked to FOXP2 mutations have emerged, including one associated with a mutant that results in an abnormally truncated protein [15]. In the KE family, affected members are heterozygous for an R553H mutation in the DNA-binding domain of FOXP2, and exhibit a number of expressive and receptive linguistic abnormalities. The most prominent deficit lies in the sequencing of orofacial movements required for speech articulation. The neurobiological basis of these deficits appears to lie in structural and functional abnormalities of cortico-striatal and cortico-cerebellar circuitries important for learning and execution of sequential movements [16].
In their recent study, Groszer et al. [3] characterized the basic motor aspect of this phenotype through analogy, by investigating motor-skill learning in Foxp2 mutant mice. In collaboration with the Mammalian Genetics Unit of the Medical Research Council in the United Kingdom, they took over 5,000 F1 offspring from N-ethyl-N-nitrosourea randomly mutagenized founders, and examined genomic DNAs for mutations in Foxp2. Incredibly, they identified a mouse that carried the R552H mutation identical to the one harbored by the KE family (the 552nd amino acid in the mouse protein is equivalent to the 553rd in the human protein). After a series of backcrosses with wild-type mice to remove potentially confounding mutations, the R552H line was established, ready for anatomical, cellular, and behavioral investigations.
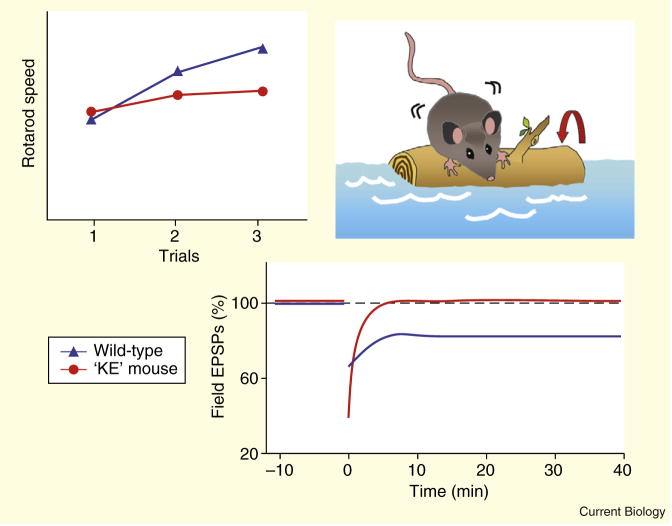
R552H homozygous mice show profound deficits, including reduced weight gain and delayed maturation of the righting reflex, and die a few weeks after birth. Interestingly, the cerebellum of these mice is disproportionately small, while its gross histoarchitecture remains intact. Heterozygotes, on the other hand, are fully viable with no gross neuroanatomical changes and normal base-line motor abilities. Strikingly, when it comes to learned motor skills, these mice are impaired in performance on two assays: the tilted voluntary running-wheel and the accelerating rotarod. On both, heterozygotes perform no worse than normal mice on the first trial, but they make slower improvements over subsequent trials compared to their wild-type littermates (Figure 1).
Figure 1. Impaired motor-skill learning in Foxp2 heterozygous mice.
Heterozygous mice carrying the same point mutation in Foxp2 as affected members of the KE family (‘KE’ mice) exhibited markedly impaired motor-skill learning which may be explained by abnormal neural circuitry (upper panel). On the accelerating rotarod test, ‘KE’ mice performed comparably to wild-type mice on the first trials, but over subsequent trials showed slower improvement (lower panel). In ‘KE’ mice, the dorsal striatum, which is implicated in this type of motor-skill learning, lacked normal plasticity as measured by induction of long-term depression. (Adapted from [3].)
In accordance with these behavioral results, in the heterozygous mice, neurons in the dorsal striatum, a region implicated in skill learning on the accelerating rotarod [17], were found to lack a form of plasticity known as long-term depression. The impairment is ‘experience-dependent’ as non-tetanized ‘naïve’ neurons exhibit normal intrinsic properties and input-output curves. In another circuit, this time in the cerebellum, parallel fiber-to-Purkinje cell synapses also show altered plasticity, evident in enhanced paired-pulse facilitation. Pieced together, these observations depict a role for Foxp2 in motor-skill learning involving cortico-striatal and cortico-cerebeller circuitries, two networks implicated in human speech. Planned work in conditional mutants promises to reveal any real-time contributions of Foxp2 to these behaviors.
The new work of Groszer et al. [3] addresses a controversy from an earlier study on Foxp2 knockout mice [18]. The latter study found that stranded heterozygous knockout pups had reduced ultrasonic vocalizations known as isolation calls, which typically cause the mother mouse to locate the pup. In contrast, the R552H heterozygote pups emit normally structured isolation calls at similar numbers to their wild-type littermates. Careful analysis of different calls and call properties suggest that severe Foxp2 mutations (heterozygous null or homozygous R552H) cause developmental delays that leave the pups less physically able to produce isolation calls. Assuming such calls are unlearned (which is likely given their function in newly born mice), the normal calls of the KE-like heterozygotes are not surprising as they reinforce the notion that FoxP2 is critical for learned motor skills.
Each emerging piece of information has helped to fill in our incomplete picture of FoxP2 function. The identification of genes regulated by FoxP2 provides additional pieces to the puzzle [19,20]. Pioneers of this approach used chromatin immunoprecipitation coupled with promoter microarrays to identify the downstream gene targets to which FOXP2 directly binds. The profiles of these genes suggest roles in a wide range of functions including morphogenesis, neurite growth, axon guidance, synaptic plasticity and neurotransmission. Through multidisciplinary approaches, more pieces of the jigsaw puzzle have been turned over. Now we are to interconnect them and complete the picture.
References
- 1.Chomsky N. Language and Mind. 1. New York: Harcourt, Brace & World; 1968. [Google Scholar]
- 2.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 3.Groszer M, Keays DA, Deacon RMJ, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, et al. Impaired motor learning and synaptic plasticity in mice carrying a point mutation implicated in human speech deficits. Curr Biol. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferland RT, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- 5.Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- 6.Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haesler S, Wada K, Nshdegan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teramitsu I, White SA. FoxP2 regulation during undirected singing in adult songbirds. J Neurosci. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haesler S, Rochefort C, Licznerski P, Georgi B, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Wang J, Rossiter SJ, Jones G, Zhang S. Accelerated FoxP2 evolution in echolocating bats. PLoS ONE. 2007;2:e900. doi: 10.1371/journal.pone.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Pääbo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Webb DM, Podlaha O. Accelerated protein evolution and origins of human-specific features: FOXP2 as an example. Genetics. 2002;162:1825–1835. doi: 10.1093/genetics/162.4.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb DM, Zhang J. FoxP2 in song-learning birds and vocal-learning mammals. J Hered. 2005;96:212–216. doi: 10.1093/jhered/esi025. [DOI] [PubMed] [Google Scholar]
- 14.Krause J, Lalueza-Fox C, Orlando L, Enard W, Green RE, Burbano HA, Hublin JJ, Hanni C, Fortea J, de la Rasilla M, et al. The derived FOXP2 variant of modern humans was shared with Neandertals. Curr Biol. 2007;17:1908–1912. doi: 10.1016/j.cub.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Macdermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, et al. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- 17.Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu W, Cho JY, Jiang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiteri E, Konopka G, Coppola G, Bomar J, Oldham M, Ou J, Vernes SC, Fisher SE, Ren B, Geschwind DH. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am J Hum Genet. 2007;81:1144–1157. doi: 10.1086/522237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernes SC, Spiteri E, Nicod J, Groszer M, Taylor JM, Davies KE, Geschwind DH, Fisher SE. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am J Hum Genet. 2007;81:1232–1250. doi: 10.1086/522238. [DOI] [PMC free article] [PubMed] [Google Scholar]