Abstract
Hematopoietic SCT from a partially HLA-mismatched (HLA-haploidentical) first-degree relative offers the benefits of rapid and near universal donor availability but also the risks that result from traversing the HLA barrier; namely, graft failure, severe GVHD and prolonged immunodeficiency. Improvements over the last 10 years in conditioning regimens, graft engineering and pharmacological immuno-prophylaxis of GVHD have substantially reduced the morbidity and mortality of HLA-haploidentical SCT. Highly immunosuppressive but nonmyeloablative conditioning extends the availability of HLA-haploidentical SCT to elderly hematologic malignancy patients lacking HLA-matched donors and permits recovery of autologous hematopoiesis in the event of graft failure. Current regimens for HLA-haploidentical SCT are associated with a 2-year non-relapse mortality of 20 ± 5%, relapse of 35 ± 15% and overall survival of 50 ± 20%. Major developmental areas include harnessing natural killer cell alloreactivity to reduce the risk of disease relapse and improving immune reconstitution by delayed infusions of lymphocytes selectively depleted of alloreactive cells. Hematologic malignancy patients who lack suitably matched related or unrelated donors can now be treated with HLA-haploidentical related donor or unrelated umbilical cord blood SCT. Future clinical trials will assess the relative risks and benefits of these two graft sources.
Keywords: allo-SCT, alternative donors, natural killer cells, conditioning regimens, GVHD, immune reconstitution
Introduction
Allo-SCT, following either myeloablative or reduced intensity conditioning, is a potentially curative therapy for a variety of hematologic malignancies and non-hematologic disorders. Of all the potential sources of allografts, transplantation of stem cells from an HLA-matched sibling has generally produced the best overall and progression-free survivals.1 Unfortunately, only about one-third of candidates for allo-SCT have HLA-matched siblings.2 For patients who lack HLA-matched siblings, there are three alternative sources of stem cells for allo-SCT: (1) volunteer unrelated donors (VUDs); (2) umbilical cord blood and (3) partially HLA-mismatched, or HLA-haploidentical, related donors. Next to HLA-matched related donors, phenotypically matched VUDs are the most widely sought for allo-SCT.3 However, the chance of finding an HLA-matched VUD varies significantly depending upon the racial and ethnic background of the recipient, ranging from over 60–70% in Caucasians, to about 10–20% for ethnic minorities.4 The search for an HLA-matched VUD is also hindered by the amount of time it takes from search initiation to donor identification.5,6 In contrast, a partially HLA-compatible first-degree relative can be identified and mobilized immediately for transplantation in nearly all situations. This is because a patient shares exactly one HLA haplotype with each biological parent or child, and each sibling of the patient has a 50% likelihood of sharing one HLA haplotype while being variably mismatched for HLA genes of the other haplotype. Thus, when a patient lacks an HLA-identical sibling, the treating physician must balance the risks that the patient’s disease will progress or health will deteriorate while searching for a VUD versus the risk of crossing HLA barriers with the use of an HLA-haploidentical donor. The aim of this article is to review the history and recent progress of allo-SCT using HLA-haploidentical donors. Included in this review will be a discussion of efforts to improve the outcome of HLA-haploidentical SCT and a comparison of the relative advantages and disadvantages of partially HLA-mismatched related donor vs unrelated umbilical cord blood (UCB) SCT.
Evolution of HLA-haploidentical SCT
Table 1 lists some of the largest published studies of HLA-haploidentical SCT after either myeloablative or nonmyelo-ablative conditioning. The table illustrates the substantial progress that has been made in improving the safety, efficacy and utility of the procedure for patients with hematologic malignancies. Much of the progress in HLA-haploidentical SCT can be attributed to advances in supportive care such as monitoring and preemptive therapy against CMV13 and EBV-related lymphoproliferative dis-ease14 and improved detection and treatment of invasive fungal infections.15
Table 1.
Selected published studies of HLA-haploidentical SCT
| Authors | N | Median age (years) |
T depletion |
Graft failure (%) |
GVHD (%) | NRM (%)* |
Relapse (%)* |
Overall survival (%)* |
EFS (%)* |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute II–IV | Acute III and IV | Chronic | |||||||||
| Myeloablative conditioning | |||||||||||
| Szydlo et al.1 | 340 | 25 | Ex vivo (49%) | ||||||||
| 1 Ag MM | 9 | 44 |
|
52 |
 b b
|
28–65 | — | 15–36 | |||
| 2 Ag MM | 16 | 56 | 60 | 37–45 | — | 20–25 | |||||
| O’Reilly et al.7 | 52 | Ex vivo |
|
9 | 3 | 20 | |||||
| Mehta et al.8 | 201 | 23 | In (71%)+ ex vivo | 2 | 13 | — | 15 | 31 | 18 | 19 | |
| Aversa et al.9 | 104 | 33 | Ex vivo | 9 | 8 | — | 7 | 37 | 25 | 39 | — |
| Lu et al.10 | 135 | 24 | In vivo | 1 | 40 | 16 | 55 | 22 | 18 |
|
|
| Nonmyeloablative conditioning | |||||||||||
| Rizzieri et al.11 | 49 |
|
In vivo | 14 | 16 | — | 14 | 31 | — | 31 | — |
| Luznik et al.12 | 68 | In vivo | 13 | 34 | 6 | 22 |
|
51 | 36 | 26 | |
Boxes illustrate salient results:
effect of HLA mismatch on severe GVHD after myeloablative, T-cell-replete BMT
excessive NRM in early trials of haploidentical SCT
increased risk of graft failure with ex vivo graft TCD without intensive immunosuppressive conditioning
improved outcome of myeloablative SCT using in vivo TCD
nonmyeloablative conditioning permits transplantation of older patients with reduced treatment-related mortality. See text for details of the studies.
Data on NRM (non-relapse mortality) are for 1–2 years after transplantation. Data on relapse, overall survival and EFS are for 1–2 years (all studies except Lu et al.10) or for 5 years after transplantation (Lu et al.). 1 Ag MM = 1 HLA Ag mismatch.
Haploidentical SCT after myeloablative conditioning
The earliest studies of T-cell-replete HLA-haploidentical SCT for hematologic malignancies were characterized by high risks of graft rejection, GVHD, and treatment-related mortality.1,16–19 Outcomes were significantly worse after HLA-haploidentical related donor as compared to HLA-matched sibling SCT, and increasing HLA disparity in the host-vs-graft and graft-vs-host directions were associated with increasing risks of graft failure16,20 and GVHD,20,21 respectively. However, overall survival was similar after HLA-matched vs 1 HLA Ag-mismatched SCT for patients with acute leukemia in remission.18 The unacceptably high incidence of severe acute GVHD after haploidentical SCT motivated early trials of graft T-cell depletion (TCD). Encouraging results were obtained for patients with SCID,22 but patients with leukemia experienced a high incidence of fatal graft rejection, up to or exceeding 30%.7 Although patients who receive T-cell-depleted HLA-haplo-identical SCT have a reduced risk of acute and chronic GVHD compared to recipients of T-cell-replete grafts, the incidence of graft failure is increased and there is no improvement in leukemia-free survival20 because of a high mortality from infection,8 EBV-related lymphoproliferative disease23 and possibly an increased risk of relapse.24
Megadose SCT: a turning point in HLA-haploidentical SCT
A solution to the problem of graft failure after T-cell-depleted allo-SCT was provided by Reisner et al.,25 who found that graft rejection could be obviated by administering an extremely high dose or ‘megadose’ of MHC-incompatible stem cells. ‘Megadose’ SCTs in humans, piloted by Aversa et al.26 in Perugia, Italy, initially consisted of G-CSF-mobilized PBSC and BM cells, both depleted of T-cells ex vivo by soybean agglutination and E-rosetting and a conditioning regimen, including TBI, CY, thiotepa and antithymocyte globulin (ATG), with no post transplant immunosuppression. The Perugia group subsequently modified this regimen extensively, with fludarabine replacing CY in the TBI-based conditioning regimen in an attempt to reduce the conditioning regimen toxicity without jeopardizing its immunosuppressive effect.27 Other advances included implementation of a CD34+ cell selection device that provides a 4.5 log TCD and the elimination of G-CSF administration after transplantation.9 This cytokine impairs DC production of IL-12, leading to abnormalities in Ag-presenting function and T-cell activation.28 Over the past decade, the Perugia group has demonstrated that full HLA-haplotype mismatched transplants can be successful in patients with acute leukemia in first or second CR when a megadose of stem cells, typically > 107 CD34+ cells per kilogram of recipient body weight, is infused after an immunomyeloablative conditioning regimen. However, the profound depletion of host and donor T cells that was required to reduce GVHD and graft rejection was accompanied by significant infectious morbidity and mortality and a prolonged time to immune reconstitution. Early results showed a non-relapse mortality (NRM) rate of 40%,27 with infection the leading cause of death. Somewhat improved immune reconstitution and fewer deaths secondary to infection occurred when G-CSF was eliminated from the regimen.
Other approaches using myeloablative conditioning and high-dose CD34+ cell-selected grafts described similarly favorable engraftment and GVHD rates, but unfortunately, recurrent malignancy and problems with infectious-related deaths were reported. In a Canadian multicenter study, all 11 study patients engrafted without GVHD but 10 of 11 patients died from leukemic relapse or infection.29 Waller et al.30 reported a 93% mortality rate in patients who received T-cell-depleted, CD34+ -enriched HLA-haploidentical SCT after an ATG-based regimen, with most deaths a result of infection or relapse. In a retrospective analysis from Japan, severe infections occurred in 20 of 32 patients receiving CD34-selected PBSCs from 2–3 HLA-antigen-mismatched related donors.31 Seventeen of 32 patients (53%) died from treatment-related causes, including 10 (31%) from infection, and 9 patients died from complications of progressive disease. These results suggest that transplantation of highly purified CD34+ PBSCs from haploidentical donors is associated with a low incidence of GVHD but an increased risk of disease progression or fatal infection. Recently, methods of depleting CD3+ T cells and CD19+ B cells from megadose PBSC collections have been developed.32 CD3/CD19 depleted grafts contain not only CD34+ stem cells but also CD34− progenitors, natural killer (NK) cells, DCs and graft facilitating cells, all of which may enhance immune reconstitution after HLA-haploidentical SCT. Preliminary results of HLA-haploidentical SCT using CD3/CD19-depleted grafts are encouraging in this regard.
Blood vs marrow from G-CSF-primed donors
Treatment of BM donors with G-CSF before donation increases marrow CD34+ and CFU-GM cells, reduces total lymphocytes and reverses the CD4+ /CD8+ T-cell ratio. To enhance engraftment by increasing the dose of transplanted HSCs, 15 patients with high-risk leukemia received myeloablative conditioning with cytarabine, Cy, and 1000 cGy TBI, G-CSF-primed BM from haploidentical donors, and GVHD prophylaxis with rabbit ATG (5 mg/kg/day on days −4 to −1), CsA, MTX and mycophenolate mofetil.33 All 15 patients had prompt trilineage hematopoietic engraftment, the cumulative incidence of GVHD was 33%, and nine of 15 patients were alive at a median follow-up of 22 months (range 13–35 months) at the time of reporting. Based upon these results, Lu et al.10 at Peking University in Beijing, China compared the outcomes of 293 patients with leukemia receiving HLA-matched sibling (n = 158) or HLA-haploidentical related grafts (n = 135) from G-CSF-primed donors. Patients undergoing haploidentical SCT were conditioned with cytarabine, oral BU, CY and methyl-CCNU, received G-CSF-primed BM on day 0 (n = 134) and/or G-CSF-primed PB on day 1 (n = 131) and GVHD prophylaxis with ATG 2.5 mg/kg/day on days −4 to −1, CsA, MTX and mycophenolate mofetil. All but two haploidentical SCT patients had sustained engraftment of donor neutrophils. The cumulative incidences of acute grades II–IV, grades III–IV and chronic GVHD in recipients of matched vs mismatched SCT were 32 vs 40% (P = 0.13), 11 vs 16% (no P-value provided) and 56 vs 55% (P = 0.90). Mismatched patients had a higher incidence of CMV antigenemia (65 vs 39%; P < 0.001) and hemorrhagic cystitis (35 vs 13%; P < 0.001) but not of CMV disease. Two-year rates of relapse and NRM were 13 vs 18% (P = 0.40) and 14 vs 22% (P = 0.10) for recipients of matched vs mismatched transplants, respectively. The 2-year probabilities of overall survival were 72 vs 71% (P = 0.72) and of leukemia-free survival were 71 vs 64% in the matched and mismatched cohorts, respectively. In a follow-up report of 157 consecutive recipients of G-CSF-primed BM and PB from haploidentical related donors, recipients of CD3+ T-cell doses higher than the median (1.77 × 108/kg) had a significantly lower NRM, better leukemia-free survival and better overall survival.34 The Beijing results are extremely encouraging and this regimen for haploidentical SCT needs to be evaluated at other centers. Novel aspects of the regimen that may contribute to the low rates of graft failure and GVHD may be the use of low-dose rabbit ATG,35 the use of G-CSF-mobilized BM and PB36,37 and the combination of CSP, MTX and mycophenolate mofetil.
Haploidentical SCT after nonmyeloablative conditioning
In an effort to reduce the regimen-related mortality while retaining a graft-vs-tumor effect, several recent clinical trials have evaluated the efficacy of nonmyeloablative conditioning for HLA-haploidentical SCT. Clinical trials at the Massachusetts General Hospital have been performed using nonmyeloablative conditioning with CY+/− fludarabine, in vivo TCD, pretransplant thymic irradiation and most recently, ex vivo TCD.38 The rationale for this approach, pioneered in mouse models by Sykes et al.,39,40 has included (1) the reduction of regimen-related toxicities with nonmyeloablative conditioning, (2) prevention of GVHD with in vivo and ex vivo TCD and (3) the capture of an optimal graft-vs-tumor effect with the use of delayed DLI, when clinically indicated. Their current protocol includes CY, fludarabine, MEDI-507 (a MoAb against the CD2 Ag on T cells) and thymic irradiation, which has resulted in a high incidence of mixed chimerism without early GVHD and the potential for conversion of T-cell chimerism with manageable or no GVHD. Recurrent malignancy and late infections have been the main reasons for treatment failure with this approach.41
Rizzieri et al.11 at Duke University developed a nonmyeloablative conditioning regimen incorporating fludarabine, CY and alemtuzumab for 49 hematologic malignancy patients receiving PBSCs from HLA-haploidentical donors. Mycophenolate mofetil, with (n = 25) or without CsA (n = 24), was used for post transplantation GVHD prophylaxis. A total of seven patients (14%) experienced either primary or secondary graft failure, and the incidences of acute grades II–IV and chronic GVHD were 16 and 14%, respectively. Fifteen patients (31%) died of causes unrelated to disease progression. Twenty-five percent of patients experienced a severe infection, reactivation of CMV occurred in 86% and CMV disease developed in 14%. Overall survival of patients 1 year after transplantation was 31%. Absence of GVHD was associated with improved recovery of CD4+ and CD8+ T cells and CD56+ NK cells following transplantation.
Selective allodepletion using CY-induced immunologic tolerance
Luznik et al.12 exploited the protocol of drug-induced immunological tolerance, first described in 1959 by Schwartz and Dameshek,42 to achieve selective in vivo depletion of alloreactive T cells after nonmyeloablative HLA-haploidentical BMT. In this protocol, in vivo exposure to antigen induces the proliferation of Ag-specific lymphocytes, which are then killed by the timely administration of a drug that is selectively toxic to proliferating over resting cells. Studies in mice established that high-dose, post transplantation CY inhibits both graft rejection and GVHD after either MHC-matched or -mismatched SCT.43–46 Based upon these studies, 68 patients with hematologic malignancies (n = 67) or paroxysmal nocturnal hemoglobinuria (n = 1) received CY 50 mg/kg on day 3 (n = 28) or days 3 and 4 (n = 40) after nonmyeloablative conditioning and transplantation of T-cell-replete BM from HLA-haploidentical related donors.12 Graft failure occurred in 9 of 66 (13%) evaluable patients, and was fatal in one. The cumulative incidences of acute grades II–IV and grades III–IV GVHD were 34 and 6%, respectively, and of chronic GVHD was 22%. Serious infections were relatively infrequent: there were no cases of CMV disease and only five cases of invasive fungal infection, two of which were fatal. NRM and relapse at 1 year after transplantation were 15 and 51%, respectively. Actuarial overall and EFS at 2 years after transplantation were 36 and 26%, respectively. These results suggest that post transplantation CY induces selective allodepletion in vivo, inhibiting fatal graft rejection and severe GVHD, while sparing functional immunity to infection.
Methods to reduce GVHD and improve immune reconstitution after HLA-haploidentical SCT
As discussed above, nonselective depletion of grafted T cells significantly reduces the incidence and severity of GVHD after partially HLA-mismatched related donor SCT but also increases the risk of graft failure and fatal opportunistic infection from prolonged immune compromise. Aside from selecting donors with the least degree of HLA mismatch with the patient, a number of additional strategies have been envisioned or used to reduce the risk of GVHD without causing profound immune compromise. These strategies include: (1) selection of donors based upon the principle of tolerance to non-inherited maternal Ags, or NIMA; (2) selective depletion of alloreactive T cells from the graft; (3) reconstitution of T-cell-depleted grafts with T cells that protect against infection but do not cause GVHD; or (4) adding cells that suppress GVHD to T-cell-replete grafts.
Selection of donors tolerant to non-inherited maternal antigens
Exposure of the developing fetus to maternal cells, which occurs during pregnancy,47 can lead to either immunity or tolerance of non-inherited maternal HLA Ags (NIMA) and subsequently have an effect on transplant outcome. Two separate studies have demonstrated that approximately 50% of individuals with antibodies against a large number of HLA Ags do not have antibodies against NIMA.48,49 Reactivity against non-inherited paternal antigens (NIPA) is significantly higher. Siblings who are HLA-haploidentical to each other share either the paternal or the maternal HLA haplotype. When siblings share the paternal HLA haplotype, they are mismatched for both inherited and non-inherited maternal antigens (NIMAs). Thus, HLA typing of both of the patient’s parents is required to assign parental haplotypes for determining whether a sibling is mismatched for NIMA or for NIPA. It has been speculated that there should be less GVHD and less graft rejection with NIMA- rather than with NIPA-mismatched transplantations. Because graft failure and GVHD affect the outcome of HLA-haploidentical SCT, NRM and overall survival might also differ between NIMA- and NIPA-mismatched transplants. To date, several population studies have provided evidence in favor of the presence of the tolerogenic ‘NIMA’ effect. Such evidence includes low rates of aGVHD in T-cell-replete, HLA-haploidentical SCT from a NIMA-mismatched sibling,50,51 as well as in unmanipulated marrow transplantation from fully HLA-haploidentical mothers using standard preparative regimens combined with peritransplantation ATG,52 and a significantly lower risk of cGVHD in recipients of non-T-cell depleted maternal transplants vs paternal transplants.50 Several recent studies53–56 have also demonstrated sustained remissions of chemorefractory hematologic malignancies with acceptable rates of GVHD after T-cell-replete, HLA-haploidentical SCT from microchimeric NIMA-mismatched family members. Further studies are required to evaluate more precisely the effects of NIMA- or NIPA-specific allotolerance and to identify genetic factors associated with GVHD, NRM and relapse-free survival in a given NIMA-mismatched donor–recipient pair.
Selective graft allodepletion
Another strategy to reduce GVHD after HLA-haploidentical SCT is to induce tolerance, or ‘anergy,’ in host-reactive T cells by exposing the graft ex vivo to host alloantigens (‘signal 1’), while simultaneously blocking the delivery of T-cell costimulatory signals (‘signal 2’).57 Guinan et al.57–59 conducted two pilot trials of myeloablative, HLA-haploidentical SCT in which donor marrow was incubated with irradiated recipient mononuclear cells in the presence of CTLA-4-Ig, a fusion molecule that blocks interaction of the T-cell costimulatory receptor CD28 with its ligands, B7-1 and B7-2, on APCs (n = 19) or with a combination of MoAbs against B7-1 and B7-2 (n = 5). GVHD developed in only 9 of 21 evaluable patients (four grade II, four grade III, one grade IV) and eight patients were alive at a median of 8 years after transplantation. Ex vivo tolerance induction with the combination of antibodies against B7-1 and B7-2 resulted in a 99% reduction in T cells capable of proliferating to host Ags with no significant loss of reactivity to third-party alloantigens, viral Ags or the WT-1 tumor Ag.60,61 These in vitro results correlated with the low incidence of late viral infections or of opportunistic infections requiring admission.58 However, there were 12 early deaths due to bacterial or fungal infection and/or regimen-related toxicity. The investigators are currently studying the effects of administering allo-anergized T cells after CD34-selected haploidentical SCT, to determine the optimal dose for augmenting immune reconstitution without causing GVHD.62
Graft TCD followed by infusion of allodepleted lymphocytes
An alternative to selectively depleting the stem cell graft of alloreactive lymphocytes is to administer a TCD stem cell graft followed by delayed infusion of mature lymphocytes selectively depleted of alloreactive cells. Selective allodepletion has been achieved by activating donor lymphocytes ex vivo with host APCs, followed by targeted removal based upon differential expression of surface activation markers, proliferation or retention of photoactive dyes. Methods of alloreactive cell elimination include treatment with immunotoxins,63,64 immunomagnetic separation,65–69 activation of suicide genes,70,71 activation-induced cell death,72 flow cytometric sorting73,74 or photodynamic purging.75,76 To achieve selective allodepletion, the group at Hôpital Necker in Paris, France, cocultured donor and irradiated host lymphocytes ex vivo, followed by addition of a ricin A-chain-coupled MoAb against CD25, the α-chain of the IL-2 receptor.77 This procedure results in a > 2 log depletion of host-reactive cells, while sparing reactivity to viral and bacterial Ags as well as third-party alloantigens.63 Allodepleted lymphocytes in doses ranging from 1 to 8 × 105 cells per kg were infused into 15 patients from 15 to 47 days after myeloablative conditioning and transplantation of CD34-selected stem cell grafts from HLA-haploidentical donors. Grades I and II acute GVHD occurred in four patients, correlating with antihost residual proliferation above 1% in a mixed lymphocyte reaction, and limited chronic GVHD in one. Compared to controls, recipients of allodepleted T cells had a faster recovery of CD4+ and CD8+ T cells, and infections from EBV, CMV and adenovirus were eliminated following infusion. At the time of reporting, 8 of 15 patients were alive and well at a median of 24 months of follow-up. A trial currently enrolling patients uses immunomagnetic depletion of CD25+ cells from HLA-haploidentical donor lymphocyte infusions (DLI), which are then given to improve immune reconstitution after HLA-haploidentical SCT.78 Two recipients of 3 × 105 allodepleted T cells per kg achieved > 200 CD3+ CD4+ T cells per µl blood as early as 60 days after SCT, and one developed grade II GVHD.
Amrolia et al.80 conducted a clinical trial of infusing allodepleted lymphocytes, using the ricin A chain-conjugated anti-CD25 moAb, into 16 recipients of T-cell-depleted haploidentical SCT.79 Eight patients received a dose of 104 T cells per kg, whereas another eight patients received 105 T cells per kg. Recipients of the higher dose demonstrated improved T-cell recovery resulting from expansion of the effector memory population without evidence of new T-cell generation in the thymus. In vitro T-cell responses to CMV- and EBV-associated Ags were detected as early as 2–4 months after transplantation in four of six recipients of the higher T-cell dose but not until 6–12 months after transplantation among recipients of the lower T-cell dose. Acute and chronic GVHD occurred in only two patients each. More recently, the same group has found that immunomagnetic depletion of alloactivated lymphocytes expressing CD25 and/or CD71 is more effective at reducing alloreactivity than strategies based on depleting only CD25+ T cells. This double depletion strategy may facilitate infusion of larger doses of T cells to promote immune reconstitution while avoiding GVHD.
A potentially promising strategy for enhancing immune reconstitution and preserving GVL after TCD haploidentical SCT arises from the observation that effector memory (CD44+ CD62L−) CD4+ T cells do not cause GVHD following their transfer into irradiated MHC-mismatched recipients.81–84 Their inability to cause GVHD stands in contrast to their ability to mediate GVL effects83,85 as well as protection from infection. These results suggest a strategy of augmenting immune reconstitution and GVL by infusing effector memory T cells into recipients of TCD haploidentical SCT.
Adding T cells that suppress GVHD to T-cell replete grafts
MSCs and CD4+ CD25+ foxp3+ regulatory T cells are two types of cells that can inhibit T-cell responses to alloantigens and so could be infused with or after T-cell-replete grafts to inhibit GVHD after haploidentical SCT. MSCs are BM stromal cells that can differentiate into other cells derived from mesoderm, including chondrocytes, tenocytes and myoblasts.86 MSCs can be immunosuppressive, inhibiting the proliferation of human T cells stimulated by irradiated allogeneic PBMCs.87–91 Third-party MSCs have been cotransplanted with stem cell grafts to suppress GVHD after HLA-matched sibling SCT92 and to treat established GVHD after HLA-matched or mismatched donor SCT.93 In contrast to their effects on T-cell responses to alloantigens, MSCs have little effect on established T-cell responses to EBV and CMV.94
Regulatory T cells have an established role in the maintenance of self-tolerance and the prevention of autoimmunity.95 Addition of large numbers of donor regulatory T cells to stem cell grafts suppresses the development of acute GVHD after MHC-mismatched allogeneic SCT in mice without impairing GVL activity.96–99 Successful translation of these findings to haploidentical SCT in humans requires protocols to expand regulatory T cells ex vivo to sufficient numbers to suppress alloreactivity in vivo.
Strategies to decrease relapse after haploidentical SCT
NK cells
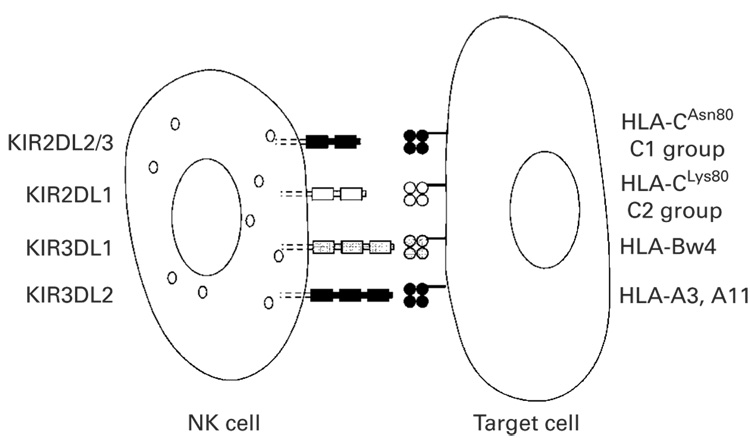
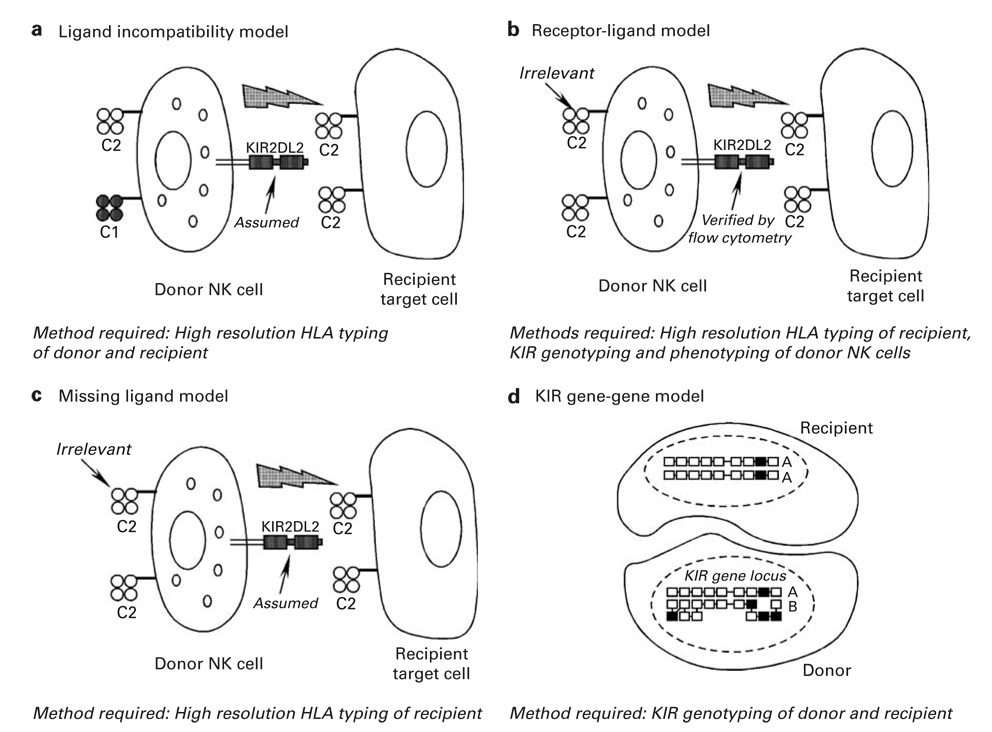
HLA-haploidentical transplants have the potential to trigger beneficial donor-vs-recipient NK cell-mediated alloreactions. NK cells express activating and inhibitory receptors, termed killer immunoglobulin-like receptors (KIRs), that stimulate or inhibit NK cell cytotoxicity, respectively. The nomenclature for the KIRs describes the number of extracellular immunoglobulin-like domains (2D or 3D) and the length of the cytoplasmic tail (L for long, S for short). The KIR family (Figure 1) includes inhibitory receptors, or iKIRs, for polymorphic determinants of HLA-A (KIR3DL2), HLA-B (KIR3DL1) and HLA-C (KIR2DL1, KIR2DL2 and KIR2DL3).100 In HLA-haploidentical SCT, the potential for NK cell alloreactivity in the graft-vs-host direction exists when the recipient’s cells lack expression of an HLA allele that is required to deliver an inhibitory signal through a donor iKIR. Figure 2 depicts several models described in the literature to predict the potential for NK cell alloreactivity in the setting of SCT. For instance, the ‘ligand–ligand’ or ‘ligand incompatibility’ model (Figure 2a) incorporates information from high resolution HLA typing and predicts donor NK cell alloreactivity when a known HLA ligand for an iKIR is present on donor but not on recipient cells. In contrast, the ‘receptor-ligand’ model (Figure 2b) predicts donor NK cell alloreactivity when an HLA ligand is absent on recipient cells and the corresponding iKIR is expressed by the donor, as determined by genotyping of iKIRs or by flow cytometry for surface expression of the iKIR. In studies by the Perugia group, KIR ligand incompatibility (HLA ligand present in the donor but absent in the recipient; Figure 2a) reduced the risk of relapse in 57 AML patients while improving engraftment and protecting against GVHD.104 Their updated analysis of greater than 90 HLA-haploidentical transplants for high-risk AML showed that transplantation from NK alloreactive donors was associated with control of AML relapse and improved EFS, with a greater than 65% EFS of AML patients transplanted in remission from NK alloreactive donors and a 30% EFS of chemoresistant AML patients. This was compared to an EFS of 18% in AML patients transplanted from non-NK alloreactive HLA-haploidentical donors.105 The ‘receptor-ligand’ model (Figure 2b) was better able to predict relapse in pediatric AML and ALL patients than the ligand incompatibility model.101 Still, utilizing a third method where genotyping of inhibitory KIR was performed (Figure 2d), patients with KIR gene mismatches (that is, KIR gene present in the donor but absent in the recipient, or vice versa) had a higher incidence of GVHD than those without mismatches.102 Among patients receiving nonmyeloablative haploidentical SCT with high-dose post transplantation CY, inhibitory KIR gene mismatches between donor and recipient were associated with improved overall survival and EFS.106
Figure 1.
Interactions between inhibitory killer immunoglobulin-like receptors (iKIRs) and their HLA ligands of relevance to natural killer (NK) cell alloreactivity after allo-SCT. For convenience, a single NK cell expressing four distinct iKIRs is shown. Each NK cell need only express one molecular species of iKIR for functional maturation to occur. High resolution HLA typing is required to determine whether specific alleles of HLA-B and HLA-Cw are ligands of specific iKIRs. Group 2 HLA-C alleles (C2; for example, -Cw2, -Cw4, -Cw5 and -Cw6) are the ligands for KIR2DL1, whereas group 1 HLA-C alleles (C1; for example, -Cw1, -Cw3, -Cw7, -Cw8) are the ligands for KIR2DL2 and KIR2DL3. High resolution typing of HLA-B and -Cw loci are incorporated into the ligand incompatibility, receptor-ligand and missing ligand models of NK cell alloreactivity (Figure 2). Interactions between KIR3DL2 and HLA-A3 or -A11 are generally not considered in these models.
Figure 2.
Models of natural killer (NK) cell alloreactivity after allo-SCT. Models of NK cell alloreactivity incorporate some or all of the following information: (1) high resolution HLA typing of donor and recipient;103 (2) genotyping of the killer immunoglobulin-like receptor (KIR) locus by PCR of genomic DNA using sequence-specific oligonucleotide probes (SSP)102 and (3) phenotyping of KIR expression by flow cytometry using commercially available antibodies.101 (a) The ligand incompatibility model predicts NK cell alloreactivity in the graft-vs-host direction (depicted) when the recipient lacks expression of an HLA ligand for inhibitory KIR, in this case a member of the HLA-C1 group, that is present in the donor. The presence of functional donor NK cells expressing KIR2DL2, the receptor for molecules of the HLA-C1 group, is assumed in this model. (b) The receptor-ligand model predicts NK cell alloreactivity in the graft-vs-host direction when the recipient lacks an HLA ligand for donor inhibitory KIR, whose presence is verified by KIR genotyping and flow cytometry of donor NK cells. The HLA type of donor cells is irrelevant to this model. (c) The missing ligand model predicts NK cell alloreactivity in the graft-vs-host direction when recipient cells lacks expression of at least one of the HLA ligands (C1, C2 or -Bw4) for inhibitory KIR. (d) The KIR gene–gene model predicts NK alloreactivity when the donor and recipient are mismatched for KIR gene content. Inhibitory KIR genes are shown as unshaded boxes, whereas black boxes represent activating KIR genes. In the example shown, the recipients KIR genotype is said to be ‘included’ in the donor’s KIR genotype.103
Activating KIRs also deserve evaluation in HLA-haploidentical transplantation. Activating KIRs exhibit allelic polymorphisms in specific genes and extensive variation in gene number and content, which lead to heterogeneity within the general population and within diverse ethnic groups. In some studies, transplantation from donors carrying activating KIR genes was associated with improved control of leukemia relapse after HLA-identical transplantation,107 and improved survival after unrelated donor transplantation.108 Other reports have shown that transplantation from donors carrying activating KIR genes adversely affected transplantation outcomes after partially TCD HLA-haploidentical transplants, mainly through an increased risk of GVHD.109 Conversely, it has been shown that transplantation from donors carrying activating KIR genes (group B haplotype) did not cause GVHD but was surprisingly associated with less infectious mortality and better survival.105
Since non-transformed tissues generally do not over-express ligands for activating receptors on NK cells,110,111 NK cell adoptive immunotherapy has the potential to induce GVL effects without causing GVHD.104,112 There are several strategies available to enhance the antitumor effects of NK cells in the context of HLA-haploidentical SCT. Since each patient has on average five HLA-haploidentical first-degree relatives who are eligible to donate stem cells (HJS and EJF, unpublished observations), donors could be selected on the basis of optimal NK cell alloreactivity, as determined by models (Figure 2) or by in vitro assays. Chemotherapy can enhance the antitumor efficacy of subsequent NK cell infusions through multiple mechanisms, including the induction on tumor cell expression of stress ligands for NK cell-activating receptors,113 sensitization of tumor cells to NK cell-induced apoptosis114–116 or enhancement of the survival of adoptively transferred NK cells through lymphopenia-induced cytokines.117 Finally, therapeutic MoAbs, such as rituximab, may enhance the tumoricidal activity of NK cells by engaging activating Fc receptors, such as FcγRIII (CD16).118 More study is clearly needed to define the optimal conditions and strategies for enhancing the antitumor effect of NK cells in the context of HLA-haploidentical SCT.
Donor T-cell infusions
The published literature on DLI after HLA-haploidentical SCT is scanty. In a study from Israel, 28 patients received prophylactic (n = 6) or therapeutic DLI (n = 22) in doses ranging from 100 to 1.5 × 109 T cells per kg.119 Of the six patients receiving prophylactic DLI, three patients remain in remission, one relapsed and two died of GVHD. CR was achieved in only four of the 22 recipients of therapeutic DLI, and only one remains in CR. The group in Beijing administered G-CSF-primed DLI prophylactically to 29 patients120 and therapeutically to 20 patients.121 Two-year EFSs were 37.3 vs 40% of recipients of prophylactic vs therapeutic DLI, respectively. Severe GVHD occurred in six patients in each group. Further studies to define dose–response relationships for both GVHD and antitumor efficacy are clearly required before DLI can be routinely recommended for the prevention or treatment of relapse after HLA-haploidentical SCT.
It is worth noting that the presence of T cells in allogeneic stem cell grafts affects NK cell reconstitution and function after unrelated122 and HLA-haploidentical related donor SCT.123 Cross talk between T cells, NK cells and DCs occurs at the interface of innate and adaptive immunity,124 and these complicated interactions are only beginning to be explored in the context of allo-SCT. Both T cell and NK cell adoptive immunotherapies will benefit from an improved understanding of these cellular interactions. The ready availability of the original transplant donor for repeated lymphocyte donations is a distinct advantage of HLA-haploidentical related over unrelated donor SCT.
Unrelated donor UCB vs haploidentical related donor SCT
Patients who lack suitably HLA-matched related or unrelated donors have a choice between two sources of alternative donor stem cells: unrelated UCB or HLA-haploidentical related stem cells. Is there any a priori or empirical basis for choosing between these two alternatives?
Unrelated UCB has an established track record in the treatment of hematologic malignancies of children. A retrospective analysis compared the outcomes of unrelated UCB transplantation (UCBT; n = 503) vs eight of eight HLA allele (HLA-A, -B, -C and -DRB1)-matched unrelated donor marrow transplantation (n = 116) for children under the age of 16 with leukemia.125 Typing of the UCB grafts was performed at low resolution (antigen level) for HLA-A and -B and at high resolution (allele level) for HLA-DRB1, and results for 1 HLA locus mismatch grafts were analyzed according to cell dose (> 3.0 × 107 nucleated cells per kg vs ≤ 3.0 × 107 nucleated cells per kg). The results in Table 2 show that, at the very least, HLA-matched and high-dose, single locus-mismatched UCB grafts produce overall and leukemia-free survivals that are at least as good as is seen after 8/8 allele-matched unrelated BMT. Leukemia-free survival after 1 or 2 HLA locus-mismatched UCBT was not significantly worse than after HLA-matched unrelated donor SCT. These results establish 4–6/6 HLA Ag-matched UCB as a viable alternative to the use of HLA-matched unrelated donor BM for the transplantation of children with acute leukemia. Further, the results suggest that HLA-matched UCB is the new ‘gold standard’ among alternative graft sources for allo-SCT in childhood leukemia.
Table 2.
Clinical outcomes of unrelated adult marrow vs cord blood transplantation for leukemia in children
| Graft | TRM (%) | Relapse (%) | LFS (%) | OS (%) |
|---|---|---|---|---|
| BM, allele matched at HLA-A, -B, -C, -DRB1 | 19 | 41 | 38 | 45 |
| CB, A, B, antigen-matched, DRB1 allele matched | 6 | 34 | 60 | 63 |
| CB, 1-locus mismatched, high cell dose | 29 | 31 | 41 | 45 |
| CB, 1-locus mismatched, low cell dose | 43 | 21 | 37 | 36 |
| CB, 2-loci mismatched any cell dose | 49 | 20 | 33 | 33 |
Abbreviations: CB = cord blood; LFS = leukemia-free survival; OS = overall survival; TRM = treatment-related mortality. Low cell dose, ≤ 3.0 × 107 nucleated cells per kg; high cell dose, > 3.0 × 107 nucleated cells per kg. From Eapen et al.125
There are not enough data at present to make statistically valid comparisons of the outcomes of HLA-haploidentical related vs HLA-matched unrelated donor SCT in the treatment of childhood leukemia. Therefore, HLA-haploidentical related donor SCT for childhood leukemia should only be conducted in the context of carefully designed clinical trials.
For adult patients, cell dose is a major limitation in the use of UCBT. Most single UCB units simply do not contain enough hematopoietic stem cells to guarantee reliable engraftment in older adults. In the first series of US adults receiving UCBT, median UCB graft cell dose was 10-fold lower than among recipients of HLA-matched or mismatched marrow (0.22 vs 2.4 and 2.2 × 108 cells per kg, respectively), sustained neutrophil engraftment occurred in < 70% of UCBT recipients, and NRM occurred in 95 of 150 patients, many due to infection within the first 100 days after transplantation.126 HLA matching is also a significant limitation of UCBT. In an analysis of 1511 recipients of single cord blood units from the New York Blood Center National Cord Blood Program, the degree of HLA mismatch was found to correlate adversely with engraftment, GVHD, relapse, treatment-related mortality and overall survival.127 Although cell dose did not affect the outcome of fully HLA-matched UCB transplants, a twofold increase in the cell dose was required to overcome differences in treatment-related mortality and survival for 2 vs 1 HLA Ag-mismatched grafts. These findings are significant because the likelihood of finding a single cord blood unit that is mismatched for at most 1 HLA Ag and that contains > 3 × 107 nucleated cells per kg for an adult recipient is low.
Recently, adult transplantation protocols incorporating the infusion of two UCB units, each containing ≥ 1.5 × 107 nucleated cells per kg, have been a major step toward overcoming the limitations of inadequate cell dose in individual units. Double unit UBCT after myeloablative conditioning was associated with improved engraftment and lower NRM compared to historical controls receiving a single unit, and 1-year disease-free survival among 23 patients was 57%.128 In that study, the median total nucleated cell dose was 4.8 × 107/kg, and 13 patients received at least one unit that was matched to the patient at 5–6/6 HLA Ags. Two recent studies have demonstrated the feasibility of double unit UCBT after nonmyeloablative conditioning in adults.129,130 Among 110 patients studied by Brunstein et al.,130 93 (85%) required two units to achieve the target nucleated cell dose of 3 × 107/kg. Fifty-three (57%) of these patients received at least one unit matched for 5–6/6 HLA Ags, whereas the remainder received two units matched to the patient and each other at 4/6 HLA Ags. Among the total group of patients, neutrophil engraftment occurred in 92%, treatment-related mortality was 19% at 180 days and 26% at 3 years, and overall and EFSs at 3 years after transplantation were 45 and 38%, respectively. Importantly, receipt of double UCBT was associated with favorable EFS.
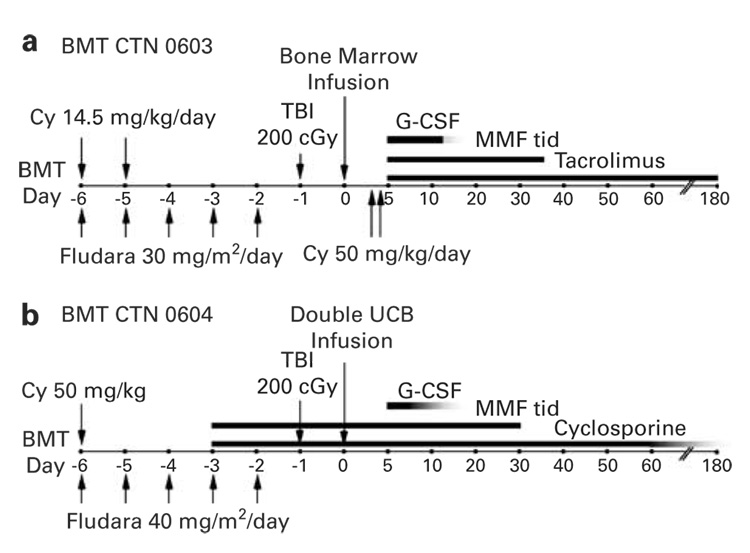
The encouraging preliminary results of nonmyeloablative alternative donor SCT may at last provide the means to offer a therapeutic graft-vs-tumor effect to a major portion of the hematologic malignancies universe; that is, elderly patients who lack HLA-matched siblings. To address this issue of donor availability, the US Blood and Marrow Transplant Clinical Trials Network (BMT CTN) is sponsoring parallel multicenter phase II trials of double unit UCB vs HLA-haploidentical marrow transplantation after nonmyeloablative conditioning for leukemia or lymphoma. Patients between the age of 21 and 70 with a diagnosis of acute leukemia or Burkitt’s lymphoma in CR, Hodgkin’s or large cell lymphoma in chemosensitive relapse, or multiply-relapsed follicular or marginal zone lymphoma are potentially eligible for either trial if autologous or HLA-matched allogeneic SCT is not a feasible option. The treatment schemas for the HLA-haploidentical related (BMT CTN 0603) and double unit UCB (BMT CTN 0604) trials are shown in Figure 3. The primary objective of each trial is to estimate the survival of patients 6 months after transplantation. The 6 month survival of patients receiving VUD SCT after nonmyeloablative conditioning is approximately 60%;131 thus, comparable survival rates after UCB or haploidentical related donor SCT would justify further testing of either or both graft sources in the nonmyeloablative SCT setting.
Figure 3.
Treatment schemata for Blood and Marrow Transplant Clinical Trials Network (BMT CTN) multicenter clinical trials of nonmyeloablative conditioning and transplantation of (a) partially HLA-mismatched (haploidentical) BM (BMT CTN 0603) or (b) double unit unrelated umbilical cord blood (UBC) (BMT CTN 0604) for adults with leukemia or lymphoma.
In summary, UCB transplantation is an acceptable therapy for children with leukemia who lack an HLA-matched sibling donor. In light of the availability of 4–6/6 HLA-matched unrelated cord blood units, haploidentical SCT in children should only be performed in the context of clinical trials. In adults, unrelated double cord blood or haploidentical related donor SCT is a reasonable therapeutic option for patients who lack an HLA-matched sibling or an 8/8 HLA allele-matched unrelated donor. Nonmyeloablative SCT using UCB or haploidentical marrow can provide long-term disease-free survival for hematologic malignancy patients who are ineligible for intensive conditioning and who lack an HLA-matched donor. The relative merits of these two graft sources will be evaluated in multicenter clinical trials.
Conclusions
HLA-haploidentical related donor SCT has come a long way in the last 20 years. The problems of excessive graft rejection and severe GVHD have been addressed by transplanting megadoses of T-cell-depleted stem cells into intensively conditioned recipients or by selective allodepletion techniques. Nonmyeloablative conditioning safeguards against the possibility of fatal graft rejection and has extended the application of haploidentical SCT to older or more infirm patients and to those who have failed a prior autologous SCT procedure. The main developmental challenges for the future are to enhance immune reconstitution and to prevent relapse after haploidentical SCT. The respective contributions of UCB vs haploidentical related donor SCT for adult patients lacking HLA-matched donors need to be defined. Both of these graft sources offer the advantages of rapid and easy availability for nearly all patients in need of transplantation. Going forward, no patient should be denied access to hematopoietic SCT for lack of an available donor.
Acknowledgements
This study was supported by Grant 1KL2RR025006-01 to HJS from the National Center for Research Resources and P01 CA15396 to EJF from the National Cancer Institute. EJF is a Clinical Research Scholar of the Leukemia and Lymphoma Society of America.
References
- 1.Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 2.Beatty PG. The immunogenetics of bone marrow transplantation. Transfusion Med Rev. 1994;8:45–58. doi: 10.1016/s0887-7963(94)70097-3. [DOI] [PubMed] [Google Scholar]
- 3.Hansen JA, Petersdorf E, Martin PJ, Anasetti C. Hematopoietic stem cell transplants from unrelated donors. Immunol Rev. 1997;157:141–151. doi: 10.1111/j.1600-065x.1997.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 4.Beatty PG, Mori M, Milford E. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995;60:778–783. [PubMed] [Google Scholar]
- 5.Tiercy JM, Bujan-Lose M, Chapuis B, Gratwohl A, Gmur J, Seger R, et al. Bone marrow transplantation with unrelated donors: what is the probability of identifying an HLA-A/B/Cw/DRB1/B3/B5/DQB1-matched donor? Bone Marrow Transplant. 2000;26:437–441. doi: 10.1038/sj.bmt.1702529. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Krepski TP, Defor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly RJ, Keever C, Kernan NA, Brochstein J, Collins N, Flomenberg N, et al. HLA nonidentical T cell depleted marrow transplants: a comparison of results in patients treated for leukemia and severe combined immunodeficiency disease. Transplant Proc. 1987;19:55–60. [PubMed] [Google Scholar]
- 8.Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, Rhee FF, et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant. 2004;33:389–396. doi: 10.1038/sj.bmt.1704391. [DOI] [PubMed] [Google Scholar]
- 9.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 10.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 11.Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 12.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 15.Sable CA, Strohmaier KM, Chodakewitz JA. Advances in antifungal therapy. Annu Rev Med. 2008;59:361–379. doi: 10.1146/annurev.med.59.062906.071602. [DOI] [PubMed] [Google Scholar]
- 16.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 17.Clift RA, Hansen JA, Thomas ED, Buckner CD, Sanders JE, Mickelson EM, et al. Marrow transplantation from donors other than HLA-identical siblings. Transplantation. 1979;28:235–242. doi: 10.1097/00007890-197909000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 19.Powles RL, Morgenstern GR, Kay HE, McElwain TJ, Clink HM, Dady PJ, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;1:612–615. doi: 10.1016/s0140-6736(83)91793-2. [DOI] [PubMed] [Google Scholar]
- 20.Ash RC, Horowitz MM, Gale RP, van Bekkum DW, Casper JT, Gordon-Smith EC, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7:443–452. [PubMed] [Google Scholar]
- 21.Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 22.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 23.Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 24.Henslee PJ, Thompson JS, Romond EH, Doukas MA, Metcalfe M, Marshall ME, et al. T cell depletion of HLA and haploidentical marrow reduces graft-versus-host disease but it may impair a graft-versus-leukemia effect. Transplant Proc. 1987;19:2701–2706. [PubMed] [Google Scholar]
- 25.Bachar-Lustig E, Rachamim N, Li HW, Lan F, Reisner Y. Megadose of T cell-depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nat Med. 1995;1:1268–1273. doi: 10.1038/nm1295-1268. [DOI] [PubMed] [Google Scholar]
- 26.Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, et al. Successful engraftment of T-cell-depleted haploidentical ‘three- loci’ incompatible transplants in leuke-mia patients by addition of recombinant human granulocyte colony-stimulating factor- mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84:3948–3955. [PubMed] [Google Scholar]
- 27.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 28.Volpi I, Perruccio K, Tosti A, Capanni M, Ruggeri L, Posati S, et al. Postgrafting administration of granulocyte colony-stimulating factor impairs functional immune recovery in recipients of human leukocyte antigen haplo-type-mismatched hematopoietic transplants. Blood. 2001;97:2514–2521. doi: 10.1182/blood.v97.8.2514. [DOI] [PubMed] [Google Scholar]
- 29.Walker I, Shehata N, Cantin G, Couture F, Dhédin N, Barty R, et al. Canadian multicenter pilot trial of haploidentical donor transplantation. Blood Cells Mol Dis. 2004;33:222–226. doi: 10.1016/j.bcmd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Waller EK, Giver CR, Rosenthal H, Somani J, Langston AA, Lonial S, et al. Facilitating T-cell immune reconstitution after haploidentical transplantation in adults. Blood Cells Mol Dis. 2004;33:233–237. doi: 10.1016/j.bcmd.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki S, Ohno Y, Taniguchi S, Yoshida T, Hayashi S, Ogawa H, et al. Allogeneic peripheral blood stem cell transplantation from two- or three-loci-mismatched related donors in adult Japanese patients with high-risk hematologic malignancies. Bone Marrow Transplant. 2004;33:279–289. doi: 10.1038/sj.bmt.1704342. [DOI] [PubMed] [Google Scholar]
- 32.Barfield RC, Otto M, Houston J, Holladay M, Geiger T, Martin J, et al. A one-step large-scale method for T- and B-cell depletion of mobilized PBSC for allogeneic transplantation. Cytotherapy. 2004;6:1–6. doi: 10.1080/14653240310004411. [DOI] [PubMed] [Google Scholar]
- 33.Ji SQ, Chen HR, Wang HX, Yan HM, Zhu L, Liu J, et al. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: An excellent alternative for high-risk leukemia. Bone Marrow Transplant. 2002;30:861–866. doi: 10.1038/sj.bmt.1703769. [DOI] [PubMed] [Google Scholar]
- 34.Dong L, Wu T, Zhang MJ, Gao ZY, Lu DP. CD3+ cell dose and disease status are important factors determining clinical outcomes in patients undergoing unmanipulated haploidentical blood and marrow transplantation after conditioning including antithymocyte globulin. Biol Blood Marrow Transplant. 2007;13:1515–1524. doi: 10.1016/j.bbmt.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Nachbaur D, Eibl B, Kropshofer G, Meister B, Mitterschiffthaler A, Schennach H, et al. In vivo T cell depletion with low-dose rabbit antithymocyte globulin results in low transplant-related mortality and low relapse incidence following unrelated hematopoietic stem cell transplantation. J Hematother Stem Cell Res. 2002;11:731–737. doi: 10.1089/15258160260194884. [DOI] [PubMed] [Google Scholar]
- 36.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98:3186–3191. doi: 10.1182/blood.v98.12.3186. [DOI] [PubMed] [Google Scholar]
- 37.Pan L, Delmonte JJ, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 38.Sykes M, Preffer F, McAfee S, Saidman SL, Weymouth D, Andrews DM, et al. Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 39.Pelot MR, Pearson DA, Swenson K, Zhao G, Sachs J, Yang YG, et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based non-myeloablative conditioning regimen. Biol Blood Marrow Transplant. 1999;5:133–143. doi: 10.1053/bbmt.1999.v5.pm10392959. [DOI] [PubMed] [Google Scholar]
- 40.Mapara MY, Kim YM, Wang SP, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100:1903–1909. doi: 10.1182/blood-2002-01-0023. [DOI] [PubMed] [Google Scholar]
- 41.Spitzer TR. Haploidentical stem cell transplantation: the always present but overlooked donor. Hematology. 2005;2005:390–395. doi: 10.1182/asheducation-2005.1.390. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;183:1682–1683. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- 43.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820–2829. [PubMed] [Google Scholar]
- 44.Colson YL, Wren SM, Schuchert MJ, Patrene KD, Johnson PC, Boggs SS, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155:4179–4188. [PubMed] [Google Scholar]
- 45.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 46.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complexidentical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 47.Ichinohe T, Maruya E, Saji H. Long-term feto-maternal microchimerism: nature’s hidden clue for alternative donor hematopoietic cell transplantation? Int J Hematol. 2002;76:229–237. doi: 10.1007/BF02982792. [DOI] [PubMed] [Google Scholar]
- 48.Claas FH, Gijbels Y, van der Velden-de Munck J, Van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 49.van Rood JJ, Zhang L, van LA, Claas FH. Neonatal tolerance revisited. Immunol Lett. 1989;21:51–54. doi: 10.1016/0165-2478(89)90011-4. [DOI] [PubMed] [Google Scholar]
- 50.van Rood JJ, Loberiza FR, Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 51.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–4678. [PubMed] [Google Scholar]
- 52.Polchi P, Lucarelli G, Galimberti M, Giardini C, Baronciani D, Angelucci E, et al. Haploidentical bone marrow transplantation from mother to child with advanced leukemia. Bone Marrow Transplant. 1995;16:529–535. [PubMed] [Google Scholar]
- 53.Ichinohe T, Uchiyama T, Shimazaki C, Matsuo K, Tamaki S, Hino M, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104:3821–3828. doi: 10.1182/blood-2004-03-1212. [DOI] [PubMed] [Google Scholar]
- 54.Umeda K, Adachi S, Ishihara H, Higashi Y, Shiota M, Watanabe KI, et al. Successful T-cell-replete peripheral blood stem cell transplantation from HLA-haploidentical microchimeric mother to daughter with refractory acute lymphoblastic leukemia using reduced-intensity conditioning. Bone Marrow Transplant. 2003;31:1061–1063. doi: 10.1038/sj.bmt.1704057. [DOI] [PubMed] [Google Scholar]
- 55.Narimatsu H, Morishita Y, Saito S, Shimada K, Ozeki K, Kohno A, et al. Conditioning regimen of melphalan, fludarabine and total body irradiation in unmanipulated HLA haploidentical stem cell transplantation based on feto-maternal tolerance. Intern Med. 2004;43:1063–1067. doi: 10.2169/internalmedicine.43.1063. [DOI] [PubMed] [Google Scholar]
- 56.Obama K, Utsunomiya A, Takatsuka Y, Takemoto Y. Reduced-intensity non-T-cell depleted HLA-haploidentical stem cell transplantation for older patients based on the concept of feto-maternal tolerance. Bone Marrow Transplant. 2004;34:897–899. doi: 10.1038/sj.bmt.1704692. [DOI] [PubMed] [Google Scholar]
- 57.Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 58.Guinan EC, Gribben JG, Brennan LL, Nadler LM. Patients (pts) surviving haploidentical stem cell transplantation (SCT) after ex vivo costimulatory blockade to induce anergy experience few long-term complications. ASH Annual Meeting Abstracts. 2005;106:599. [Google Scholar]
- 59.Davies J, Gribben J, Brennan L, Nadler L, Guinan E. Alloanergized HLA-mismatched bone marrow transplantation — low incidence of clinically significant GVHD and viral infection resulting in long term disease free survival. Biol Blood Marrow Transplant. 2008;14:16–17. [Google Scholar]
- 60.Davies JK, Gorgun G, Nadler LM, Guinan EC. Effective control of mismatched alloreactivity via ex vivo alloantigen-specific co-stimulatory blockade does not significantly impact pathogen-specific immunity. ASH Annual Meeting Abstracts. 2006;108:3177. [Google Scholar]
- 61.Davies J, Yuk D, Nadler L, Guinan E. Donor-derived T cells can be rendered hyporesponsive to alloantigen without loss of pathogen or tumor immune responses. ASH Annual Meeting Abstracts. 2007;110:771. [Google Scholar]
- 62.Davies J, Yuk D, Brennan L, Nadler L, Guinan E. 42: In vivo expansion of CD4+ foxp3+ regulatory T cells may contribute to control of acute GVHD after HLA-mismatched alloanergized HSCT. Biol Blood Marrow Transplant. 2008;14:18. [Google Scholar]
- 63.Montagna D, Yvon E, Calcaterra V, Comoli P, Locatelli F, Maccario R, et al. Depletion of alloreactive T cells by a specific anti-interleukin-2 receptor p55 chain immunotoxin does not impair in vitro antileukemia and antiviral activity. Blood. 1999;93:3550–3557. [PubMed] [Google Scholar]
- 64.Mavroudis DA, Jiang YZ, Hensel N, Lewalle P, Couriel D, Kreitman RJ, et al. Specific depletion of alloreactivity against haplotype mismatched related individuals by a recombinant immunotoxin: a new approach to graft-versus-host disease prophylaxis in haploidentical bone marrow transplantation. Bone Marrow Transplant. 1996;17:793–799. [PubMed] [Google Scholar]
- 65.Koh MB, Prentice HG, Lowdell MW. Selective removal of alloreactive cells from haematopoietic stem cell grafts: graft engineering for GVHD prophylaxis. Bone Marrow Transplant. 1999;23:1071–1079. doi: 10.1038/sj.bmt.1701749. [DOI] [PubMed] [Google Scholar]
- 66.van Dijk AMC, Kessler FL, Stadhouders-Keet SAE, Verdonck LF, de Gast GC, Otten HG, et al. Selective depletion of major and minor histocompatibility antigen reactive T cells: towards prevention of acute graft-versus-host disease. Brit J Haematol. 1999;107:169–175. doi: 10.1046/j.1365-2141.1999.01675.x. [DOI] [PubMed] [Google Scholar]
- 67.Davies JK, Koh MBC, Lowdell MW. Antiviral immunity and T-regulatory cell function are retained after selective alloreactive T-cell depletion in both the HLA-identical and HLA-mismatched settings. Biol Blood Marrow Transplant. 2004;10:259–268. doi: 10.1016/j.bbmt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Ge X, Brown J, Sykes M, Boussiotis VA. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol Blood Marrow Transplant. 2008;14:518–530. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wehler TC, Nonn M, Brandt B, Britten CM, Grone M, Todorova M, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109:365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 70.Bonini C, Ciceri F, Marktel S, Bordignon C. Suicide-gene-transduced T-cells for the regulation of the graft-versus-leukemia effect. Vox Sang. 1998;74 Suppl 2:341–343. doi: 10.1111/j.1423-0410.1998.tb05440.x. [DOI] [PubMed] [Google Scholar]
- 71.Gendelman M, Yassai M, Tivol E, Krueger A, Gorski J, Drobyski WR. Selective elimination of alloreactive donor T cells attenuates graft-versus-host disease and enhances T-cell reconstitution. Biol Blood Marrow Transplant. 2003;9:742–752. doi: 10.1016/j.bbmt.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Hartwig UF, Nonn M, Khan S, Link I, Huber C, Herr W. Depletion of alloreactive donor T lymphocytes by CD95-mediated activation-induced cell death retains antileukemic, antiviral, and immunoregulatory T cell immunity. Biol Blood Marrow Transplant. 2008;14:99–109. doi: 10.1016/j.bbmt.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Godfrey WR, Krampf MR, Taylor PA, Blazar BR. Ex vivo depletion of alloreactive cells based on CFSE dye dilution, activation antigen selection, and dendritic cell stimulation. Blood. 2004;103:1158–1165. doi: 10.1182/blood-2003-04-1098. [DOI] [PubMed] [Google Scholar]
- 74.Martins SLR, St John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ, et al. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood. 2004;104:3429–3436. doi: 10.1182/blood-2004-05-1918. [DOI] [PubMed] [Google Scholar]
- 75.Mielke S, Nunes R, Rezvani K, Fellowes VS, Venne A, Solomon SR, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008;111:4392–4402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roy DC, Cohen S, Busque L, Fish D, Kiss T, Lachance S, et al. Phase I clinical trial of haplotype mismatched myeloablative stem cell transplantation: higher doses of donor lymphocyte infusions depleted of alloreactive cells using ATIR may improve outcome without causing GVHD. ASH Annual Meeting Abstracts. 2007;110:2976. [Google Scholar]
- 77.André-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360:130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 78.Dal Cortivo L, Mahlaoui N, Picard C, Neven B, André-Schmutz I, Luby JM, et al. Adoptive immunotherapy with donor allodepleted T cells. ASH Annual Meeting Abstracts. 2005;106:479. [Google Scholar]
- 79.Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samarasinghe SR, Nawroly N, Karlsson H, Openshaw P, Vitteta E, Veys P, et al. Functional characterisation of alloreactive T-cells identifies CD25 and CD71 as the optimal targets for allodepletion strategies. ASH Annual Meeting Abstracts. 2007;110:2183. [Google Scholar]
- 81.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Joe G, Zhu J, Carroll R, Levine B, Hexner E, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 84.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland C, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 87.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 88.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 89.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 90.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 91.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 92.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 94.Karlsson H, Samarasinghe S, Ball LM, Sundberg B, Lankester AC, Dazzi F, et al. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T cell responses. Blood. 2008 doi: 10.1182/blood-2007-10-119370. (in press) [DOI] [PubMed] [Google Scholar]
- 95.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 96.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 98.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+ CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 99.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+ CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nat Rev Immunol. 2003;3:108. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- 101.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 102.Gagne K, Brizard G, Gueglio B, Milpied N, Herry P, Bonneville F, et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Hum Immunol. 2002;63:271–280. doi: 10.1016/s0198-8859(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 103.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 105.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–218. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 106.Symons HJ, Leffell MS, Rossiter ND, Sugar E, Luznik L, Phelps M, et al. Impact of killer immunoglobulin receptor (KIR) ligand incompatibility in nonmyeloablative bone marrow transplantation (BMT) from haploidentical donors. ASH Annual Meeting Abstracts. 2006;108:604. [Google Scholar]
- 107.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19:1446–1451. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 108.De Santis D, Bishara A, Witt CS, Nagler A, Brautbar C, Slavin S, et al. Natural killer cell HLA-C epitopes and killer cell immunoglobulin-like receptors both influence outcome of mismatched unrelated donor bone marrow transplants. Tissue Antigens. 2005;65:519–528. doi: 10.1111/j.1399-0039.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 109.Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63:204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 110.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 111.Gasser S, Raulet D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 2006;16:344–347. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 112.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 113.Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Res. 2006;66:3959–3962. doi: 10.1158/0008-5472.CAN-05-4603. [DOI] [PubMed] [Google Scholar]
- 114.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, et al. Bortezomiband depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–7325. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 115.Hallett WHD, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–170. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]
- 116.Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, et al. Bortezomibdown-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in cancer patients. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 118.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 119.Or R, Hadar E, Bitan M, Resnick IB, Aker M, Ackerstein A, et al. Safety and efficacy of donor lymphocyte infusions following mismatched stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1295–1301. doi: 10.1016/j.bbmt.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 120.Huang XJ, Liu DH, Liu KY, Xu LP, Chen YH, Wang Y, et al. Modified donor lymphocyte infusion after HLA-mismatched/haploidentical T cell-replete hematopoietic stem cell transplantation for prophylaxis of relapse of leukemia in patients with advanced leukemia. J Clin Immunol. 2008;28:276–283. doi: 10.1007/s10875-007-9166-z. [DOI] [PubMed] [Google Scholar]
- 121.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007;92:414–417. doi: 10.3324/haematol.10570. [DOI] [PubMed] [Google Scholar]
- 122.Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen S, Kuentz M, Vernant JP, Dhedin N, Bories D, Debre P, et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia. 2007;22:344–352. doi: 10.1038/sj.leu.2405041. [DOI] [PubMed] [Google Scholar]
- 124.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 125.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 126.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 127.Stevens CE, Rubinstein P, Scaradavou A. HLA matching in cord blood transplantion: clinical outcome and implications for cord blood unit selection and inventory size and ethnic composition. ASH Annual Meeting Abstracts. 2006;108:3104. [Google Scholar]
- 128.Barker JN, Weisdorf DJ, Defor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 129.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giralt S, Logan B, Rizzo D, Zhang M-J, Ballen K, Emmanouilides C, et al. Reduced intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the National Marrow Donor Program. Biol Blood Marrow Transplant. 2007;13:844–852. doi: 10.1016/j.bbmt.2007.03.011. [DOI] [PubMed] [Google Scholar]