Abstract
Two-pore potassium channels can influence neuronal excitability by regulating background leakage of potassium ions and resting membrane potential. The present study used quantitative real time PCR and in situ hybridization to determine if the decreased activity from deafness would induce changes in two-pore potassium channel subunit expression in the rat inferior colliculus. Ten subunits were assessed with qRT-PCR at 3 days, 3 weeks and 3 months following bilateral cochlear ablation. TASK-1, TASK-5 and THIK-2 showed significant decreases in expression at all three times assessed. TASK-5, relatively specific to auditory neurons, had the greatest decrease. TWIK-1 was significantly decreased at 3 weeks and 3 months following deafness and TREK-2 was only significantly decreased at 3 days. TASK-3, TWIK-2, THIK-1, TRAAK and TREK-1 did not show any significant changes in gene expression. In situ hybridization was used to examine TASK-1, TASK-5, TWIK-1 and THIK-2 in the central nucleus, dorsal cortex and lateral (external) cortex of the IC in normal hearing animals and at 3 weeks following deafening. All four subunits showed expression in neurons throughout IC subdivisions in normal hearing rats, with TASK-5 having the greatest overall number of labeled neurons. There was no co-localization of subunit expression with GFAP immunostaining, indicating no expression in glia. Three weeks following deafening there was a significant decrease in the number of neurons expressing TASK-1 and THIK-2 in the IC, while TASK-5 had significant decreases in the central nucleus and dorsal cortex and TWIK-1 in the lateral and dorsal cortices.
Two-pore potassium channels (K2p) are a class of open rectifying potassium selective channels (Ketchum et al., 1995) that, when activated, allow a background leakage of potassium ions that raises the resting membrane potential to hyperpolarizing levels, resulting in decreased neuronal excitability (see Lesage and Lazdunski, 2000; Goldstein et al., 2001; Patel and Honore, 2001; Plant et al., 2005 for reviews). There are, to date, 18 subunits in the K2p channel family that have been divided into different classes based on what is know about their sensitivities. The TASK-1 (K2p3.1, KCNK3), TASK-3 (K2p9.1, KCNK9) and TWIK-1 (K2p1.1, KCNK1) subunits are widely expressed throughout the brain but have been reported to have only moderate expression in the auditory brain stem (Karschin et al., 2001; Talley et al., 2001). The TASK-5 (K2p15.1, KCNK15) subunit has a relatively selective expression, primarily found in auditory brain stem neurons and Purkinje cells of the cerebellum, with additional expression in only a few neurons of the spinal trigeminal nucleus, the mammillary nucleus and the olfactory bulb (Karschin et al., 2001). Gene expression for TWIK-1, TREK-1 (K2p2.1, KNCK2), TASK-1, TRAAK (K2p4.1, KCNK4), TWIK-2 (K2p6.1, KCNK6), TASK-3, TREK-2 (K2p10.1, kcnk10), THIK-2 (K2p12.1, KCNK12), THIK-1 (K2p13.1, KCNK13) and TASK-5 mRNAs has recently been reported for the rat cochlear nucleus (Holt et al., 2006) and TASK-1 was reported as selectively elevated in spherical bushy cells (Pal et al., 2005).
The expression of K2p channels can be regulated by biochemical and physical cues as well as activity (Enyeart et al., 2003; Holt et al., 2006; Kang et al., 2004; Li et al., 2005; Liu and Saint, 2004; Xu et al., 2004 and Yeom et al., 2005). These channels could play a role in activity-dependent synaptic plasticity, where intracellular signaling induced by changes in activity level can alter the properties of target neurons. Neurons in the inferior colliculus (IC) have been reported to have increased excitability following deafness (Bledsoe et al., 1995; Bledsoe et al., 1997; Mossop et al., 2000; Salvi et al., 2000; Syka and Rybalko, 2000; Vale and Sanes, 2002 and Vale et al., 2004; for reviews Moller, 2005 and Syka, 2002). Decreases in inhibitory influences, such as GABA input, have been suggested as a mechanism for the increased neuronal excitability in the IC after deafness (Bledsoe et al., 1995, 1997; Mossop et al 2000; Salvi et al., 2000; Syka, 2002). Changes in intrinsic neuronal properties responsible for cellular excitability, however, could be another underlying mechanism. Down-regulation of K2p channels or their function could increase excitability by dampening a mechanism that decreases excitability. We therefore examined deafness associated changes in K2p channel expression in the rat IC at 3 days, 3 weeks and 3 months after bilateral deafening, using quantitative real-time PCR. Four subunits showed sustained changes in expression and were further examined at the cellular level in specific IC subdivision with in situ hybridization.
Experimental Procedures
Animals
Male Sprague-Dawley rats, 250–350 g, with normal hearing, were obtained from Charles River Laboratories (Wilmington, MA, USA). Hearing was assessed by auditory brain stem responses (ABR) recorded from all animals prior to the beginning of the study and normal hearing thresholds were required for inclusion in the study. All studies were approved by the University Committee on the Use and Care of Animals. Animals were randomly assigned to one of the four groups: Bilateral cochlear ablation with assessment at 3 days following deafening; Bilateral cochlear ablation with assessment at 3 weeks following deafening; Bilateral cochlear ablation with assessment at 3 months following deafening; or Normal hearing age matched controls.
Cochlear ablation
For cochlear ablation, rats were anesthetized with an i.m. injection of xylazine (8mg/kg) and ketamine (75mg/kg). Local injections of 1% lidocaine–HCl solution were made at the site of each surgical incision. Surgical procedures were performed under aseptic conditions. Bilateral cochlear ablation, by mechanical dissection, was performed through the lateral wall of the bulla under a dissection microscope. ABR thresholds were measured at frequencies of 2, 10 and 20 kHz from 0 to 100dB both before and after 3, 21 or 90 days surgery to confirm that all animals had normal hearing before surgery and loss of hearing after surgery. Only animals that sustained a threshold shift of 80dB or greater were included in the study to provide a homogenous group of deafened animals.
Quantitative RT-PCR (Q RT-PCR)
Twelve animals were randomly assigned to each of the four groups. Each group of 12 rats was then further divided into four sub-groups of three rats each to generate four different pools of IC mRNA for each condition. Rats were heavily anesthetized with 0.8 cc of sodium pentobarbital (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI), decapitated, the brain removed and placed in ice cold DEPC-treated PBS. The entire IC from both hemispheres was rapidly removed and placed in microcentrifuge tubes containing RNAlater (Ambion Inc., Austin, TX). Tissue from three animals (six IC) was combined into a pool and homogenized in Trizol (Invitrogen) for 10 sec. RNA was isolated as described previously (Holt et al., 2006). Inclusion of an RNA pool in the study depended on the integrity of the 18S and 28S ribosomal RNA bands, determined using a RNA 6000 Nano LabChip in a Bioanalyzer (Agilent, Palo Alto, CA). First strand cDNA was synthesized from total RNA (2 μg) using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) that utilizes 250 units of MultiScribe reverse transcriptase and random primers in the presence of RNasin (20 units; Invitrogen, Carlsbad, CA) in a total reaction volume of 100μl. The reactions were incubated at 25°C for 10 min followed by 37°C for 2h. The PCR primer sequence for TASK-5 (Forward Primer Sequence-GGCTCTTTCTACTTCGCCATCAC; Reverse Primer Sequence-GGTGCCTGGAGCAG CAT; Probe Sequence-ATCACCACCATCGGGTATG) was designed such that the amplicon crossed the boundary between exons 1 and 2 near the 5′ end of the gene sequence (Assays-by-Design, Applied Biosystems). The primer probe pairs for the other nine genes of interest were acquired through Assays-on-Demand (TaqMan Gene Expression Assays, Applied Biosystems). For each gene assayed, quantitative real-time PCR (qRT-PCR) was performed on four cDNA samples in a Prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA). Experimental variability among multiple pools of RNA samples was minimized by assaying the expression levels of each gene across each experimental group simultaneously, i.e. in the same PCR plate. The expression levels of the genes of interest were normalized using a house-keeping gene encoding ribosomal protein S16. Levels of S16 mRNA remained relatively constant among experimental groups. The threshold cycle (CT) is defined as the PCR cycle number at which the fluorescence intensity crosses a manually determined threshold value, at a level where the fluorescent signal is appreciably above the background level but is still in the early exponential phase of amplification. Each of the four samples in each group was assayed in triplicate and the resulting CT values were averaged and the standard deviation calculated. If the standard deviation of the triplicate values was more than 0.3 then the value for that sample was not used in the final analysis. Next, for each pool of RNA the difference in the averaged CT values (CT-avg) for a gene of interest and S16 for a given experimental group was calculated and defined as ΔCT for each gene of interest. The ΔCT value for each of four pools for each experimental group was then averaged and the standard deviation (SD) calculated. For each gene the average ΔCT value for the normal, non-deafened control was subtracted from the average ΔCT value of the deafened group resulting in the ΔΔCT. The error associated with ΔΔCT was equivalent to the standard deviation of the mean of the ΔCT for the experimental group. The data are reported as fold change, which was calculated as 2-ΔΔCT. This assumes doubling of products every amplification cycle or 100% amplification efficiency. The standard error is represented as 2-(ΔΔCT±SD). This results in an asymmetric distribution of the error across the bar graph (Livak and Schmittgen, 2001). The significance of the ΔΔCT values was evaluated by performing a student T-test (StatView, SAS Institute, Cary, NC) with a p value of p ≤ 0.05 considered significant.
In Situ Hybridization
The four subunits showing significant sustained changes in gene expression with qRT-PCR were further assessed using in situ hybridization (ISH). Three age-matched normal hearing animals were compared with three 3 week deaf animals. Rats were anesthetized with sodium pentobarbital (Fatal Plus, 0.5cc) and then received vascular perfusion with phosphate buffer followed by a fixative containing 4% paraformaldehyde in phosphate buffer. Brains were removed, immersed in the same fixative for 2h and then placed in 30% sucrose in PBS for 12–16h at 4°C. Thirty-μm coronal sections through the IC were cut on a cryostat, thaw-mounted onto Fisher Plus slides, and dried at 40°C in a desiccation box over night. Twelve adjacent cryostat sections from each animal were chosen as comparable based on morphological appearance and landmarks. Each section contained five IC regions of interest to be assessed: 1) high frequency central nucleus; 2) low frequency central nucleus; 3) ventral lateral cortex; 4) dorsal lateral cortex; 5) dorsal cortex, as shown in Figure 1. Sections were then processed for ISH, with sections 1, 5 and 9 hybridized with antisense oligonucleotide probe to TASK-1, sections 2, 6 and 10 hybridized with antisense oligonucleotide probe to TASK-5, sections 3, 7 and 11 hybridized with antisense oligonucleotide probe to TWIK-1 and sections 4, 8 and 12 hybridized with antisense oligonucleotide probe to THIK-2. The synthetic oligonucleotide probes were chosen from the open reading frame with least homology to other known sequences to minimize cross-hybridization. Antisense oligonucleotides designed for a minimal tendency of forming hairpins and self-dimers were as follows (base position on coding strand indicated): TASK-1: (103-143) 5’-AGCTCCAGCTGCCGCAGCTCCAGCCGCTGCCGCTCGAT CAT3’; TASK-5: (684-730) 5’-GGACCACCAGGTTGAGGAAAGCACCAATGACT GTGAGCCCCAGCAGG3’; TWIK-1: (209-246) 5’-CTGTTTCATCTCCCTGAGCAC CATCGGCCTGGG3’; THIK-2: (834-879) 5’- TATGGTCGACACCACGGTGCCCAC GAAGTAGAAGGCTCCAGGGAAG3’. Oligonucleotides were 5’ end-labeled with Biotin (Invitrogen USA) for hybridization at concentrations of 1-1.5ng/μl (30μl/section).
Figure 1.
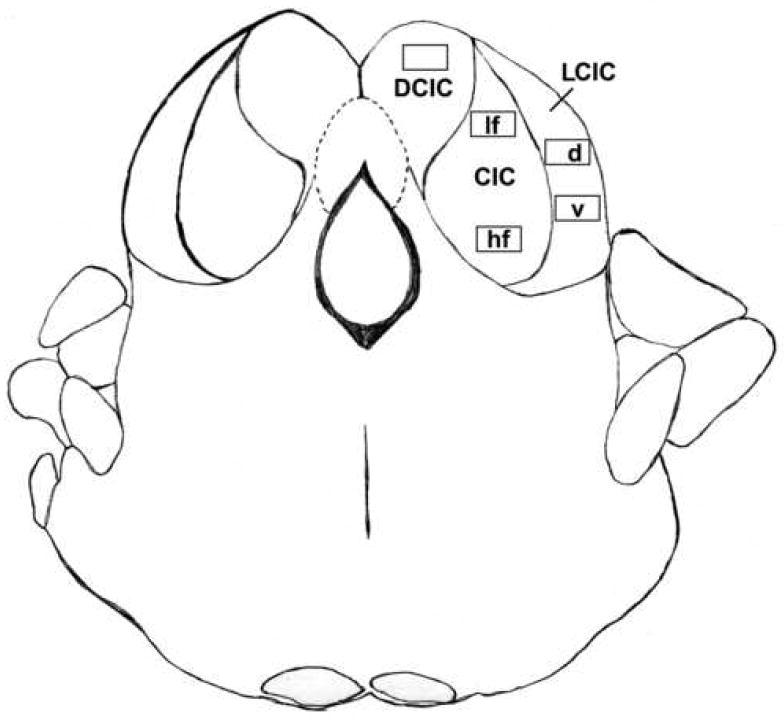
Schematic showing the location of the five regions of interest in the inferior colliculus used for quantitative assessment of the number of cells (within each region of interest) labeled with in situ hybridization. DCIC = dorsal cortex of inferior colliculus; CIC = central nucleus of the inferior colliculus; LCIC = lateral cortex (external cortex) of the inferior colliculus; lf = low frequency region; hf = high frequency region; d = dorsal region; v = ventral region.
Slides were pretreated with 0.1M PBS, Glycine-PBS and Triton-PBS for 10 minutes at room temperature (RT) respectively, then immersed in proteinase K (1μg/ml)-TE buffer for 30 minutes at 37°C, and 4% PFA for 5 min at 4°C. Slides were next treated with 170μl prehybridization solution containing 47% Formamide Deionized, 2×saline-sodium citrated (SSC), 0.05M phosphate buffer (PB) for 2 hours at 37°C followed wash in 2×SSC for 5 minutes. Sections were then hybridized for 18 h at 37°C with antisense probe to TASK-1, TASK-5, TWIK-1 or THIK-2 in 170μl of buffer containing 47% Formamide Deionized, 2×SSC, 0.05M PB, 1×Denhard’s solution, 10% dextran sulfate, 0.05M Dithiothreitol (DTT), 0.25mg/ml yeast tRNA, 0.5mg/ml denatured salmon sperm DNA, 1-1.5μg/ml probe. Control sections were digested with RNase A (100ng /ml) for 30 min at 37 °C before hybridization or hybridized with a sense probe to TASK-5: (764-810) 5’-CCTGCTGGGGCTCACAGTCATTGGTGCTTTCCTCAACCTGGTGGTCC 3’. Slides were then washed twice in 2×SSC, 1×SSC, 0.5×SSC and 0.1×SSC respectively for 15 minutes at 37°C. This was followed by two rinses in PBS-Tween (0.1% Tween20) for 10 minutes at RT and a rinse in blocking buffer (1×PBS with 1% BSA, 0.1% Tween 20) for 30 minutes at 37°C. Sections were then incubated with 30μl Avidin- FITC antibody (1:100, Roche Applied Science) for 2 hours at 37°C, followed by three five minute washes in PBS. Slides were covered-slipped and stored in the dark.
Morphometry
Five regions of interest were assessed in each section (Figure 1): 1) high frequency region of the central nucleus; 2) low frequency region of the central nucleus; 3) dorsal region of the lateral cortex; 4) ventral region of the lateral cortex; 5) dorsal cortex. Images of each region of interest were acquired using a Zeiss LSM confocal microscope with image gathering parameters kept constant and entered into a Metamorph Image Analysis Workstation. The number of all cells with intensity of fluorescent labeling at least 5X over background was determined for each region of interest in each section assessed. We further defined cells based on size as small (less than 10 microns), medium (10-20 microns), or large (over 20 microns) (Osen 1972; Malmierca et al., 1993; Faye-Lund and Osen, 1985).
Co-Labeling with GFAP
Sections from two normal hearing animals were processed for combined in situ hybridization and immunocytochemistry to test if there was any expression of TASK-1, TASK-5, TWIK-1 or THIK-2 in glial cells. Sections were processed with ISH for the four subunits, as above, and then rinsed in phosphate-buffered saline (1× PBS). The sections were processed for immunofluorescence staining. Sections were incubated with a primary antibody to glial fibrillary acidic protein (GFAP, Sigma Inc., 1:1000 dilution) with 5% normal goat serum (Jackson Laboratories, USA) in PBS with 0.1% triton X-100 for 2 h at 4°C in a humid chamber. The sections were then rinsed three times for 5 min in PBS at room temperature and incubated for 1h with Alexa fluor®568 goat anti-rabbit secondary antibody (1:100, Molecular Probes) in 0.1% triton X-100. After rinsing, slides were cover slipped using gel/mount (Biomedia Corp., Foster City, CA, USA) and examined with a Zeiss laser scanning confocal microscope for co-localization of staining. Sections without primary antibody served as controls.
Results
PCR Results
TASK subunits
All three TASK subunits examined (TASK-1, TASK-3 and TASK-5), were expressed in the IC of normal hearing rats (Figure 2A). This is consistent with previous studies in the inferior colliculus using in situ hybridization (Karschin et al., 2001, Pal et al., 2005 and Talley et al., 2001).
Figure 2.
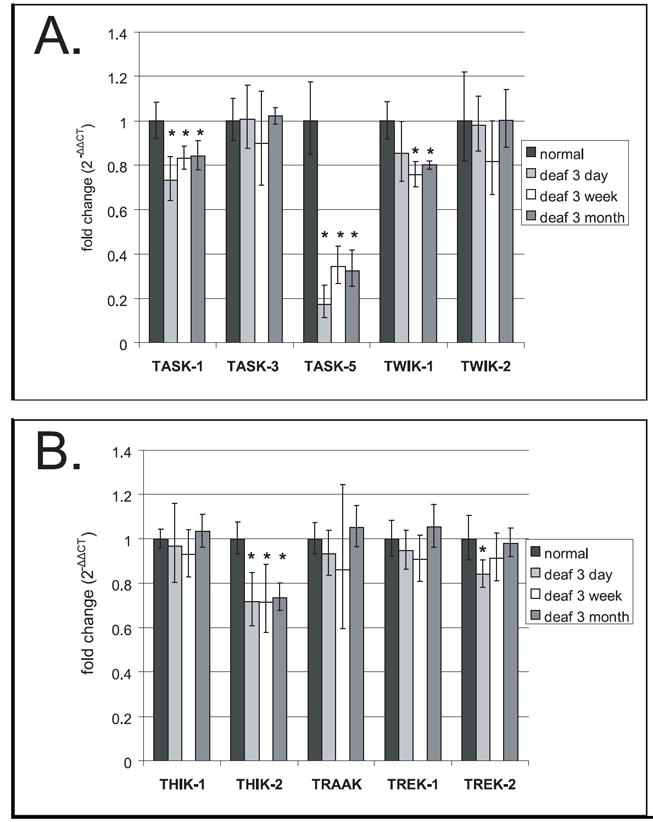
Changes in the gene expression of selected 2-pore domain potassium channel subunits in the rat inferior colliculus following deafness, as shown by quantitative real-time PCR. Asterisks indicated statistically significant differences when compared to normal expression levels. Error bars indicate standard deviation.
In this study, TASK-1 and TASK-5 subunits showed significant and sustained decreases in expression in the inferior colliculus following deafening at all three times assessed (Figure 2A). TASK-5 subunit gene expression was decreased by 83% (p < 0.001) at 3 days following deafening, 66% (p < 0.001) at 3 weeks and 68% (p < 0.001) at 3 months. TASK-1 expression was decreased by 27% at 3 days following deafness (p=0.008), there was a 17% decrease at 3 weeks (p = 0.02) and 16% decrease at 3 months (p = 0.02). TASK-3 did not show significant changes.
TWIK subunits
TWIK-1 and TWIK-2 were expressed in the IC of normal hearing rats (Figure 2A). Previous RT-PCR and in situ hybridization studies have revealed that TWIK-1 is present in the mammalian brain and also in a wide variety of tissues outside the nervous system (Lesage et al, 1996; Orias et al, 1997, Arrighi et al, 1998; Wang et al,1998; Medhurst et al, 2001 and Talley et al, 2001). TWIK-1 expression has been previously shown in the cochlear nucleus using PCR (Holt et al., 2006) and in the inner ear and cochlear nucleus using in situ hybridization (Nicolas MT et al., 2003; Talley et al., 2001). In the present study, a decrease in TWIK-1 expression was found which did not reach significance until 3 weeks following deafening (24% decrease, p=0.003) and remained significantly decreased by 20% (p=0.002) at 3 months following deafness (Figure 2A). TWIK-2 expression did not show any significant deafness-associated changes.
TREK and TRAAK subunits
Previous studies using in situ hybridization reported labeling for TREK-1, TREK-2 and TRAAK in the IC and cochlear nucleus (Talley et al., 2001). These subunits were also shown to be expressed in the rat cochlear nucleus with PCR (Holt et al., 2006) and TREK-1 immunostaining was shown in the rat cochlea (Kanjhan et al., 2004a) and cochlear nucleus (Kanjhan et al., 2004b). In the current study all three subunits are expressed in the IC (Figure 2B). TREK-2 expression showed a significant deafness-associated decrease of 16% at 3 days (p=0.03) after deafening, however it returned to normal levels of expression by 3 months, with a small decrease at 3 weeks that did not reach significance (Figure 2B). The TREK-1 and TRAAK subunits did not show significant changes in expression following deafness.
THIK subunits
The THIK-1 and THIK-2 halothane sensitive 2-pore domain potassium channels showed expression in the rat IC (Figure 2B). Distribution of THIK-2 subunit expression has previously been reported in the rat IC using in situ hybridization (Rajan et al., 2001). In the present study the THIK-2 subunit showed deafness-associated decreases in expression at all three times assayed following deafness with a 28% decrease at 3 days (p=0.01), a 29% decrease at 3 weeks (p=0.02) and a 26% decrease at 3 months (p=0.001) (Figure 2B). The THIK-1 subunit, on the other hand, did not show significant deafness-associated decreases at any of the times assessed, perhaps because of the limited distribution of this subunit in the IC (Rajan et al., 2001).
In Situ Hybridization
General
In situ hybridization resulted in cells labeled for TASK-1, TASK-5, TWIK-1 and THIK-2 across all regions of the rat IC. Control sections digested with RNase A or hybridized with a sense probe to TASK-5 did no show any labeling of cells.
TASK-5
TASK-5 was abundantly expressed in neurons in all three subdivisions in the IC of normal hearing rats, with the greatest number and density of labeled cells (Figures 3a, 4) of the four subunits assessed. In the high frequency region of the central nucleus of the IC, large, medium and small sized neurons were labeled. In the low frequency region, however, expression was found only in medium and small sized neurons, primarily in the medium sized neurons. Interestingly, the low frequency area had a greater number of positive cells than the high frequency area, not seen with the other subunits assessed (Figures 3b-3d). The cells responsible for the increased labeling in lower frequency central nucleus were small-medium sized with an oval shape. There was also a difference between dorsal and ventral regions of the lateral cortex with more neurons expressing TASK-5 in the dorsal than the ventral lateral cortex. The neurons expressing TASK-5 in the lateral cortex were primarily medium in size with fewer small or large labeled cells. In the dorsal cortex most of the neurons with TASK-5 expression were medium sized and fewer were large or small.
Figure 3.
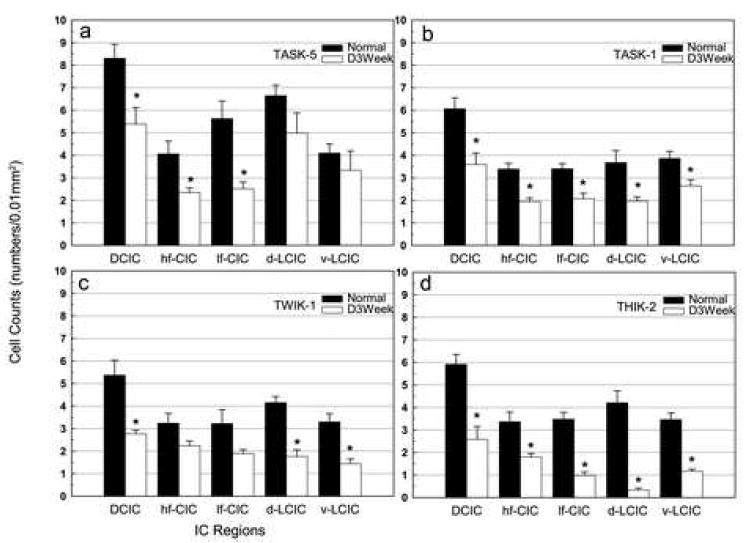
Graph showing the number of TASK-5, TASK-1, TWIK-1 and THIK-2 (a-d) cells labeled with in situ hybridization in five regions of the rat inferior colliculus in normal hearing animals (Normal) and in animals assessed three weeks after bilateral cochlear ablation (D3week). Cell counts are given as the number of labeled cells per 0.01mm2 of tissue assessed (y axis). Error bars represent standard deviations. Asterisks indicated statistically significant differences (p value below 0.05) in the number of labeled cells in the deafened group when compared to the levels in normal hearing animals.
Figure 4.
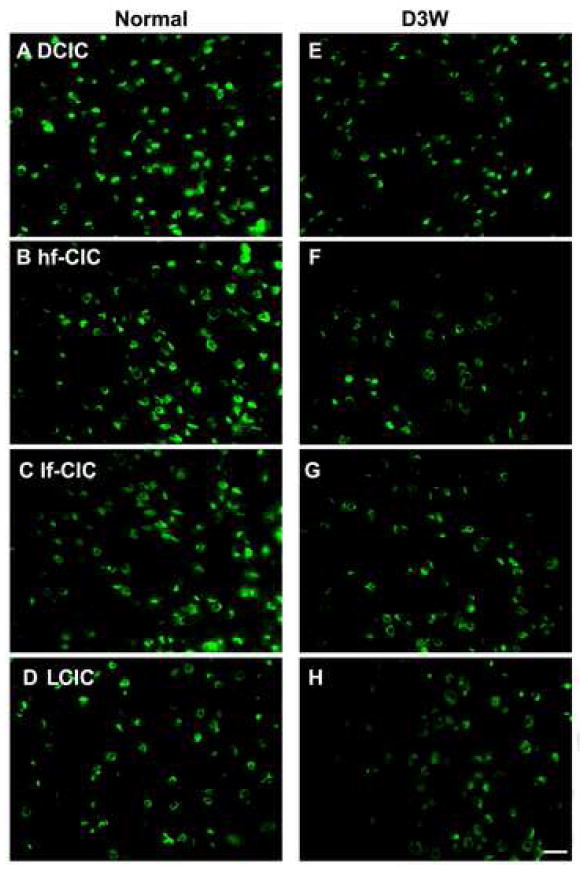
Photomicrographs of representative in situ hybridization labeling of cells for TASK-5 in four regions of the rat inferior colliculus in normal hearing animals (Normal) (A-D)and in animals assessed three weeks following deafening (D3W) (E-H)from bilateral cochlear ablation in the Dorsal Cortex (DCIC) (A,E); high frequency region of the Central Nucleus of the Inferior Colliculus (hf-CIC) (B,F); low frequency region of the Central Nucleus of the Inferior Colliculus (lf-CIC) (C,G); lateral (external) cortex of inferior colliculus (LCIC) (D,H), scale bar = 25 μm.
At 3 weeks following deafening there was a significant decrease in the number of neurons meeting criteria for positive labeling. Even in labeled neurons both the intensity of label and the amount of labeling within the soma often decreased. In the central nucleus of 3 week deaf rats there was significant decreases of 55% (p < 0.01) in the low frequency region and of 42% (p = 0.01) in the high frequency region. While the number of positive cells started out higher in the low than the high frequency regions of the central nucleus (in normal hearing animals), the greater decrease in the low frequency region resulted in comparable numbers of labeled cells in high and low frequency regions following deafening. The decrease was most notable in medium and small cells. In the dorsal cortex there was a significant 35% decrease of TASK-5 labeled cells (p < 0.01) with the medium sized round / olivary shaped neurons no longer detected and the majority of labeled neurons that remained appearing to be medium sized flat / stellate shaped cells. Although there were changes in the number of TASK-5 labeled cells in the lateral cortex following deafness, the decreases did not reach significance in either dorsal or ventral regions.
TASK-1
TASK-1 expression in normal hearing animals was found in central nucleus, lateral cortex and dorsal cortex of the IC (Figures 3b, 5) with expression in small, medium and large neurons. In the central nucleus of the IC, TASK-1 expression was found in similar neuronal types as for TASK-5 (Figure 5), although fewer cells were labeled, particularly in the low frequency area. As with TASK-5, in the high frequency region of the central nucleus, large, medium and small sized neurons were labeled while in the low frequency region expression was found only in the medium and small sized neurons, with the medium sized neurons most numerous. In the lateral cortex, labeling was primarily in medium-large and medium sized neurons while TASK-1 labeled neurons in the dorsal cortex were predominantly medium sized.
Figure 5.
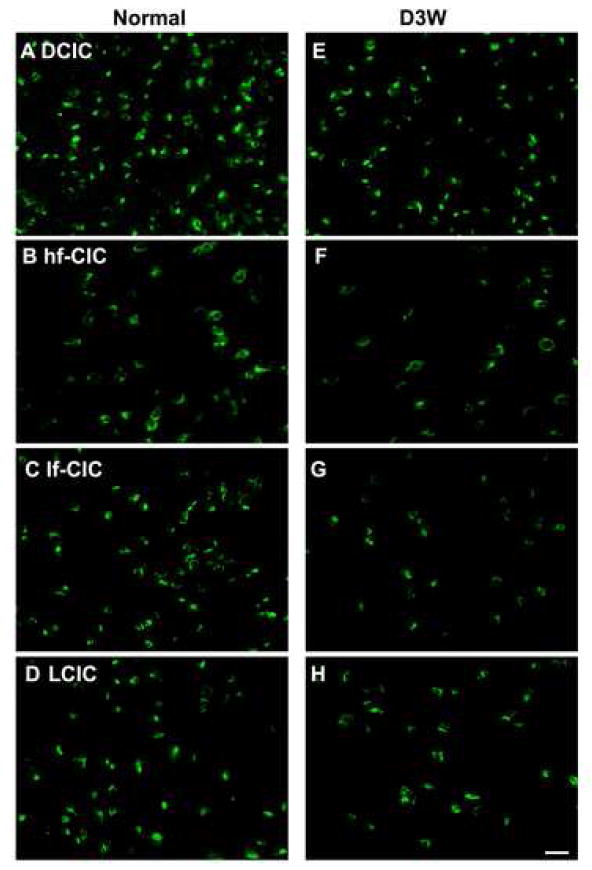
Photomicrographs of representative in situ hybridization labeling of cells for TASK-1 in four regions of the rat inferior colliculus in normal hearing animals (Normal) (A-D)and in animals assessed three weeks following deafening (D3W) (E-H)from bilateral cochlear ablation in the Dorsal Cortex (DCIC) (A,E); high frequency region of the Central Nucleus of the Inferior Colliculus (hf-CIC) (B,F); low frequency region of the Central Nucleus of the Inferior Colliculus (lf-CIC) (C,G); lateral (external) cortex of inferior colliculus (LCIC) (D,H), scale bar = 25 μm.
At 3 weeks following deafening there was a significant decrease in the number of neurons meeting criteria for positive labeling within all three IC subdivisions (Figures 3b, 5). In the central nucleus there were significant decreases in both the low frequency region (39%, p < 0.01) and the high frequency region (42%, p < 0.01). The small cells appeared least effected by deafness, becoming a greater percentage of the cells retaining label. The number of TASK-1 labeled cells decreased significantly in the dorsal cortex (41%, p < 0.01). There were also significant decreases in labeled cells in the lateral cortex in both the ventral (32%, p < 0.01) and dorsal (47%, p <0.04) regions. In the lateral cortex the medium sized cells were most affected by deafness, becoming a smaller proportion of those retaining label, However, we cannot rule out that there may be decreases in cell size, as reported to occur with deafening (Kawano et al., 1997; Lesperance et al., 1995; Lustig et al., 1994; Niparko, 1999; Niparko et al., 1997; Sie et al., 1992; Willott et al., 1994). Such a decrease in cell size could bring neurons from a previous classification of medium sized to the class of small neurons.
TWIK-1
Normal expression of TWIK-1 was found in all three subdivision of the IC, with the number of labeled neurons less numerous than for TASK-5 and comparable to those for TASK-1 and THIK-2 (Figures 3c & 6). In the central nucleus large neurons were found in the high frequency region, while both medium and small cells were found in high and low frequency regions. In the dorsal cortex many large neurons were positive in addition to fewer medium sized neurons. In the external cortex there was expression in medium sized neurons with fewer small sized neurons also labeled. In the three week deaf animals, TWIK-1 was significantly decreased in the dorsal cortex (48%, p < 0.01) as well as in both the dorsal (57%, p < 0.01) and ventral (56%, p < 0.01) external cortex (Figure 3c & 6). The decreases in TWIK-1 labeled neurons closely approached but did not reach significance in the either high or low frequency regions of the central nucleus (p = 0.053, p = 0.057).
Figure 6.
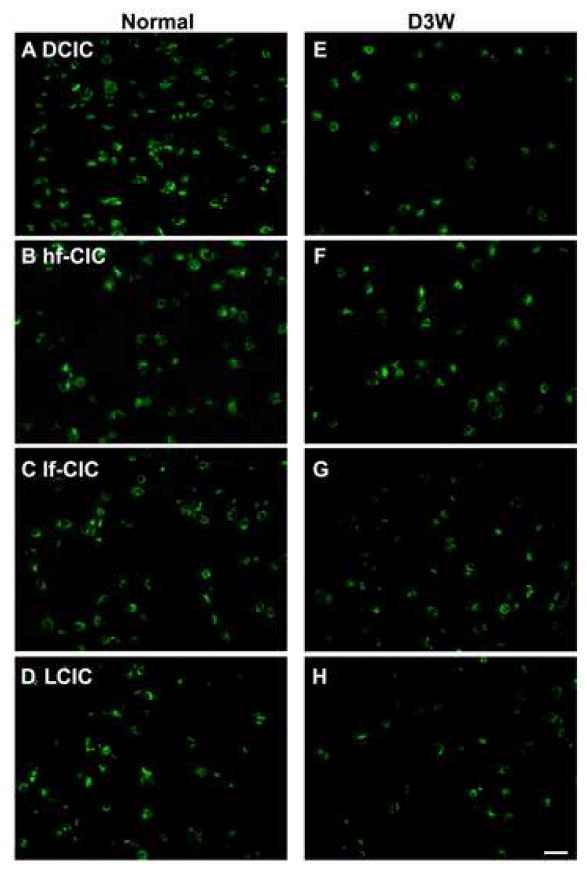
Photomicrographs of representative in situ hybridization labeling of cells for TWIK-1 in four regions of the rat inferior colliculus in normal hearing animals (Normal) (A-D)and in animals assessed three weeks following deafening (D3W) (E-H)from bilateral cochlear ablation in the Dorsal Cortex (DCIC) (A,E); high frequency region of the Central Nucleus of the Inferior Colliculus (hf-CIC) (B,F); low frequency region of the Central Nucleus of the Inferior Colliculus (lf-CIC) (C,G); lateral (external) cortex of inferior colliculus (LCIC) (D,H), scale bar = 25 μm.
THIK-2
Expression of THIK-2 was found in neurons in all subdivision of the IC in normal hearing animals, with the number of labeled cells as well as the cell types similar to that of TASK-1 and TWIK-1 (Figures 3d, 7). Of the four subunits assessed, THIK-2 had the largest deafness-associated decrease in labeled cells (Figure 3d). The remaining labeled neurons were sparse, particularly in the external cortex and low frequency regions of the central nucleus (Figure 7). At 3 weeks following deafening there was a decrease of 46% (p < 0.01) in high frequency and 72% (p < 0.01) in low frequency regions of the central nucleus, 56% (p < 0.01) in dorsal cortex and 92% (p < 0.01) in the dorsal external cortex and 66% (p < 0.01) in the ventral external cortex.
Figure 7.
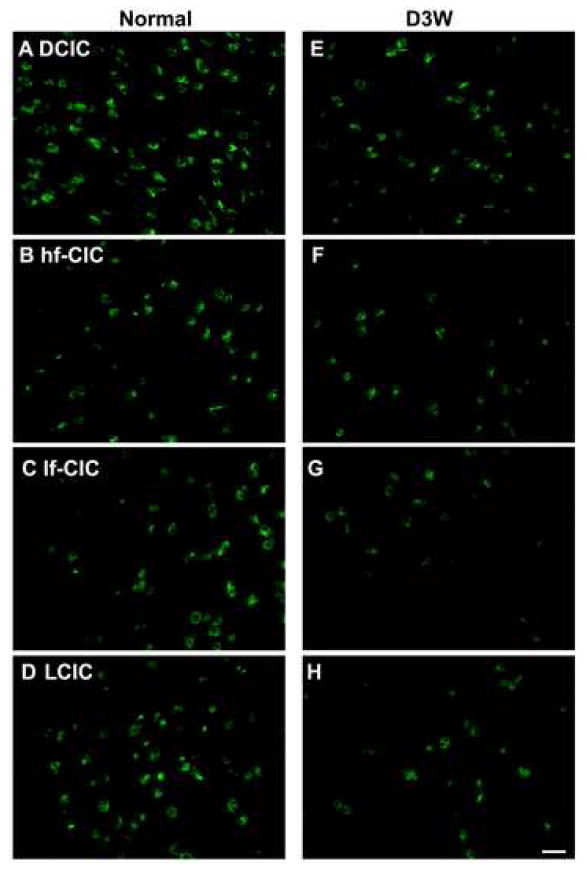
Photomicrographs of representative in situ hybridization labeling of cells for THIK-2 in four regions of the rat inferior colliculus in normal hearing animals (Normal) (A-D)and in animals assessed three weeks following deafening (D3W) (E-H)from bilateral cochlear ablation in the Dorsal Cortex (DCIC) (A,E); high frequency region of the Central Nucleus of the Inferior Colliculus (hf-CIC) (B,F); low frequency region of the Central Nucleus of the Inferior Colliculus (lf-CIC) (C,G); and the lateral (external) cortex of inferior colliculus (LCIC) (D,H), scale bar = 25 μm.
GFAP
GFAP immunostaining revealed many labeled cells in all division of the IC. ISH labeling for TASK-1, TASK-5, TWIK-1 or THIK-2 expression was restricted to soma, while GFAP immunolabeled smaller soma as well as processes. When images of ISH labeling and GFAP immunolabeling were merged there was no co-labeling observed (Figure 8), although labeling was often in close apposition. This suggests that expression of TASK-1, TASK-5, TWIK-1 and THIK-2 in the IC is neuronal.
Figure 8.
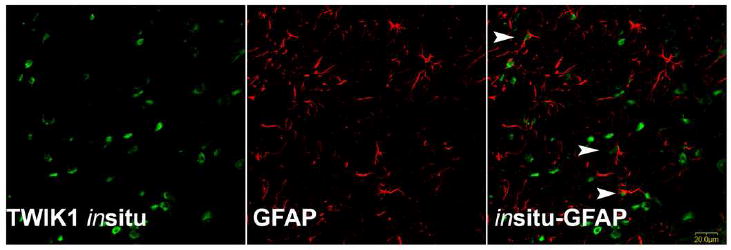
Photomicrographs of merged imaged from the same section showing immuno-labeling for glial fibrillary acidic protein (GFAP) with a red chromophore and in situ hybridization labeling for TWIK-1 with a green chromophore. There is no overlap of labeling suggesting that TWIK-1 label is not present in glia and may be restricted to neurons. Scale bar = 20 μm.
Discussion
Quantitative real-time PCR was used to show that ten K2p channel subunits across 4 classes are expressed in the rat IC, five of these show deafness-associated decreases in expression, and four have sustained decreases through three months of deafness. Using this method, TASK-5, the subunit reported to be relatively specific to auditory neurons (Karschin et al., 2001), showed the greatest decrease in expression. In situ hybridization showed that the four subunits with sustained decreases were expressed in multiple neuronal types across the central nucleus, lateral cortex and dorsal cortex of the IC and confirmed their decreases following deafening.
While the present study found significant deafness-associated decreases in five of the ten subunits assessed, an earlier study, using only qRT-PCR, found significant decreases in nine of these ten subunits in the cochlear nucleus (CN). TRAAK, TASK-3, TWIK-2 and TREK-1 did not show significant decreases in the IC while in the CN TRAAK and TASK-3 had significant decreases at 3 days and 3 weeks following deafness, and TREK-1 and TWIK-2 at 3 weeks and 3 months. TREK-2 had a different pattern of change in CN and IC, significantly decreased only at 3 days following deafness in the IC, and significantly decreased in the CN at 3 weeks and 3 months. THIK-1 showed no changes in CN or IC. There are several possible explanations for the different responses in the IC and CN. The IC and CN have different inputs, outputs and neurons with different properties. Deafening the cochlea would influence both excitatory and inhibitory inputs to the IC, while primarily excitatory inputs to the CN are affected. Also, the method of deafening applied, cochlear ablation causes not only loss of excitation but also a physical degeneration and loss of auditory nerve terminals onto the CN neurons. In the IC, however, the terminals remain, they just become inactive. Given the multiple sensitivities of 2-pore domain channels it is therefore not unexpected to find different responses in CN and IC.
In the present study, in the rat IC, in situ hybridization for TASK-5 labeled more cells than were labeled for the other three subunits in all IC subdivisions assessed. The TASK-1, THIK-2 and TWIK-1 subunits had very similar patterns, labeling approximately equal number of cells, with similar appearances. This suggests that TASK-1, THIK-2 and TWIK-1 may be expressed in the same neurons with TASK-5 expressed in those neurons as well as in additional ones. All four subunits assessed showed deafness-associated decreases in labeling, consistent with the decreases in expression shown by qRT-PCR. While TASK-5 showed the greatest deafness-associated decrease in expression with qRT-PCR (assessed in whole IC), THIK-2 showed the greatest deafness-associated decrease in neurons labeled by in situ hybridization, particularly in lateral cortex. TASK-5, on the other hand, did not have changes in the lateral external cortex that reached the level of significance. The lateral cortex is less associated with the lemniscal ascending auditory pathway and more associated with multimodal integration. It is therefore interesting that while TWIK-1, TASK-1 and particularly THIK-2 showed large and significant deafness-associated decreases in lateral cortex, the relatively auditory specific TASK-5 subunit did not.
Rat hearing generally uses high frequency more than low frequency and this would suggest there could be more normal activity in neurons in high versus low frequency regions of the central nucleus. It is therefore interesting that TASK-5 (but not TASK-1, TWIK-1 or THIK-2) has more labeled neurons in the low frequency than high frequency region of the central nucleus. While the additional TASK-5 neurons in the low frequency region fell in the class of small to medium sized neurons with an oval shape, we cannot make any conclusion as to whether they fall within or across specific functional populations because IC neurons of similar size and shape can have different response properties (e.g. Peruzzi et al., 2000). While these extra cells were found in the lower activity region of the central nucleus, they were still greatly influenced by the large decrease in activity, with the number of cells remaining labeled becoming equivalent in both low and high frequency regions of central nucleus following deafening. Labeling for all four subunits was seen in large cells found in the high frequency region of the central nucleus while these cells were not labeled in the low frequency region. This suggests a K2p channel function in this cell type that is needed only in the more active region and that has a complex regulation requiring K2p channel subunits from multiple categories. The expression of the four subunits in large cells was not greatly influenced by deafening, which predominantly decreased their expression in small and medium sized cells.
K2p channel subunits have been previously identified in astrocytes (Gnatenco et al., 2002; Rusznak et al., 2004, Kindler et al., 2000) where they have been suggested to play a role in ionic homeostasis. Skatchkov et al (2006) reported that K2p channel subunits are functionally expressed in retinal glial cells. Our immunostaining for GFAP on sections hybridized for TASK-1, TASK-5, TWIK-1 or THIK-2 expression did not reveal any co-labeling (Figure 8), suggesting that their expression was restricted to neurons. We cannot, however, rule out the possibility that any of the other six subunits we find expressed in IC are found in glia.
The ten transcripts were shown to be expressed in the rat IC, representing four groups from the six classes of K2p channel subunits, divided based on some differences in sensitivities (Lesage et al., 2000; Rajan et al., 2001; Goldstein et al., 2001; Patel et al., 2001). This suggests that neurons in the inferior colliculus are sensitive to the associated modalities and that the intrinsic excitability of IC neurons can be modulated by multiple factors. Physiological roles for K2p channels extend beyond regulation of resting potential to include metabolic signaling, cell volume regulation, pain and heat sensation, G protein signaling, and potassium homeostasis (Kim, 2005). These functions are mediated by sensitivities to endogenous and exogenous factors, including activation by pH, unsaturated fatty acids, and membrane stretch, and inhibition by anesthetics, neurotransmitter-receptor signaling, and alterations in cytosolic calcium. (Lesage and Lazdunski, 2000; Goldstein et al., 2001; Patel and Honore, 2001). In cerebellar granule cells (Millar et al., 2000) and cranial motoneurons (Talley et al., 2000) receptor-mediated inhibition of TASK-1 has been reported, which suggests an important role for the TASK family of K2p channels in neuromodulation. Experiments testing functional expression of TASK-5 and THIK-2 have been unsuccessful to date (Ashmole et al., 2001; Rajan et al., 2001) and so their biophysical properties remain undefined. However, sequence homology and similarities in topology with other K2p channel family members strongly suggests conserved function. TASK-5 has been placed into the category of acid-sensitive channels based on its sequence homology to TASK-1 and 3. In cerebellar granule cells (Millar et al., 2000) and cranial motoneurons (Talley et al., 2000) receptor-mediated inhibition of TASK-1 has been reported and TASK-3 is activated by depolarization and modulated by extracellular divalent cations (Rajan et al., 2000). TASK-5 might then be similarly sensitive to activity and neuromodulation. It is interesting that TASK-5 is relatively specific for neurons in the ascending auditory pathways, which are among the most highly active in the central nervous system. TASK-5 also showed the greatest decrease in expression, when assessed by PCR, of any of the K2p channel subunits following the decreased activity from deafening. This suggests that its function, once ascertained, may be tied to high activity.
The TREK-2 K2p channel subunit falls into the class of unsaturated fatty acid and stretch-activated K2p channel subunits. As such, one modality for TREK-2 activation is through mechanical gating via stretching the cell membrane (Kim et al., 2005). Decreases in cell size (Kawano et al., 1997; Lesperance et al., 1995; Lustig et al., 1994; Niparko, 1999; Niparko et al., 1997; Sie et al., 1992; Willott et al., 1994) or even flattening of synapses (Gulley et al, 1978; Ryugo et al, 2005) that have been reported following deafness could therefore influence TREK-2 activity and expression. It is interesting that the deafness-associated decrease in TREK-2 expression was transient, significant only at 3 days following deafness, while decreases in the other three subunits were more sustained.
When K2p channels are active they provide a “leak” of potassium ions, decreasing the excitability of neurons. Their down-regulation following deafening would reduce the potential for this leak and therefore be associated with improving intrinsic neuronal excitability when extrinsic excitation is removed. There is now extensive literature showing increased excitability of IC neurons following deafness (Bledsoe et al., 1995, Bledsoe et al., 1997, Mossop et al., 2000, Salvi et al., 2000, Syka and Rybalko, 2000, Vale and Sanes, 2002 and Vale et al., 2004; for reviews Moller, 2005 and Syka, 2002), with these reports often suggesting increased excitability is a consequence of decreases in inhibitory influences (Bledsoe et al., 1995, 1997; Mossop et al 2000, Salvi et al., 2000, Syka, 2002). The results of the current study showing deafness-associated decreases in “leak” channels suggests that changes in intrinsic membrane properties could also contribute to decreased neuronal excitability.
Acknowledgments
These studies were supported by NIDCD Grant DC00383 and core center grant P30 DC05188 from the NIDCD, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrighi I, Lesage F, Scimeca JC, Carle GF, Barhanin J. Structure, chromosome localization, and tissue distribution of the mouse twik K+ channel gene. FEBS Lett. 1998;425:310–316. doi: 10.1016/s0014-5793(98)00260-9. [DOI] [PubMed] [Google Scholar]
- Ashmole I, Goodwin PA, Stanfield PR. TASK-5, a novel member of the tandem pore K+ channel family. Pflugers Arch. 2001;442:828–833. doi: 10.1007/s004240100620. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system. Neuroreport. 1995;7:225–229. [PubMed] [Google Scholar]
- Bledsoe SC, Nagase S, Altschuler RA, Miller JM. Changes in the central auditory system with deafness and return of activity via a cochlear prosthesis. In: Syka J, editor. The Language of Science. New York: Plenum Press; 1997. pp. 513–529. [Google Scholar]
- Enyeart JA, Danthi S, Enyeart JJ. Corticotropin induces the expression of TREK-1 mRNA and K+ current in adrenocortical cells. Mol Pharmacol. 2003;64:132–142. doi: 10.1124/mol.64.1.132. [DOI] [PubMed] [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol (Berl) 1985;171:1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Gnatenco C, Han J, Snyder AK, Kim D. Functional expression of TREK-2 K+ channel in cultured rat brain astrocytes. Brain Res. 2002;931:56–67. doi: 10.1016/s0006-8993(02)02261-8. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gulley RL, Wenthold RJ, Neises GR. Changes in the synapses of spiral ganglion cells in the rostral anteroventral cochlear nucleus of the waltzing guinea pig following hair cell loss. Brain Res. 1978;158:279–294. doi: 10.1016/0006-8993(78)90675-3. [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Keith Duncan R, Lomax CA, Juiz JM, Altschuler RA. Deafness associated changes in expression of two-pore domain potassium channels in the rat cochlear nucleus. Hear Res. 2006;216-217:146–153. doi: 10.1016/j.heares.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjhan R, Balke CL, Housley GD, Bellingham MC, Noakes PG. Developmental expression of two-pore domain K+ channels, TASK-1 and TREK-1, in the rat cochlea. Neuroreport. 2004a;15:437–441. doi: 10.1097/00001756-200403010-00011. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Anselme AM, Noakes PG, Bellingham MC. Postnatal changes in TASK-1 and TREK-1 expression in rat brain stem and cerebellum. Neuroreport. 2004b;15:1321–1324. doi: 10.1097/01.wnr.0000127462.15985.dc. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K(+) channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Hakuhisa E, Funasaka S. Effects of chronic electrical stimulation on cochlear nuclear neuron size in deaf kittens. Adv Otorhinolaryngol. 1997;52:33–35. doi: 10.1159/000059001. [DOI] [PubMed] [Google Scholar]
- Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SA. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Pietruck C, Yost CS, Sampson ER, Gray AT. Localization of the tandem pore domain K+ channel TASK-1 in the rat central nervous system. Brain Res Mol Brain Res. 2000;80:99–108. doi: 10.1016/s0169-328x(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lesage F, Reyes R, Fink M, Duprat F, Guillemare E, Lazdunski M. Dimerization of TWIK-1 K+ channel subunits via a disulfide bridge. Embo J. 1996;15:6400–6407. [PMC free article] [PubMed] [Google Scholar]
- Lesperance MM, Helfert RH, Altschuler RA. Deafness induced cell size changes in rostral AVCN of the guinea pig. Hear Res. 1995;86:77–81. doi: 10.1016/0378-5955(95)00056-a. [DOI] [PubMed] [Google Scholar]
- Li ZB, Zhang HX, Li LL, Wang XL. Enhanced expressions of arachidonic acid-sensitive tandem-pore domain potassium channels in rat experimental acute cerebral ischemia. Biochem Biophys Res Commun. 2005;327:1163–1169. doi: 10.1016/j.bbrc.2004.12.124. [DOI] [PubMed] [Google Scholar]
- Liu W, Saint DA. Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin Exp Pharmacol Physiol. 2004;31:174–178. doi: 10.1111/j.1440-1681.2004.03964.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lustig LR, Leake PA, Snyder RL, Rebscher SJ. Changes in the cat cochlear nucleus following neonatal deafening and chronic intracochlear electrical stimulation. Hear Res. 1994;74:29–37. doi: 10.1016/0378-5955(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK, Karagulle T, Molowny RL. The central nucleus of the inferior colliculus in rat: a Golgi and computer reconstruction study of neuronal and laminar structure. J Comp Neurol. 1993;333:1–27. doi: 10.1002/cne.903330102. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci U S A. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR. Anatomical and physiological basis for neural plasticity, Neural Plasticity and Disorders of the Nervous System. New York: Cambridge University Press; 2005. pp. 1–38. [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Nicolas MT, Barhanin J, Reyes R, Dememes D. Cellular localization of TWIK-1, a two-pore-domain potassium channel in the rodent inner ear. Hear Res. 2003;181:20–26. doi: 10.1016/s0378-5955(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Niparko JK, Finger PA. Cochlear nucleus cell size changes in the dalmatian: model of congenital deafness. Otolaryngol Head Neck Surg. 1997;117:229–235. doi: 10.1016/s0194-5998(97)70179-7. [DOI] [PubMed] [Google Scholar]
- Niparko JK. Activity influences on neuronal connectivity within the auditory pathway. Laryngoscope. 1999;109:1721–1730. doi: 10.1097/00005537-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Orias M, Velazquez H, Tung F, Lee G, Desir GV. Cloning and localization of a double-pore K channel, KCNK1: exclusive expression in distal nephron segments. Am J Physiol. 1997;273:F663–666. doi: 10.1152/ajprenal.1997.273.4.F663. [DOI] [PubMed] [Google Scholar]
- Osen KK. Projection of the cochlear nuclei on the inferior colliculus in the cat. J Comp Neurol. 1972;144:355–372. doi: 10.1002/cne.901440307. [DOI] [PubMed] [Google Scholar]
- Pal B, Por A, Pocsai K, Szucs G, Rusznak Z. Voltage-gated and background K+ channel subunits expressed by the bushy cells of the rat cochlear nucleus. Hear Res. 2005;199:57–70. doi: 10.1016/j.heares.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2p domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Sivaramakrishnan S, Oliver DL. Identification of cell types in brain slices of the inferior colliculus. Neuroscience. 2000;101:403–416. doi: 10.1016/s0306-4522(00)00382-1. [DOI] [PubMed] [Google Scholar]
- Plant LD, Rajan S, Goldstein SA. K2p channels and their protein partners. Curr Opin Neurobiol. 2005;15:326–333. doi: 10.1016/j.conb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Muller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Rusznak Z, Pocsai K, Kovacs I, Por A, Pal B, Biro T, Szucs G. Differential distribution of TASK-1, TASK-2 and TASK-3 immunoreactivities in the rat and human cerebellum. Cell Mol Life Sci. 2004;61:1532–1542. doi: 10.1007/s00018-004-4082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cat by cochlear implants. Science. 2005;310:1490–2. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Sie KC, Rubel EW. Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. J Comp Neurol. 1992;320:501–508. doi: 10.1002/cne.903200407. [DOI] [PubMed] [Google Scholar]
- Skatchkov SN, Eaton MJ, Shuba YM, Kucheryavykh YV, Derst C, Veh RW, Wurm A, Iandiev I, Pannicke T, Bringmann A, Reichenbach A. Tandem-pore domain potassium channels are functionally expressed in retinal (Muller) glial cells. Glia. 2006;53:266–276. doi: 10.1002/glia.20280. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N. Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hear Res. 2000;139:59–68. doi: 10.1016/s0378-5955(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Juiz JM, Moore DR, Sanes DH. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. Eur J Neurosci. 2004;20:2133–2140. doi: 10.1111/j.1460-9568.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–2428. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- Willott JF, Bross LS, McFadden SL. Morphology of the inferior colliculus in C57BL/6J and CBA/J mice across the life span. Neurobiol Aging. 1994;15:175–183. doi: 10.1016/0197-4580(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Xu X, Pan Y, Wang X. Alterations in the expression of lipid and mechano-gated two-pore domain potassium channel genes in rat brain following chronic cerebral ischemia. Brain Res Mol Brain Res. 2004;120:205–209. doi: 10.1016/j.molbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Yeom M, Shim I, Lee HJ, Hahm DH. Proteomic analysis of nicotine-associated protein expression in the striatum of repeated nicotine-treated rats. Biochem Biophys Res Commun. 2005;326:321–328. doi: 10.1016/j.bbrc.2004.11.034. [DOI] [PubMed] [Google Scholar]


