Abstract
Mutation of the reeler gene (Reln) disrupts neuronal migration in several brain regions and gives rise to functional deficits such as ataxic gait and trembling in the reeler mutant mouse. Thus, the Reln product, reelin, is thought to control cell–cell interactions critical for cell positioning in the brain. Although an abundance of reelin transcript is found in the embryonic spinal cord [Ikeda, Y. & Terashima, T. (1997) Dev. Dyn. 210, 157–172; Schiffmann, S. N., Bernier, B. & Goffinet, A. M. (1997) Eur. J. Neurosci. 9, 1055–1071], it is generally thought that neuronal migration in the spinal cord is not affected by reelin. Here, however, we show that migration of sympathetic preganglionic neurons in the spinal cord is affected by reelin. This study thus indicates that reelin affects neuronal migration outside of the brain. Moreover, the relationship between reelin and migrating preganglionic neurons suggests that reelin acts as a barrier to neuronal migration.
The autosomal recessive mouse mutant reeler, which exhibits ataxia of gait, dystonic posture, and tremor (1), has provided a genetic model for neural development for half a century (2–4). Characteristic of the reeler mutant are abnormal lamination of the cerebral, cerebellar, and hippocampal cortices and neuronal ectopia in several brainstem nuclei (5–13). The gene Reln, the mutation of which is responsible for the reeler phenotype, has recently been cloned (14). Its protein product, reelin, has been identified as an extracellular matrix molecule (15). Despite such progress, the role of reelin in neuronal migration remains unknown. The migration of sympathetic preganglionic neurons reported here provides a simple model system that could facilitate studies of reelin function.
Sympathetic preganglionic neurons undergo extensive migration during the development of the spinal cord. In the rat (16–18), it has been shown that postmitotic preganglionic neurons first migrate from the neuroepithelium along radial glial fibers to the ventrolateral spinal cord. There, along with somatic motor neurons, they form a primitive motor column. Preganglionic neurons next segregate from the somatic motor neurons and undergo a secondary dorsolateral migration toward the intermediolateral column (IML) region. This secondary migration is perpendicular to radial fibers and is independent of radial glial fibers. Upon terminal migration, the majority of preganglionic neurons become localized to the IML. A small number of preganglionic neurons settle in areas adjacent to the central canal.
Results from our present study show that the migration of preganglionic neurons in the reeler mutant is disrupted. Moreover, reelin expression and in vitro function blocking studies suggest that reelin acts as a barrier to migrating preganglionic neurons.
Materials and Methods
Animals.
The reeler mouse colony was originally derived from heterozygous B6C3Fe-a/a-rl adults (The Jackson Laboratory). Homozygous and heterozygous mice were obtained by mating homozygous males with heterozygous females. The day on which a vaginal plug was detected was designated as embryonic day 0.5 (E0.5). Embryonic staging was verified by using the criteria of Rugh (19). Embryos were genotyped by using PCR (20).
Sympathetic Nervous System of the Mouse.
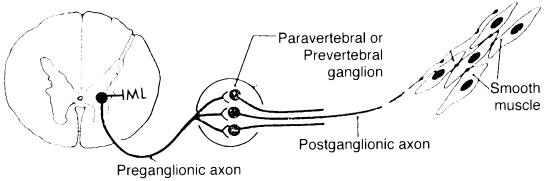
The anatomy of the sympathetic nervous system is shown in Fig. 1. Sympathetic preganglionic neurons are located primarily in the IML region of the thoracic spinal cord. Their axons exit the spinal cord in the ventral roots to enter into the paravertebral ganglia. Most preganglionic axons terminate in the paravertebral ganglia. Some axons pass through the paravertebral ganglia to innervate the prevertebral ganglia. Postganglionic neurons innervate smooth muscle, cardiac muscle, and glands.
Figure 1.
Anatomy of the mouse sympathetic nervous system.
Identification of Preganglionic Neurons in the Mouse Embryo.
Preganglionic neurons in embryos were retrogradely labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) or fluorescent dextran amines (Molecular Probes). For dextran amine labeling, a small amount (30% in 0.1% Triton X-100 in water) was injected into the sympathetic ganglia. The preparation was maintained in an oxygenated culture at 37°C for 4 h to allow for the transport of the dextran amines. Tissue was then fixed in 4% paraformaldehyde for 30 min at 4°C and equilibrated in 30% phosphate-buffered sucrose. Twenty-micrometer-thick serial sections were cut in the transverse plane with a cryostat. For DiI labeling, tissue was fixed in 4% paraformaldehyde, and a few crystals of DiI were embedded in the sympathetic ganglia. In some embryos, 3,3′-dioctadecyloxacarbocyanine (DiO) crystals were applied to spinal nerves to retrogradely label somatic motor neurons. Dye-injected embryos were maintained at 37°C for 2 days to allow for diffusion of the dye. Thereafter, they were serially sectioned in the transverse plane with a Vibratome at a thickness of 100 μm.
Identification of Preganglionic Neurons in Postnatal Mice.
Preganglionic neurons in 1 month postnatal mice were identified by i.p. injection of 10 μl of 2% Fluorogold in sterile saline (Fluorochrome, Denver). Animals were killed 1 week after and were perfused through the heart with 4% paraformaldehyde. The spinal cord was then removed, postfixed for 1 h in 4% paraformaldehyde at 4°C, and equilibrated in 30% phosphate-buffered sucrose. The thoracic spinal cord was serially sectioned at 20 μm in the transverse plane with a cryostat. This method has previously been shown to label all preganglionic neurons (21).
Embryo Slices Cultured with Reelin Function-Blocking Antibody.
Spinal cord slices 400–600 μm thick were cut from the T1–T2 spinal levels of E11.5 embryos and embedded in 2% low-melting-point agarose (SeaPrep; FMC) in defined medium (MEM/progesterone 20 nM/insulin 40 μg/ml/putrescine 1 μg/ml/sodium selenite 20 nM/transferrin 40 μg/ml; all materials from GIBCO) supplemented with nerve growth factor (15 ng/ml; Sigma) (22). The agarose preparation containing the embedded tissue slices was allowed to gel at 4°C for 6 min. Blocks containing individual tissue slices were cultured in the same medium saturated with 95% O2/5% CO2 at 37°C. Experimental slices were cultured in the presence of CR-50 (a monoclonal antibody against reelin), at a concentration of 0.5–2.0 mg/ml, similar to that used by Miyata et al. (23). After 2 days in culture, preganglionic neurons in the experimental and control cultures were retrogradely labeled with dextran amine as described above.
Immunostaining.
For immunostaining, embryos were fixed in periodate-lysine-paraformaldehyde (24) or 4% paraformaldehyde for 1 h at 4°C. The embryos were cryoprotected with 30% phosphate-buffered sucrose solution and sectioned at 20 μm in the transverse plane with a cryostat. Sections were washed three times in 0.01% Triton X-100 in PBS for 10 min each and blocked with 0.5% horse serum in 0.01% Triton X-100 in PBS for 1–2 h at room temperature. Sections were then incubated at 4°C overnight with primary antibodies [CR-50, 1:200; nitric oxide synthase antibody, 1:100 (Santa Cruz Biotechnology)] and washed three times in 0.01% Triton X-100 in PBS for 10 min each. Thereafter, sections were incubated with appropriate secondary antibodies for 2 h at room temperature, washed three times in 0.01% Triton X-100 in PBS for 10 min each, and mounted in glycerol-based mounting medium.
Results
Migratory Pathways of Sympathetic Preganglionic Neurons in Wild-Type and Reeler Mutant Mice.
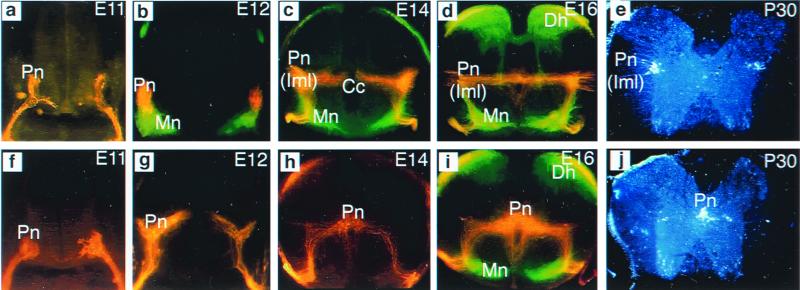
As a first step toward understanding the role of reelin in the migration of preganglionic neurons, the migratory pathways of preganglionic neurons in upper thoracic levels of wild-type and reeler mutant embryos were examined. Retrograde labeling of preganglionic neurons at progressive stages of development revealed that, in wild-type embryos, preganglionic neurons first migrate from the neuroepithelium to the ventrolateral spinal cord, where they become colocalized with somatic motor neurons, forming a single, primitive motor column (Fig. 2a). Next, they segregate from the somatic motor neurons and undergo a secondary dorsolateral migration toward the IML region, where the majority of these neurons remain (Fig. 2b). A small number of preganglionic neurons become oriented in a mediolateral direction and settle in areas adjacent to the central canal (Fig. 2 c and d). Counts of Fluorogold-labeled preganglionic neurons in the thoracic spinal cord (T1–T12) of two P30 wild-type mice (Fig. 2e) showed that on average 87% of the preganglionic neurons are located in the IML, 6% adjacent to the central canal, and 7% between the IML and the central canal region. The migratory pathway of sympathetic preganglionic neurons in the mouse is, therefore, similar to that of the rat. In the reeler mutant, the initial migration of preganglionic neurons from the neuroepithelium to the ventrolateral spinal cord is the same as in the wild-type embryos (Fig. 2f). However, as development progresses, preganglionic neurons in the reeler mutant are found migrating back toward the central canal instead of toward their normal destination in the IML (Fig. 2g). The majority of the preganglionic neurons settle in areas adjacent to the central canal; only a few are found in the IML (Fig. 2 h and i). The ectopic location of preganglionic neurons in the reeler mutant persists after birth (Fig. 2j). Cell counts of two P30 reeler mutants showed that 89% of preganglionic neurons cluster around the central canal, 8% in the IML, and 3% between the IML and the central canal region. Thus, in the absence of reelin, preganglionic neurons do migrate, albeit inappropriately.
Figure 2.
Migratory pathways of sympathetic preganglionic neurons in upper thoracic spinal cord of wild-type and reeler mutant. Preganglionic neurons in embryos were retrogradely labeled with DiI crystals applied to the sympathetic ganglia; somatic motor neurons were retrogradely labeled with DiO crystals applied to the spinal nerves (a–d and f–i). Preganglionic neurons in postnatal mice were labeled by i.p. injection of Fluorogold (e and j). (a) E11 wild type. Transverse section shows that preganglionic neurons (Pn) have undergone their primary migration from the neuroepithelium to the ventrolateral spinal cord. (b) E12 wild type. Many preganglionic neurons (red) have migrated dorsally to separate from the somatic motor neurons (Mn, green). (c and d) E14 (c) and E16 (d) wild type. The majority of preganglionic neurons have completed their dorsal migration to arrive at their final location in the IML (Iml); a small number of neurons become localized to areas between the IML and the central canal (Cc). Note that in these micrographs, DiI labeling is found spanning the middle of the spinal cord as well as around the central canal. However, microscopic examination at higher magnification revealed that this is mostly fiber labeling. (e) Fluorogold labeling in a P30 mouse shows that the majority of sympathetic neurons are indeed located in the IML. (f) E11 reeler. Preganglionic neurons migrated, as in the wild-type mouse, to the ventrolateral spinal cord. (g) E12 reeler. Many preganglionic neurons stream toward the central canal, instead of migrating toward the IML as they normally do. The secondary migration of preganglionic neurons in the reeler mutant is, therefore, abnormal. (h and i) E14 (h) and E16 (i) reeler. The majority of preganglionic neurons have arrived at their final location adjacent to the central canal; a few neurons remain in the IML. Dh, dorsal horn. (j) Fluorogold labeling in a P30 reeler mutant confirms that the majority of preganglionic cell bodies are located adjacent to the central canal.
Embryo Slices Cultured with Reelin Function-Blocking Antibody.
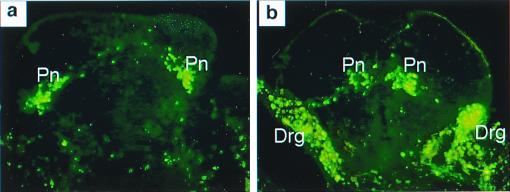
Recent studies on reelin function have shown that CR-50, a monoclonal antibody that recognizes an epitope in the N-terminal region of the reelin molecule (15), disrupts the laminar pattern formation in cortical reaggregation cultures (25), Purkinje cell alignment in cerebellar explant cultures (23), axonal branching pattern in cultures of the entorhinohipppocampal pathway (26), and hippocampal development in vivo (27). These studies indicate that the CR-50 epitope region of reelin is responsible for the reeler phenotype in these brain structures. To determine whether the CR-50 epitope region of reelin is also responsible for the normal migration of sympathetic preganglionic neurons, spinal cord slices were prepared from wild-type embryos before the secondary migration of sympathetic preganglionic neurons and cultured for 2 days with or without CR-50. Results show that in control slices cultured without CR-50, preganglionic neurons migrated to the IML (Fig. 3a). However, in those slice cultures treated with CR-50, the majority of neurons migrated toward the central canal (Fig. 3b), resembling preganglionic neuronal migration in the reeler mutant. Thus, the CR-50 epitope region of reelin is also responsible for the normal migration of sympathetic preganglionic neurons. The finding that the CR-50 epitope region of reelin is responsible for the reeler phenotype in both the brain and the spinal cord suggests that reelin is likely to function in a similar manner throughout the central nervous system. Moreover, results from this isolated spinal cord slice culture preparation show that the source of reelin and reelin-mediated mechanisms that control preganglionic neuronal migration lie solely in the spinal cord.
Figure 3.
Blocking reelin function in slice culture of wild-type spinal cord disrupts preganglionic neuronal migration. E11.5 spinal cord slices were cultured for 2 days with and without reelin function-blocking antibody, CR-50. Preganglionic neurons were retrogradely labeled with FITC dextran amine. (a) Control culture without CR-50 shows that preganglionic neuronal migration to the IML is normal. (b) Embryo slice cultured in the presence of CR-50 shows abnormal migration of preganglionic neurons. As in the reeler mutant, the majority of preganglionic neurons (Pn) are located adjacent to the central canal, instead of at their normal location in the IML. Note that in this slice, the dorsal root ganglia (Drg) were inadvertently labeled because of their proximity to the sympathetic ganglia where dextran amine was injected. In the spinal cord, however, only preganglionic neurons are retrogradely labeled.
Relationship Between Reelin and Migrating Sympathetic Preganglionic Neurons.
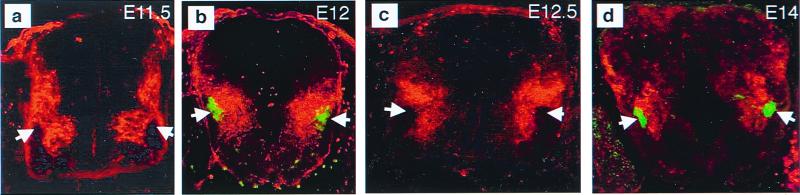
A major step in the pursuit of reelin function is to examine the relationship between reelin and migrating preganglionic neurons in wild-type embryos. Reelin was revealed by CR-50 immunostaining; preganglionic neurons were identified by retrograde dextran amine labeling or by nitric oxide synthase immunostaining (28, 29). In the E11.5 embryo, reelin is found in a triangle between the neuroepithelium and the pial surface of the ventral spinal cord (Fig. 4a). The only region that appears to be devoid of reelin is the ventrolateral spinal cord, where preganglionic and motor neurons are located. At E12.5, reelin is found in more dorsal locations of the ventral spinal cord (Fig. 4c). Again, reelin is absent from the ventrolateral region of the spinal cord, where preganglionic and somatic motor neurons are located. The relationship between reelin and migrating preganglionic neurons is illustrated more clearly by labeling both reelin and preganglionic neurons. Results showed that reelin is found adjacent to migrating preganglionic neurons and separates them from the central canal (Fig. 4 b and d). Moreover, the locations of preganglionic neurons and reelin do not overlap; reelin is absent from areas where preganglionic neurons are present. Results from these studies suggest that migrating preganglionic neurons might avoid areas of reelin.
Figure 4.
Relationship between reelin and migrating preganglionic neurons. (a) E11.5. CR-50 immunostaining shows that reelin is widely distributed in the newly formed ventral spinal cord. The only regions that are devoid of reelin are the neuroepithelium and the ventrolateral spinal cord, where preganglionic and somatic motor neurons are located (arrows). (b) E12. Retrograde dextran amine labeling of preganglionic neurons (green, arrows) combined with immunostaining of reelin (red) shows that reelin is present adjacent to migrating preganglionic neurons in the ventral spinal cord. (c) E12.5. Reelin is located more dorsally in the spinal cord. Again, the area devoid of reelin is where preganglionic neurons are located (arrows). (d) E14. Double immunostaining with nitric oxide synthase immunostaining for preganglionic neurons and CR-50 shows that reelin (red) is located more dorsally but is still adjacent to the preganglionic neurons (green, arrows) and separates them from regions of the central canal.
Discussion
The ectopic location of sympathetic preganglionic neurons reported here is clear evidence of abnormality in the reeler spinal cord. This finding shows that reelin is also important for the normal development of the spinal cord. Although several hypotheses on how reelin might affect the formation of laminar structures in the brain have been proposed (23, 25, 27, 30–32), the exact role of reelin in neuronal migration is still unclear. Our results suggest that reelin may provide an inhibitory signal to neuronal migration. In wild-type embryos, most migrating preganglionic neurons are separated from regions of the central canal by reelin. When reelin is absent from the reeler mutant, the majority of preganglionic neurons migrate toward the central canal across areas of the spinal cord that are normally occupied by reelin. Similarly, when reelin function is blocked by the CR-50 antibody, most preganglionic neurons also migrate toward the central canal, traversing an area that is occupied by CR-50-bound reelin. Taken together, these findings suggest that the majority of preganglionic neurons are prevented from migrating toward the central canal by reelin. Therefore, reelin may act as a stop signal for migrating preganglionic neurons. Note, however, in wild-type mice 13% of preganglionic neurons either cluster around the central canal or are interspersed between the IML and the region of the central canal. Why some preganglionic neurons are able to traverse areas of reelin is unclear. One possible explanation is that there are multiple classes of preganglionic neurons (33–36) and not all of them respond to reelin.
The present study suggests that reelin may act as a stop signal to terminate the migration of sympathetic preganglionic neurons on radial glia fibers. The initial migration of preganglionic neurons from the neuroepithelium to the ventrolateral spinal cord, which is generally thought to be mediated by radial glial fibers (16–18), is the same in wild-type and reeler embryos. During secondary migration in the reeler mutant, migrating preganglionic neurons are oriented in the direction of radial fibers and appear to migrate back toward the central canal along radial fibers. Radial glia fibers may, therefore, be tracks along which preganglionic neurons can migrate in either direction. However, the presence of reelin in wild-type embryos might prevent preganglionic neurons from migrating back toward the central canal. Consistent with this idea are findings that glial-guided neuronal migration is bidirectional in cocultures of dissociated neurons and glia but unidirectional in vivo (37–39). Molecules such as reelin could render glial-guided migration of neurons unidirectional in vivo. That reelin can affect the migration of neurons on radial glial fibers is also suggested by the studies of Pinto-Lord et al. (40), which showed that the extent of contact between the somatic surfaces of postmigratory neurons and the surfaces of radial glia fibers in the reeler is substantially greater than in wild-type mouse.
The function of reelin as an inhibitory or stop signal has also been suggested by studies in the cortex. During corticogenesis in the normal mouse, reelin is produced near the pial surface by Cajal–Retzius cells of the preplate. Cortical plate neurons are generated in the ventricular zone and migrate along radial glia fibers toward the pial surface. Young cortical plate neurons split the preplate and form distinct layers between the outside marginal zone and the inside subplate. It has been proposed that in the absence of reelin, the migrating cortical plate neurons do not stop and form distinct layers. Instead, they migrate past their normal destination, reverse direction, and accumulate beneath the preplate (41).
Over the last 2 years, remarkable progress has been made in the identification of the reelin receptor and other molecules involved in the reelin signaling pathway (32, 42–45). Sympathetic preganglionic neurons offer several unique advantages over neuronal populations in the brain for the analysis of reelin signaling in control of neuronal migration. The accessibility of preganglionic axon terminals at the sympathetic chain ganglia makes it possible to introduce markers or exogenous genes by retrograde uptake into migrating preganglionic neurons. Thus, migrating preganglionic neurons can be identified, and genes that are thought to be involved in the reelin signaling pathway can be manipulated selectively. Furthermore, the migration of preganglionic neurons proceeds normally in slice cultures and can therefore be analyzed in vitro. The sympathetic system provides advantages in elucidating the role of reelin and its signaling pathway in the control of neuronal migration.
Acknowledgments
This paper is dedicated to Dr. Viktor Hamburger on the occasion of his 100th birthday. We thank Willie Halfter for critical comments on the manuscript. This work was supported by National Science Foundation Grant IBN-9982673; the Competitive Medical Research Fund from the University of Pittsburgh Medical Center; and by grants from the Ministry of Education, Science, Sports and Culture of Japan; the Human Frontier Science Program; Precursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Corporation; the Uehara Memorial Foundation; and the NOVARTIS Foundation (Japan) for the Promotion of Science.
Abbreviations
- IML
intermediolateral column
- En
embryonic day n
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
- DiO
3,3′-dioctadecyloxacarbocyanine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.150040497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.150040497
References
- 1.Falconer D S. J Genet. 1951;50:192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- 2.Sidman R L, Rakic P. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 3.Caviness V S, Rakic P. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- 4.Rakic P, Caviness V S. Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 5.Caviness V S, Sidman R L. J Comp Neurol. 1973;148:141–152. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 6.Caviness V S, Sidman R L. J Comp Neurol. 1973;147:235–254. doi: 10.1002/cne.901470206. [DOI] [PubMed] [Google Scholar]
- 7.Caviness V S. Dev Brain Res. 1982;4:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 8.Mariani J, Crepel F, Mikoshiba K, Changeux J-P, Sotelo C. Philos Trans R Soc London B. 1977;281:1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- 9.Stanfield B, Cowan W M. J Comp Neurol. 1979;185:393–422. doi: 10.1002/cne.901850302. [DOI] [PubMed] [Google Scholar]
- 10.Wyss J M, Stanfield B B, Cowan W M. Brain Res. 1980;188:566–571. doi: 10.1016/0006-8993(80)90056-6. [DOI] [PubMed] [Google Scholar]
- 11.Goffinet A M. J Comp Neurol. 1983;219:10–24. doi: 10.1002/cne.902190103. [DOI] [PubMed] [Google Scholar]
- 12.Goffinet A M. J Anat. 1984;138:207–215. [PMC free article] [PubMed] [Google Scholar]
- 13.Martin M R. J Comp Neurol. 1981;197:141–152. doi: 10.1002/cne.901970111. [DOI] [PubMed] [Google Scholar]
- 14.D'Arcangelo G, Miao G G, Chen S C, Morgan J I, Curran T. Nature (London) 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 15.D'Arcangelo G, Nakajima N, Miyata T, Ogowa M, Mikoshiba K, Curran T. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markham J A, Vaughn J E. J Neurobiol. 1991;22:811–822. doi: 10.1002/neu.480220803. [DOI] [PubMed] [Google Scholar]
- 17.Barber R P, Phelps P E, Vaughn J E. J Comp Neurol. 1991;311:509–519. doi: 10.1002/cne.903110406. [DOI] [PubMed] [Google Scholar]
- 18.Phelps P E, Barber R P, Vaughn J E. J Comp Neurol. 1991;307:77–86. doi: 10.1002/cne.903070108. [DOI] [PubMed] [Google Scholar]
- 19.Rugh R. The Mouse: Its Reproduction and Development. Minneapolis: Burgess; 1968. [Google Scholar]
- 20.D'Arcangelo G, Miao G G, Curran T. Mol Brain Res. 1996;39:234–236. doi: 10.1016/0169-328x(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 21.Anderson C R, Edwards S L. J Neurosci Methods. 1994;53:137–141. doi: 10.1016/0165-0270(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 22.Sharma K, Korade Z, Frank E. Development (Cambridge, UK) 1994;120:1315–1323. doi: 10.1242/dev.120.5.1315. [DOI] [PubMed] [Google Scholar]
- 23.Miyata T, Nakajima K, Mikoshiba M, Ogawa M. J Neurosci. 1997;17:3599–3609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean I W, Nakane P K. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 26.Del Rio J A, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, et al. Nature (London) 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima K, Mikoshiba K, Miyata T, Kuo C, Ogawa M. Proc Natl Acad Sci USA. 1997;94:8196–8201. doi: 10.1073/pnas.94.15.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blottner D, Baumgarten H G. J Comp Neurol. 1992;316:45–55. doi: 10.1002/cne.903160105. [DOI] [PubMed] [Google Scholar]
- 29.Bruning G, Mayer B. Neurosci Lett. 1996;202:189–192. doi: 10.1016/0304-3940(95)12239-7. [DOI] [PubMed] [Google Scholar]
- 30.Lambert de Rouvoit C, Goffinet A M. Adv Anat Embryol Cell Biol. 1998;150:1–106. [PubMed] [Google Scholar]
- 31.Pearlman A L, Faust P L, Hatten M E, Brunstrom J E. Curr Opin Neurobiol. 1998;8:45–54. doi: 10.1016/s0959-4388(98)80007-x. [DOI] [PubMed] [Google Scholar]
- 32.Rice D S, Curran T. Genes Dev. 1999;13:2758–2773. doi: 10.1101/gad.13.21.2758. [DOI] [PubMed] [Google Scholar]
- 33.Anderson C R, McLachlan E M, Srb-Christie O. J Comp Neurol. 1989;283:269–284. doi: 10.1002/cne.902830208. [DOI] [PubMed] [Google Scholar]
- 34.Janig W, McLachlan E M. Trends Neurosci. 1992;15:475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- 35.Morris J L, Gibbins I L. In: Autonomic Neuroeffector Mechanisms. Burnstock G, Hoyle C H V, editors. Vol. 1. Reading, U.K.: Harwood Academic; 1992. pp. 33–120. [Google Scholar]
- 36.Pyner S, Coote J H. J Comp Neurol. 1994;342:15–22. doi: 10.1002/cne.903420103. [DOI] [PubMed] [Google Scholar]
- 37.Edmondson J C, Hatten M E. J Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatten M E. Trends Neurosci. 1990;13:179–187. doi: 10.1016/0166-2236(90)90044-b. [DOI] [PubMed] [Google Scholar]
- 39.Hatten M E, Mason C A. Experientia. 1990;46:907–916. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- 40.Pinto-Lord M C, Evrard P, Caviness V S. Dev Brain Res. 1982;4:379–393. doi: 10.1016/0165-3806(82)90181-x. [DOI] [PubMed] [Google Scholar]
- 41.Sheppard A M, Pearlman A L. J Comp Neurol. 1997;378:173–179. doi: 10.1002/(sici)1096-9861(19970210)378:2<173::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer R E, Richardson J A, Herz J. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 43.D'Arcangelo G, Homayouni R, Keshvara L, Rice D S, Sheldon M, Curran T. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 44.Hiesberger T, Trommsdorff M, Howell B W, Goffinet A, Mumby M C, Cooper J A, Herz J. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 45.Senzaki K, Ogawa M, Yagi T. Cell. 1999;99:635–647. doi: 10.1016/s0092-8674(00)81552-4. [DOI] [PubMed] [Google Scholar]