Abstract
Objectives
The objectives of this phase II trial were to assess the activity and tolerability of the combination of bevacizumab and erlotinib in patients with recurrent ovarian, primary peritoneal or fallopian tube cancer.
Methods
This was a single arm, multicenter phase II trial with overall objective response as the primary endpoint. Eligible patients had two or fewer prior chemotherapy regimens for recurrent or refractory disease and no prior anti-VEGF or anti- EGFR agents. Bevacizumab, 15 mg/kg, was administered intravenously every 21 days and erlotinib, 150 mg orally, was given daily.
Results
Between July and October 2005, 13 patients were enrolled. There were two major objective responses, one complete response of 16+ months duration and one partial response of 11 months duration, for a response rate of 15% (95% CI 1.9% to 45.4%). Seven patients had a best response of stable disease. The most common grade 3 or 4 toxicities included anemia (n=1), nausea (n=2), vomiting (n=1), hypertension (n=1), and diarrhea (n=2). One patient with an ileostomy was removed from the study secondary to grade 3 diarrhea. Two patients had fatal gastrointestinal perforations.
Conclusion
There was no strong suggestion that this combination was superior to single agent bevacizumab, and the rate of gastrointestinal perforation was of concern. The study was therefore stopped. Identification of risk factors for gastrointestinal perforation will be of importance for the use of bevacizumab in the treatment of ovarian cancer.
Introduction
Vascular endothelial growth factor (VEGF) has been implicated in the pathogenesis of ovarian cancer[1–3]. VEGF expression has been correlated with tumor progression, advanced stage, ascites, shortened disease-free survival and poor overall survival in advanced ovarian cancer[4–7]. Bevacizumab is a humanized recombinant antibody that prevents VEGF receptor binding and inhibits angiogenesis and tumor growth. Prospective phase II trials have already established the activity of bevacizumab in recurrent ovarian cancer with single agent response rates in the range of 16%–21% [8,9].
The human epidermal growth factor receptor (EGFR) is expressed in 35% –70% of advanced epithelial ovarian carcinomas [10,11]. High tumor EGFR expression has been correlated with advanced stage and poor survival in ovarian cancer[12–14]. Erlotinib HCI (Tarceva; Genentech, Inc, South San Francisco, CA) is an orally available, EGFR tyrosine kinase inhibitor that is FDA approved for the treatment of non-small cell lung cancer. Gordon et al evaluated erlotinib monotherapy at 150 mg per day in 34 patients with recurrent, refractory EGFR-positive ovarian cancer. Two patients had a partial response, giving an overall objective response rate of 6%. The one-year survival rate was 35.3%[15].
EGFR activation has been suggested to promote VEGF secretion [16]. Combining an anti-VEGF and an anti-EGFR therapy may provide a synergistic anti-cancer therapy with the potential to overcome resistance and improve clinical outcomes. Phase I and II studies of bevacizumab and erlotininb showed no pharmacokinetic interaction and full doses of both agents have been administered to patients with nonsquamous stage IIIB/IV non-small cell lung and renal cell carcinoma [17] [18].
This multi–center study investigated the clinical activity and safety of bevacizumab and erlotinib in patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer.
Methods
Eligibility Criteria
The clinical trial was reviewed and approved by the Institutional Review Board (IRB) at the University of Chicago Cancer Center and the IRBs of all participating institutions. All patients provided written informed consent before study participation according to institutional and federal guidelines. Eligible patients were at least 18 years old and had measurable, recurrent or progressive epithelial ovarian, primary peritoneal or fallopian tube carcinoma. Patients were also required to have: ECOG performance status of 0 to 2, absolute neutrophil count of ≥1,500/μL, platelet count of ≥100,000/μL, serum bilirubin level less than or equal to the institutional upper limits of normal (ULN), AST/ALT ≤2.5 times the ULN in patients without liver metastases and ≤5.0 times the ULN in patients with liver metastases, serum creatinine ≤1.5 mg/dL, urine protein < 1+ or 24 hour urine protein < 1000 mg. Patients must have received platinum-based chemotherapy for primary disease, and patients with a platinum-free interval of more than 12 months from primary therapy were required to have been retreated with a platinum-containing regimen. No more than two prior cytotoxic chemotherapies were allowed in the setting of recurrent disease. Patients were excluded if they had prior treatment with VEGF or EGFR directed therapy, evidence of brain metastases, a stroke, arterial thromboembolic event or myocardial infarction within the past 6 months, a major surgical procedure within 28 days prior to day 1 of therapy, uncontrolled hypertension, or increased risk of bleeding. A history of bowel obstruction or fistula was not an exclusion criterion; however, patients with gastrointestinal tract disease resulting in an inability to take oral medication or prior surgical procedures affecting absorption were not eligible.
Treatment and Monitoring
Radiologic assessment of measurable disease was performed by computed tomography scan (CT) or magnetic resonance imaging (MRI) within 28 days prior to registration. Baseline laboratory testing included CBC with differential and platelets, creatinine or calculated creatinine clearance, AST/ALT, bilirubin and CA-125. All patients received bevacizumab, 15 mg/kg in 100 mL normal saline on day 1 of each 21-day treatment cycle. The first dose was administered over 90 minutes. If this was well tolerated, the second dose was administered over 60 minutes and then subsequent doses over 30 minutes. Erlotinib, 150 mg orally per day, was administered continuously, and compliance was monitored with a patient diary. No dose reductions were indicated for bevacizumab toxicity; for most bevacizumab toxicities, including grade 3 thrombosis or hemorrhage, treatment was to be held and then restarted if/when patient met clinical parameters. Erlotinib was to be held for grade 3 rash or diarrhea until resolution to grade 1 or better, and then restarted at a dose of 100 mg per day. Bevacizumab and erlotinib were supplied by the National Cancer Institute/Division of Cancer Treatment and Diagnosis. CBC/differential, serum chemistries, and urine protein, were repeated every three weeks. Patients were evaluated for response every three cycles (nine weeks). Treatment was continued until unacceptable toxicity or progression of disease. Response was defined using the international criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST) Committee[19].
Correlative Studies
EGFR staining of archival paraffin-embedded, formalin fixed tissue (Zymed Clone 31G7 Cat no: 28-0005) [20]was performed according to previously published methods.[21] Genomic DNA was also isolated from archival formalin fixed, paraffin–embedded tumor tissues. Samples were digested overnight with Proteinase K (Qiagen: Cat no: 19133). EGFR exons 19 and 21 were amplified by PCR using primary and secondary PCR primer pairs. PCR fragments were purified and submitted for DNA sequencing against forward and reverse primers. Analysis of DNA sequence was done using Sequencherv4.2 (Gene Codes Corp., Ann Arbor, MI) as previously published. [21]
Blood and urine were collected prior to treatment and approximately nine weeks after treatment initiation. Plasma VEGF, serum VEGFR2, and urine VEGF were analyzed using ELISA (Quantikine Human Vascular Endothelial Growth Factor Immunoassay; R&D Systems, Minneapolis).
Statistical Methods
The primary end point of this phase II study was objective response rate (complete and partial responses). There were no formal stopping rules for observed toxicity. A Simon optimal two-stage design was employed. A ≤10% response rate was to preclude further study (null hypothesis), whereas a ≥30% response rate would suggest that further study would be warranted (alternative hypothesis). Using α and β errors of 0.10 and 0.10, respectively, twelve assessable patients were to be enrolled in the first stage and if ≤1 response was observed, the trial was to be terminated. Otherwise, an additional 23 patients were to be enrolled, for a total of 35 patients, and if ≥6 responses were observed the treatment would be considered sufficiently active to warrant further testing. This design had a 0.65 probability of stopping at the first stage if the true response rate was 10%. An exact 95% confidence interval (CI) was calculated for the response rate based on the binomial distribution. Overall and progression-free survival rates were estimated using the Kaplan-Meier method. Median progression-free and overall survival times and their respective 95% CIs were constructed using the method of Brookmeyer and Crowley[22]. For the analysis of the correlative data, a Wilcoxon rank-sum test was used to compare baseline levels and changes from baseline to cycle 3 between responders and non-responders. Cox proportional hazards regression models were employed to examine the association between each correlate and survival.
Results
Between July and October 2005, thirteen patients were accrued to the study. The thirteenth patient was enrolled because she signed consent before her physician was notified that the accrual for the first stage was complete. Clinical characteristics of the cohort are shown in Table 1. A median of six cycles were administered to each patient (range 2 to 22 cycles).
Table 1.
Patient Characteristics
| No. | |
|---|---|
| Patients Enrolled | 13 |
| Age (years) | |
| Median | 56 |
| Range | 45–70 |
| Primary Site | |
| Ovarian | 11 |
| Fallopian Tube | 2 |
| Histology | |
| Serous | 9 |
| Clear cell | 2 |
| Endometrioid | 1 |
| Adenocarcinoma | 1 |
| ECOG PS | |
| 0 | 6 |
| 1 | 4 |
| 2 | 3 |
| Primary Platinum Response | |
| 10 Refractory* | 4 |
| 10 Resistant (<6 mo) | 2 |
| 10 Sensitive (≥6 mo) | 7 |
| # Prior Chemo Regimens | |
| 1 | 1 |
| 2 | 8 |
| 3 | 4 |
Includes any patient who failed to obtain a complete response or progressed during primary therapy
Adverse Events
Toxicity data are shown in Table 2. The most common non-hematological toxicities were skin rash (85%), diarrhea (77%), stomatitis (31%), elevated bilirubin (23%), proteinuria (31%), headache (15%), and epistaxis (31%). The most common grade 3 and higher adverse events were anemia (8%), nausea (15%), vomiting (8%), hypertension (8%), diarrhea (15%), and bowel perforation (15%).
Table 2.
Worst-grade toxicities for any cycle with bevacizumab and erlotinib (N=13)
| Grade | 1/2 | 3/4/5 |
|---|---|---|
| Hematologic | ||
| Anemia | 4 (31%) | 1 (8%) |
| Lymphopenia | 7 (54%) | 0 (0%) |
| Neutropenia | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 2 (15%) | 0 (0%) |
| Non-hematologic | ||
| Fatigue | 8 (62%) | 0 (0%) |
| Hyperglycemia | 5 (38%) | 0 (0%) |
| Rash | 11 (85%) | 0 (0%) |
| Proteinuria | 4 (31%) | 0 (0%) |
| Bowel Perforation | 0 (0%) | 2 * (15%) |
| Headache | 2 (15%) | 0 (0%) |
| Epistaxis | 4 (31%) | 0 (0%) |
| Hypertension | 0 (0%) | 1 (8%) |
| Gastrointestinal | ||
| Nausea | 4 (31%) | 2 (15%) |
| Vomiting | 4 (31%) | 1 (8%) |
| Constipation | 2 (15%) | 1 (8%) |
| Diarrhea | 8 (62%) | 2 (15%) |
| Stomatitis | 4 (31%) | 0 (0%) |
| Elev Alk phos | 5 (38%) | 0 (0%) |
| Elev SGPT | 4 (31%) | 0 (0%) |
| Elev SGOT | 3 (23%) | 0 (0%) |
| Elev Bilirubin | 3 (23%) | 0 (0%) |
The only grade 5 (fatal) toxicities were the two bowel perforations
One patient with an ileostomy from a second debulking surgery with partial colon resection experienced grade 3 diarrhea and dehydration that required hospitalization. The patient was removed from the study for toxicity although her CT scan showed stable disease after 3 cycles of treatment (her CA-125 dropped from 214 pre-treatment to 122 after cycle #1 and 134 after cycle #2). A second patient developed grade 3 diarrhea, grade 2 rash, nausea, and vomiting within two weeks of starting her first treatment cycle. She experienced a treatment delay of eight days and her erlotinib dose was reduced to 100 mg per day, which was well tolerated. A CT scan showed stable disease after 3 cycles of treatment.
There were two patients with gastrointestinal perforations. Both were fatal. The first patient’s most recent abdominal surgery was her initial surgery for ovarian cancer, including a total abdominal hysterectomy and bilateral salpingoophorectomy (TAH/BSO) which was about one year prior to registration on study. She had two subsequent chemotherapy regimens prior to starting on study. Her baseline CT scan showed moderate ascites with omental caking and a small bowel implant causing focal narrowing, but no definite evidence of small bowel obstruction. She was admitted after cycle two (one month after starting therapy) with a small bowel obstruction (SBO) that responded to conservative management. Her CA-125 had decreased by 20% after the first cycle. One week after cycle 3 (about two months after starting therapy) she was rehospitalized with nausea, vomiting, and abdominal pain. A CT was consistent with persistent SBO and suggested bowel perforation with loculated free air within the ascitic fluid. There was progression of her liver lesions. She was treated with palliative measures only, at her request.
The second patient had no abdominal surgery since her TAH/BSO/debulking about two and a half years prior to registration on study. She had two prior chemotherapy regimens. Her baseline CT showed mesenteric nodules consistent with peritoneal carcinomatosis and bowel closely adherent to the vaginal cuff with a possible small vaginal fistula. Clinical symptoms of fistula were not reported. She was taken off treatment for disease progression after cycle three (despite a drop in CA-125 from 104 to 27 after two cycles). Two weeks later (42 days from the last dose of bevacizumab and about three months after starting on study), she was hospitalized and diagnosed with a small bowel obstruction. A CT scan revealed probable distal small bowel ischemia and pneumoperitoneum consistent with bowel perforation. She declined surgery due to the high risk and limited further treatment options.
Response and Survival
There was one confirmed complete response of 16+ months duration among the first twelve patients. This patient had fallopian tube cancer, one prior regimen, about a 6 month disease-free interval since completing primary therapy, a baseline CA-125 of 1171 and fairly low-volume disease (1.9 cm peritoneal mass and a 2.8 cm peri-rectal fluid collection). After 16 months of therapy she elected to come off treatment; four months later her CA-125 rose above normal, and her CT showed evidence of recurrent disease. Patient number thirteen (papillary serous carcinoma of the ovary, three prior regimens, potentially platinum sensitive disease) had a confirmed partial response of eleven months duration. Thus, there was an objective response rate of 15% (95% CI, 1.9% to 45.4%) among all thirteen patients and a response rate of 8% (95% CI, 0.2% to 38.5%) for the pre-defined first stage (i.e one of twelve patients). Seven patients (54%) had stable disease.
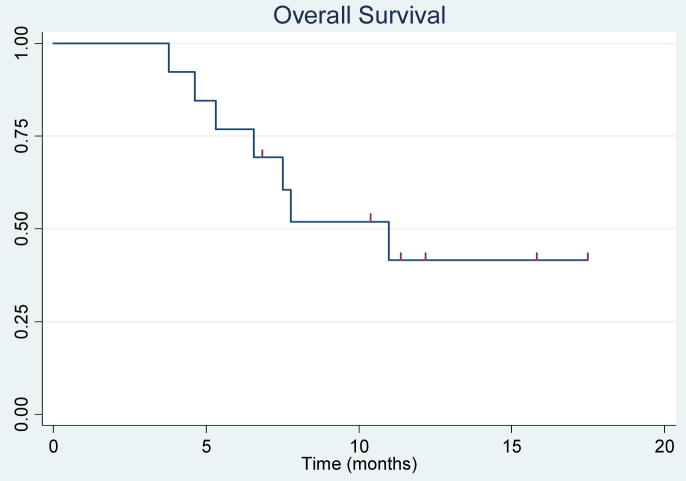
For the survival analyses, all thirteen patients were included. Seven patients have died. Median follow-up was 11.7 months (range 6.8 to 17.4 months) for survivors. The estimated median progression-free survival was 4.1 months (95% CI, 2.1 to 6.6), and median overall survival was 11.0 months (95% CI, 5.3 to not yet reached). The probability of being progression free at six months was 0.38 (95% CI, 0.14 to 0.63). The 1-year survival probability was 0.42 (95% CI, 0.14 to 0.67). A plot of CA-125 levels is shown in Figure 1. The Kaplan-Meier curves are in Figures 2 and 3.
Figure 1.
Percent change in CA-125 after two cycles of treatment
Figure 2.
Kaplan –Meier estimation of overall survival
Figure 3.
Kaplan-Meier estimation of progression- free survival.
EGFR Expression and Mutation
Genomic DNA was successfully extracted from six (1 CR, 1 PR, 3 SD, 1 PD) tumor tissue specimens to evaluate for EGFR gene somatic mutations. No EGFR mutations were found in exon 19 or exon 21 in any of the samples.
Immunohistochemical analysis revealed only one patient with EGFR positive (2+) expression (a clear cell carcinoma) out of nine specimens available for testing. The patient received three cycles of treatment prior to removal from the study for progression of disease.
Plasma VEGF, Serum VEGFR2, and Urine VEGF
Plasma VEGF levels, serum VEGFR2 levels, and urine VEGF levels were evaluated (Table 3). The value of the analyses is limited as the number of patients available for analysis was small, and no samples were available on the patient with a partial response; only serial samples for urine VEGF were available on the patient with a complete response. Thus, these analyses should be considered exploratory. The median baseline plasma VEGF level was 80.5 pg/ml (range, 16–198 pg/ml). For analysis, we combined CR+PR+SD (responders) versus PD (non-responders). There were no significant differences in baseline plasma VEGF levels (p=0.75), urine VEGF (p=0.34), or VEGFR2 (p=1.00) between responders and non-responders. There were no significant relationships between overall survival and baseline plasma VEGF (p= 0.84), urine VEGF (p=0.068), or serum VEGFR2 levels (p=0.45). There was a similar lack of association found between PFS and baseline plasma VEGF (p= 0.28), urine VEGF (p=0.34), and serum VEGFR2 levels (p=0.63).
Table 3.
Median, min/max VEGF levels, and number (n) of patients.
| Responders (CR+PR+SD) | Non-responders (PD) | p-value | |
|---|---|---|---|
| Baseline plasma VEGF | 105 16/198 (n=6) |
74 52/147 (n=4) |
0.75 |
| Baseline urine VEGF | 68 8/814 (n=7) |
227 29/355 (n=4) |
0.34 |
| Urine VEGF change | −10 −66/133 (n=5) |
19 −151/68 (n=3) |
0.65 |
| Baseline VEGFR2 | 15990 6515/19860 (n=5) |
15770 14998/18320 (n=4) |
1.00 |
| VEGFR2 change | 2648 129/5393 (n=4) |
280 −3317/1874 (n=3) |
0.16 |
Discussion
Our trial closed after the first stage of accrual and the criteria for proceeding to the second stage were not met. As the thirteenth patient entered on the trial had responded, consideration was given to amending the trial to allow continuation with the observed level of response. However, the two deaths from bowel perforation were of concern, and although they appeared clinically related to the development of small bowel obstruction, and numerous eligibility criteria revisions were discussed, we were not certain which would actually decrease the risk. The numbers were too small for any firm conclusions about the activity of the regimen. However, there was no strong signal that the combination of erlotinib plus bevacizumab was superior to single agent bevacizumab (see Table 4), and bevacizumab was (and is) being studied in the front-line treatment of ovarian cancer, which seemed likely to be a safer setting. We therefore elected to allow the trial to close as written.
Table 4.
Selected Phase II Trials of Bevacizumab in Ovarian Cancer
| Author (year) | n | #Prior Chemotherapy Regimens* | Regimen | RR | Median PFS | Median OS | Bowel Perforation and Fistula |
|---|---|---|---|---|---|---|---|
| Burger (2007) | 62 | 1–2 | Bevacizumab 15 mg/kg q 3 wk | 21% | 4.7 mos | 17 mos | 0 |
| Cannistra (2007) | 44 | 2–3 | Bevacizumab 15 mg/kg q 3 wk | 16% | 4.4 mos | 11 mos | n=5 (11%) |
| Garcia (2008) | 70 | 1–3 | Bevacizumab 10 mg/kg q 2 wk+ Cyclophosphamide 50 mg po daily | 24% | (median TTP) 7.2 mos | 17 mos | n=4 (6%) |
| Current Report | 13 | 1–3 | Bevacizumab 15 mg/kg q 3 wk+ Erlotinib 150 mg daily | 15% | 4.1 mos | 11 mos | n=2 (15%) |
all trials required measurable disease; none permitted pts with first platinum-free interval < 12 mos unless retreated
In a phase II study of gefitinib in patients with recurrent ovarian cancer reported by Schilder et al, [23] the only responding patient had a mutation in the catalytic domain of the EGFR of the tumor. It is possible that it is only this group of ovarian tumors that will benefit from EGFR tyrosine kinase inhibitors alone or in combination. The prevalence of mutations in the kinase domain of EGFR has been reported to be low in ovarian cancer[24]. We found no EGFR mutations in the six tumors on which mutational analysis was successfully performed. It is also possible that the combination is not truly synergistic; of note, the phase II randomized study of bevacizumab with or without erlotinib in renal cell carcinoma showed no evidence of benefit for the combination. [25]
Predictors of response/resistance to bevacizumab or other antiangiogenic agents are not yet established. In a GOG trial of the antiangiogenic agent, thalidomide, in the treatment of endometrial carcinoma, elevated baseline plasma VEGF (and not serum VEGF) was associated with increased risk of progression and death[26]. We did not find any such association in our ovarian cancer patients. However, the numbers were very small.
Unlike some other bevacizumab trials we did not observe any severe CNS toxicities or arterial thrombotic events. One patient had grade 3 hypertension. The rate of rash was comparable to that observed in the single agent erlotinib trial (90%).[15] The rate of diarrhea was comparable to that observed in the single agent trial of gefitinb (500 mg/day) in patients with ovarian cancer: Schilder et al reported 8 of 27 pts (30%) developing grade 3 diarrhea and 1 of 27 developing grade 3 stomatitis on gefitinib, [23] but higher than that reported with single agent erlotinib: Gordon et al reported that 38% of their patients developed diarrhea (6% grade 3); they did not report any stomatitis.[15] These differences may represent a real increase in the rate of diarrhea/stomatitis with the combination of bevacizumab and erlotinib, but they may also represent chance variation because of the small numbers of patients. The rate of grade 3 diarrhea on the erlotinib/bevacizumab arm of the randomized phase II renal cell trial was 8% [25]; Cannistra et al reported 34% diarrhea (2% ≥ gr 3) on single agent bevacizumab. We suggest that erlotinib be used very cautiously or avoided in patients with an ileostomy.
As noted above, the rate of bowel perforation in this study was concerning. While it would be possible to hypothesize that the diarrhea caused by erlotinib could increase the risk of bowel perforation, there is no evidence to support this. One of the perforations occurred after completion of therapy. Both occurred in the setting of small bowel obstruction.
The NCI alerted investigators via an investigational new drug (IND) action letter dated October 4, 2005 of the risk of gastrointestinal perforation in ovarian cancer patients treated with bevacizumab[27]. The risk appeared to be highest in heavily treated patients with extensive bowel involvement. As can be seen in Table 4, Garcia et al combined bevacizumab and metronomic oral cyclophosphamide and reported four GI perforations or fistulae among seventy patients.[28] Cannistra et al treated 44 patients with platinum resistant disease that progressed after second line topotecan or liposomal doxorubicin with single agent bevacizumab. Five had gastrointestinal perforations, one of which was fatal. [9] All had radiographic evidence of bowel involvement at study entry and stable disease at the time of perforation. A blinded independent review facility (IRF) radiologist reviewed baseline radiographs from all study patients treated on that trial, but no radiographic variables that could predict for gastrointestinal perforation risk were found. A trend for increased frequency of gastrointestinal perforation was observed for bowel wall thickening and bowel wall obstruction, but these associations were not statistically significant after accounting for multiple possible risk factors. A recent summary of the published literature to date suggested that the overall rate of bowel perforation in ovarian cancer patients treated with bevacizumab is 5–6% [29]. This is somewhat higher than the rate reported for another intra-abdominal malignancy, colon cancer. The randomized trial comparing irinotecan, fluorouracil, and leucovorin (IFL) plus bevacizumab to IFL alone in patients with previously untreated colorectal cancer noted a 1.5% rate of gastrointestinal perforation in the patients treated with bevacizumab (vs 0% in those treated with IFL alone) [30], and similar rates of gastrointestinal perforation have been observed in other large colon cancer trials. [31] Colon cancer patients may less often have bulky intra-abdominal disease (and more often, for example, extensive liver metastases) than ovarian cancer patients, and therefore be less prone to develop bowel obstructions, which appeared to be a risk factor in our series. It has been suggested that pretreated patients can be more safely given bevacizumab if they have no clinical symptoms of bowel obstruction, no evidence of rectosigmoid involvement on pelvic exam, and no evidence of bowel involvement on CT scan.[32] These recommendations are reasonable, although they will likely eliminate a fair number of platinum resistant ovarian cancer patients from bevacizumab therapy. It should also be noted that the colon cancer trials reported were in previously untreated patients, who may be more generally treatment-responsive and therefore also less likely to develop a small bowel obstruction. Hopefully front-line trials of bevacizumab in ovarian cancer, such as GOG 218 (see below) will also demonstrate a lower rate of bowel perforation.
It is possible that bevacizumab will be used to best advantage in ovarian cancer patients earlier in their disease and/or in combination with chemotherapy. Bevacizumab combined with chemotherapy has been reported to significantly prolong progression-free survival, and in some cases, overall survival in metastatic colon, breast, and lung cancer[30,33–35]. Penson et al evaluated bevacizumab in combination with carboplatin and paclitaxel in chemotherapy naïve patients with epithelial ovarian, fallopian, primary peritoneal or uterine papillary serous tumors. Bevacizumab was administered with chemotherapy for 6–8 cycles and continued for one year consolidation. Preliminary toxicity data on the 30 evaluable patients revealed 1 nasal perforation, 2 cases of delayed wound healing and no bowel perforations. Response data from this trial have not yet been published[36]. Two randomized trials, GOG 218 and ICON 7 are evaluating bevacizumab in combination with platinum-based chemotherapy in previously untreated ovarian cancer patients. Careful toxicity monitoring is built into GOG 218, and there has so far been no signal that the trial should be stopped for safety concerns. Safety and efficacy data from these trials will allow us to assess the therapeutic benefit of bevacizumab.
Acknowledgments
Research support: University of Chicago Phase II Consortium (NCI N01-CM-62201) PMH Phase II Consortium (N01-CM-17107, N01-CM-62203) California Phase II Consortium (N01-CM-62209)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abu-Jawdeh GM, Faix JD, Niloff J, et al. Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms. Lab Invest. 1996;74:1105–1115. [PubMed] [Google Scholar]
- 2.Tempfer C, Obermair A, Hefler L, et al. Vascular endothelial growth factor serum concentrations in ovarian cancer. Obstet Gynecol. 1998;92:360–363. doi: 10.1016/s0029-7844(98)00190-2. [DOI] [PubMed] [Google Scholar]
- 3.Yoneda J, Kuniyasu H, Crispens MA, et al. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 4.Cooper BC, Ritchie JM, Broghammer CL, et al. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clin Cancer Res. 2002;8:3193–3197. [PubMed] [Google Scholar]
- 5.Hefler LA, Zeillinger R, Grimm C, et al. Preoperative serum vascular endothelial growth factor as a prognostic parameter in ovarian cancer. Gynecol Oncol. 2006;103:512–517. doi: 10.1016/j.ygyno.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Konishi I, Mandai M, et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76:1221–1227. doi: 10.1038/bjc.1997.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez AA, Krigman HR, Whitaker RS, et al. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res. 1999;5:587–591. [PubMed] [Google Scholar]
- 8.Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 9.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett JM, Langdon SP, Simpson BJ, et al. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer. 1996;73:301–306. doi: 10.1038/bjc.1996.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer-Colbrie J, Witt A, Heinzl H, et al. EGFR and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res. 1997;17:613–619. [PubMed] [Google Scholar]
- 12.Psyrri A, Kassar M, Yu Z, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11:8637–8643. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 13.Niikura H, Sasano H, Sato S, Yajima A. Expression of epidermal growth factor-related proteins and epidermal growth factor receptor in common epithelial ovarian tumors. Int J Gynecol Pathol. 1997;16:60–68. doi: 10.1097/00004347-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Lassus H, Sihto H, Leminen A, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med. 2006;84:671–681. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 15.Gordon AN, Finkler N, Edwards RP, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785–792. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 16.Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203–220. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 18.Hainsworth JD, Sosman JA, Spigel DR, et al. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889–7896. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Dziadziuszko R, Hirsch FR, Varella-Garcia M, et al. Epidermal growth factor receptor (EGFR) immunohistochemistry: Comparison of antibodies (Abs) and cut points to predict benefit from gefitinib in a phase III placebo-controlled study in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(403s):abstr–57. quiz 51 p following 57. [Google Scholar]
- 22.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 23.Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11:5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 24.Mustea A, Sehouli J, Fabjani G, et al. Epidermal growth factor receptor (EGFR) mutation rate does not correlate with platinum resistance in ovarian carcinoma. Results of a prospective pilot study. Anticancer Res. 2007;27:1527–1530. [PubMed] [Google Scholar]
- 25.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25:4536–4541. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 26.McMeekin DS, Sill MW, Benbrook D, et al. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: A Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:508–516. doi: 10.1016/j.ygyno.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aghajanian C. The role of bevacizumab in ovarian cancer--an evolving story. Gynecol Oncol. 2006;102:131–133. doi: 10.1016/j.ygyno.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 29.Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol. 2007;105:3–6. doi: 10.1016/j.ygyno.2007.01.038. \. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 31.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 32.Simpkins F, Belinson JL, Rose PG. Avoiding bevacizumab related gastrointestinal toxicity for recurrent ovarian cancer by careful patient screening. Gynecol Oncol. 2007;107:118–123. doi: 10.1016/j.ygyno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 35.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 36.Penson R, Cannistra SA, Seiden MV. Phase II study of carboplatin, paclitaxel and bevacizumab as first line chemotherapy and consolidation for advanced mullerian tumors. J Clin Oncol; ASCO Annual Meeting Proceedings; 2006. pp. Abstr–5020. [Google Scholar]