Abstract
Bioactive glass is used as both a bone filler and as a coating on implants, and has been advocated as a potential osteogenic scaffold for tissue engineering. Rat derived mesenchymal stem cells (MSCs) show elevated levels of levels of alkaline phosphatase activity when grown on 45S5 bioactive glass as compared to standard tissue culture plastic. Similarly, exposure to the dissolution products of 45S5 elevates alkaline phosphatase activity and other osteogenic markers in these cells. We investigated whether human MSCs grown under the same laboratory conditions as rat MSCs would exhibit similar responses. In general, human MSCs produce markedly less alkaline phosphatase activity than rat MSCs, regardless of cell culture conditions, and do not respond to the growth factor BMP-2 in the same way as rat MSCs. In our experiments there was no difference in alkaline phosphatase activity between human MSCs grown on 45S5 bioactive glass or tissue culture plastic, in samples from five different orthopaedic patients, regardless of culture media composition. Neither was there any consistent effect of 45S5 dissolution products on human MSCs from three different donors. These results suggest that the positive effects of bioactive glass on bone growth in human patients are not mediated by accelerated differentiation of mesenchymal stem cells.
Keywords: Alkaline phosphatase, Bioactive glass, Bone tissue engineering, Cell viability, Mesenchymal stem cell, SBF (simulated body fluids)
1. Introduction
Bioactive glasses generally form a carbonated apatite layer when immersed in a solution of buffered salts representing the ion concentration of body fluids (simulated body fluids or SBF) [1,2]. In vitro, bone cells, whether primary or cell-line derived, grow well on bioactive glass and bone matrix production appears to be enhanced when compared with growth on inert glasses or plastics [3,4]. The effects of bioactive glass on bone cells has been shown to be both substrate [5] and solution mediated [6,7]. Human osteoblasts exposed only to bioactive glass dissolution products alter their gene activity compared to cells in standard media [8].
45S5 bioactive glass is a melt derived glass with composition in weight percent: 45% SiO2, 24.5%CaO, 24.5%Na2O and 6%P2O5. It was first developed in the early seventies [9] and has been shown in multiple studies to have good bone bonding properties [5,10]. The silica and phosphate compositions of this glass are within the range that allows dissolution and calcium phosphate formation at the surface while maintaining an appropriate rate of degradability. 45S5 bioactive glasses enhance bone apposition on their surface and create a strong bone/implant interface on both particles and coatings [11,12]. Bioactive glasses, including 45S5 and other compositions are used in clinical procedures such as alveolar ridge augmentation and periodontal defect filling [13–15].
It has been suggested that bioactive glasses would make good scaffolds for bone tissue engineering, either alone or in combination with a polymer scaffold [7,16,17]. Mesenchymal stem cells (MSCs) are bone marrow derived cells of the mesenchymal lineage isolated from whole bone marrow by adhesion to tissue culture plastic [18,19]. These cells can differentiate into osteoblastic cells, among other mesenchymal lineages. The good bone bonding at the surface of a biomaterial, that is seen when bioactive glasses are implanted in vivo, implies the material has allowed recruitment and/or differentiation of the osteogenic precursors that derive from MSCs. A bioactive glass scaffold seeded with MSCs has been tested in a rat model for its ability to induce bone repair with promising results [20]. In order to exploit the potential of bioactive glass scaffolds for tissue engineering the interactions between the mesenchymal stem cells and the scaffold need to be better understood.
There is evidence that cells derived from bone marrow can be stimulated towards osteogenesis when grown on bioactive glasses [21–23]. Recently, the dissolution products of 45S5 bioactive glass were shown to enhance the osteogenesis of murine embryonic stem cells [24]. Our previous experiments indicated that rat MSC differentiation is accelerated by growth on 45S5 bioactive glass or exposure to bioactive glass dissolution products [6]. Alkaline phosphatase activity, a marker of osteoblastic differentiation, was shown to be higher than in cells grown on tissue culture plastic. However, it is known that human MSCs respond differently from rodent MSCs to ‘osteoinductive’ conditions. For instance human MSCs do not react in the same way as rat MSCs to the growth factor BMP-2 [25,26]. Therefore, in these experiments we exposed human MSCs to the same bioactive glass under identical culture conditions as our previously reported rat MSCs [6]. We examined cell numbers and alkaline phosphatase activity. Our hypothesis was that human MSCs would also show elevated levels of alkaline phosphatase when exposed to 45S5 bioactive glass as a substrate or as dissolution products.
2. Materials and Methods
2.1 Bioactive Glass
Rods of 45S5 bioactive glass (MO-Sci, Rolla MO) of 12mm diameter were cut into 1mm thick disks on a diamond wheel saw. Disks were cleaned by sonication in acetone and then ethanol. To create a layer of carbonate apatite on the disks, they were immersed in a tris buffered solution (TE) containing ions at the following concentrations: 142 mM Na+, 5 mM K+, 1.5 mM Mg2+, 2.5 mM Ca2+, 148.8 mM Cl−, 4.2 mMHCO3−, 1mM HPO42− pH 7.6, [27]. This type of physiological solution is also known as simulated body fluid (SBF). Disks were immersed in TE in individual vials allowing a surface to volume ration of 0.1cm−1 (45S5 glass: TE). Vials were incubated at 37°C in a tissue culture incubator, with constant agitation. After 3 days of immersion disks were rinsed with ethanol, air dried and stored in a desiccator. All disks were checked for formation of carbonated apatite by Fourier Transform Infrared spectroscopy (FTIR, 5DXC, Nicolet, Madison, WI).
Disks were randomly assigned to rat or human derived cell experiments and were run over the same time period dependent on availability of cells in no specific order. For all experiments, disks that were pre-treated as above were immersed in 70% ethanol for 30 minutes then air-dried under UV light in a class II tissue culture cabinet, for sterilisation. Sterile disks were placed in basal tissue culture medium, alpha MEM (Invitrogen Life Technologies, Inc. Grand Island, NY), 1% antibiotics (Invitrogen) and 10% fetal bovine serum (FBS; PremiumSelect, Atlanta Biologicals, Norcross, GA) with the addition of 25mM HEPES buffer (Sigma-Aldrich, St. Louis MO) for 2 hrs. This was to allow serum proteins to adsorb to the substrate, a process which was shown to facilitate cell attachment and osteogenesis in MC3T3-E1 osteoblast-like cells [28].
2.2 Isolation and culture of mesenchymal stem cells
Human marrow cells were derived from orthopaedic patients undergoing primary hip replacement by aspiration from the femoral medullary cavity, as previously described [25, 26]. The age, gender and experimental conditions for each donor’s cells are specified in Table 1. Marrow cells were washed twice to remove fatty components, and resuspended in Hanks Balanced Salt Solution (HBSS, Invitrogen). The samples were layered on Ficoll-Paque (Amersham Pharmacia Biotech, Piscataway, NJ) and centrifuged 30min at 1900 RCF to concentrate nucleated cells at the interface. This fraction was collected, washed once with αMEM media, and resuspended in tissue culture medium. Primary cultures of these cells were established at 5×105 cells/cm2, and non-adherent cells removed after 3 days. Experiments were carried out after first or second passage.
TABLE 1.
Source of MSCs from human donors and the experiments they were used for. Where only the substrate box is checked cells were seeded on bioactive glass disks, where both the substrate and the solution only boxes are checked cell-seeded disks were placed in a Transwell® insert over a layer of cells on the well base.
| Sample | Age | Gender | Used in experiments to test effects of: | ||
|---|---|---|---|---|---|
| Substrate (standard medium) | Solution only (standard medium) | Serum free medium | |||
| H1 | 52 | Male | X | X | |
| H2 | 71 | Female | X | ||
| H3 | 66 | Male | X | X | X |
| H4 | 39 | Female | X | X | X |
| H5 | 38 | Female | X | X | |
Rat bone marrow cells were derived from the femora of female Wistar rats, 4–5 weeks old, as described previously [29]. All procedures were approved by the University of Pennsylvania’s Institutional Animal Use and Care Committee (IACUC). Briefly, after sacrifice, the ends of the femora were removed and the marrow of the midshaft flushed out with a needle. Marrow was suspended in tissue culture media and plated in a 10cm tissue culture dished at 1 femur per dish. Non adherent cells were removed after 3–5 days. Experiments were carried after first or second passage Once cells had reached confluence they were removed by 0.25% trypsin in 1 mm tetrasodium EDTA (Invitrogen) and plated on the pre-treated 45S5 bioactive glass disks 1 cm diameter or plastic tissue culture treated coverslip controls (Thermanox™, Nunc, Rochester, NY) for substrate mediated experiments. Seeding density was 10–15×104 cells per disk. Cells were allowed to adhere in a small volume of media for 1 hr. Where solution mediated effects were carried out in parallel (solution only column, Table 1), the disk with cells was placed in a porous insert (Transwell®, Corning Inc. Corning, NY) suspended above a layer of cells from the same culture which had been plated in the a standard way at the base of a 6 well plate (at 10×104 cells per cm2) and 6 mls media added such that the cells on the disk were immersed (Fig. 1). In preliminary experiments, 45S5 disks pre-treated but not seeded with cells were placed in the inserts. Statistical analysis indicated no effect of cells being present on the disk for the solution mediated responses.
FIGURE 1.
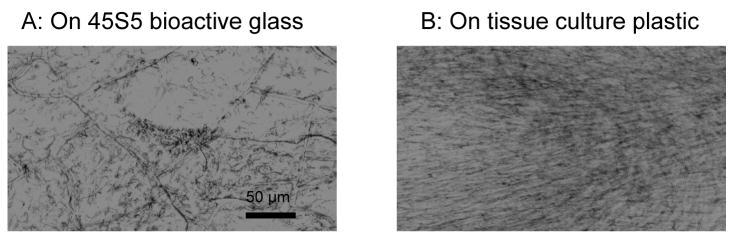
Human MSC growth on bioactive glass. Human MSCs were seeded on pre-conditioned 45S5 bioactive glass or tissue culture plastic, grown for 7 days and stained with MTT to localise metabolically active cells. While cells on tissue culture plastic were confluent and elongated with a fibroblast-like morphology, cells on 45S5 bioactive glass were more rounded with a more patchy distribution.
On the 1st day of culture the medium was exchanged and ascorbate-2-phosphate (Wako Chemicals, Richmond, VA), (100μg/ml) was added to the fresh medium. Other supplements were added depending on the medium conditions being investigated, these were: 100ng/ml BMP-2 (Genetics Institute, Cambridge, MA) and 10−8M dexamethasone (dex) for rat cells (Sigma) or 10−7M dex, for human cells. The media were exchanged on the 4th day. Usually media was replaced with the same supplements as added on day 1, however for some experiments on human cells (Table 1) a serum free media was added on day 4 as developed by Osyczka and Leboy [26]. The serum containing medium was removed and fresh medium consisting of alpha MEM, 1% antibiotics, ITS+ Premix (containing insulin, transferrin, selenious acid, linoleic acid and bovine serum albumin) (BD Biosciences, Bedford, MA) was added.
2.3 Cell staining by MTT
MTT tetrazolium salt staining was used to localise metabolically active cells on the 45S5 bioactive glass surface. Cells were rinsed with PBS and 5ug/ml MTT (Sigma) in PBS was added, cells were incubated for 1 hour at 37°C then the MTT solution removed and PBS added for viewing under a transmitted light microscope.
2.4 Cell number and alkaline phosphatase activity
On day 7 of culture, cells were harvested for total cell number and alkaline phosphatase assays. Cells numbers were assayed either by DNA quantification (CyQUANT cell proliferation assay kit, Molecular Probes, Eugene, OR) or by MTS tetrazolium salt assay of mitochondrial activity (Cell Titer 96 AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI). MTS produces a soluble formazan salt after conversion by mitochondrial dehydrogenase enzymes, as distinct from the insoluble salt produced by MTT. For DNA analysis, cells were trypsinized and a sub-sample of cell suspension centrifuged, the cell pellet lysed with the CyQUANT lysis buffer and the fluorescent DNA dye added. The resulting solution was transferred to a 96 well plate and DNA assayed fluorometrically. The remaining cells were extracted for the alkaline phosphatase assay. When MTS was used, a 1:10 dilution of MTS was applied to the monolayer of cells in phenol red-free media for 30–60 minutes, 200 μl of media plus MTS was transferred to a 96 well plate and assayed in a ‘Multiskan ascent’ plate reader (Thermolabsystems, Franklin, MA). After the respective cell quantification assays the cells were washed twice in HBSS and extracted with 0.15 M Tris, pH 9 with 0.1 mM ZnCl2, 0.1 mM MgCl2 and 1% Triton X-100 for 30 mins at 37°C, followed by overnight storage at 4°C. A sample of the cell lysate was reacted with p-nitrophenyl phosphate substrate (Sigma) in 1.5 M Tris buffer pH 9 with 1 mM ZnCl2 and 1 mM MgCl2. Phosphatase activity was measured spectrophotometrically at 410 nm with 1 absorbance unit equivalent to 64 nmol of product. Alkaline phosphatase enzyme levels were calculated as nmol p-nitrophenol product per minute normalized to MTS units or μg DNA. There were no differences in relative cell numbers between the two assays so MTS was selected as the favoured assay for the majority of experiments as it required fewer processing steps.
2.5 Statistical analysis
Statistics were performed using Minitab™ software. To combine experimental data from multiple experiments data was normalised to an internal control. Paired or two sample t-tests were performed as specified in the results section.
3. Results
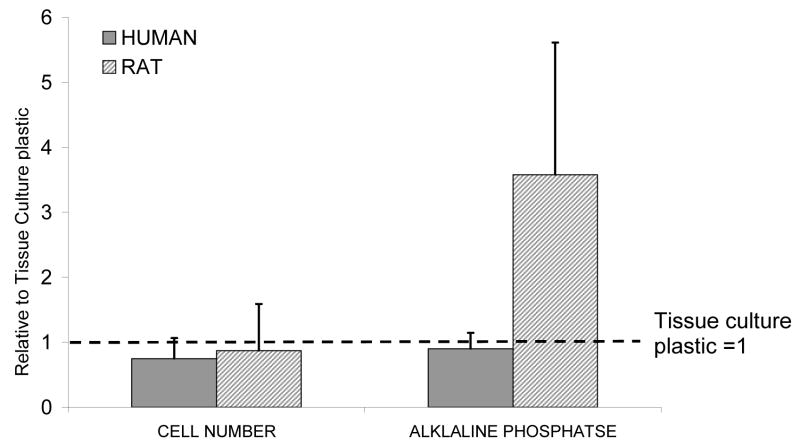
Human MSCs formed a monolayer on the bioactive glass surface and were subjectively less elongated and less confluent than on tissue culture plastic, as seen by MTT staining (Fig. 1). The MTS assay of metabolic activity also showed that the final number of active cells on bioactive glass was consistently less than that on tissue culture plastic for both rat and human cells, but the differences were small and not statistically significantly (Fig. 2a). The results presented in Fig. 2 are for cells grown in media containing dexamethasone (dex) and ascorbate (asc) but a similar pattern was seen with all treatments. Alkaline phosphatase activity per cell number in rat MSCs was higher on the 45S5 bioactive glass (Fig. 2), as reported previously [6]. However, human cell alkaline phosphatase activity did not appear to differ between substrates.
FIGURE 2.
Relative cell number and alkaline phosphatase activity, mean±S.D for 4 rat and 4 human samples grown on 45S5 bioactive glass, in standard media with ascorbate (asc) plus dexamethasone (dex) supplements, each individual mean was normalised to the tissue culture plastic controls for the respective experiment then data combined. Cell number was slightly but not significantly lower in both rat and human samples. Relative alkaline phosphatase activity was not different for human samples but was 3-fold higher in rats (no significant difference, 1 sample t-test p=0.085).
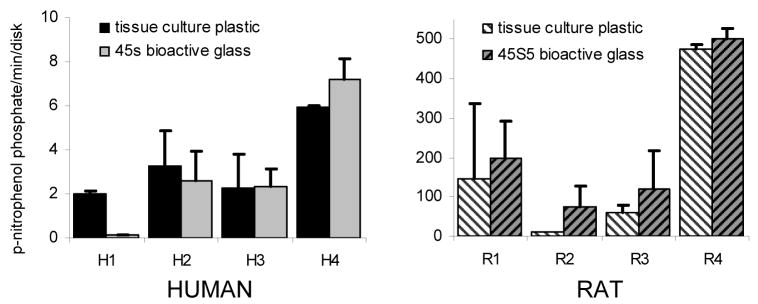
Separating the data by animal or human donor indicated that alkaline phosphatase activity levels in rats are generally 10 fold higher than that in humans (Fig. 3). Within each experiment, rat total alkaline phosphatase activity was significantly higher on bioactive glass than on tissue culture plastic (p<0.05). However human cells gave variable responses and showed no significant overall effect of being grown on 45S5 bioactive glass compared with tissue culture plastic.
FIGURE 3.
Total alkaline phosphatase activity mean±S.D. per cell-seeded disk for 4 individual rat and 4 individual human samples (n=2 per sample) grown in osteogenic medium (standard medium with asc plus dex). There is large variability between individuals. Rat samples seeded on 45S5 bioactive glass show higher alkaline phosphatase activity than samples on tissue culture plastic (paired t-test p<0.05). Human samples do not show any consistent differences. Note different y axis scales.
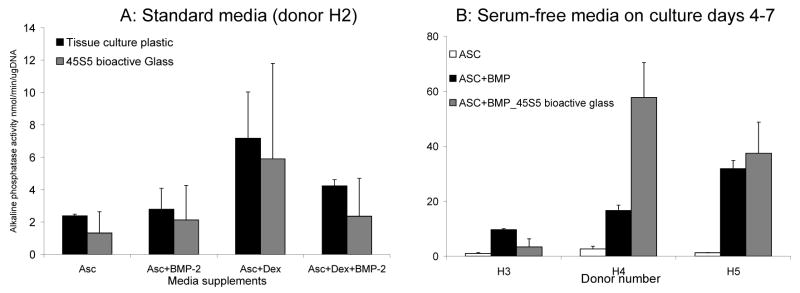
Some groups have suggested a synergistic, positive effect of bioactive glasses or hydroxyapatite and BMP-2 on bone cell differentiation [30, 31]. Therefore, we also tested human cell responses under conditions in which BMP-2 was present. Results are shown for one sample (H2) in which four medium conditions were tested: 1) ascorbate alone, 2) ascorbate plus BMP-2, 3) ascorbate plus dex and 4) all 3 supplements. There was no effect of bioactive glass on alkaline phosphatase activity in any medium conditions tested (Fig. 4a). Note that human MSCs from donor H2 did not respond to BMP-2 with an increase in alkaline phosphatase, as was the case for 90% of human MSC samples tested in our laboratory [26]. However, BMP-2 does increase alkaline phosphatase activity measured on day 7 if the medium is exchanged for a defined serum free medium on day 4. We therefore tested the effect of bioactive glass with and without BMP-2 under these serum-free conditions. As shown in Fig. 4b, bioactive glass did not have a consistent additional effect on human MSCs, although MSCs from one donor (H4) did show an additive effect of BMP + bioactive glass on levels of alkaline phosphatase with serum-free media.
FIGURE 4.
The effect of different media supplements on responses to bioactive glass. A: Alkaline phosphatase activity per μg DNA for one human sample (H2), mean and S.D. grown in 4 media conditions, Asc= ascorbate alone, Asc+Dex = ascorbate plus dexamethasone, Asc+BMP-2 = ascorbate plus BMP-2 and Asc+Dex+BMP-2 = all three supplements. There are no media conditions in which alkaline phosphatase activity is higher on bioactive glass than in tissue culture plastic (n=2). B: Alkaline phosphatase activity per unit of MTS, mean and S.D. in serum free conditions for 3 different patients. Alkaline phosphatase activity is much higher in BMP-2 treated samples in serum free medium compared with controls, the effect of bioactive glass is variable, alkaline phosphatase activity is higher in one patient (H4), n=2.
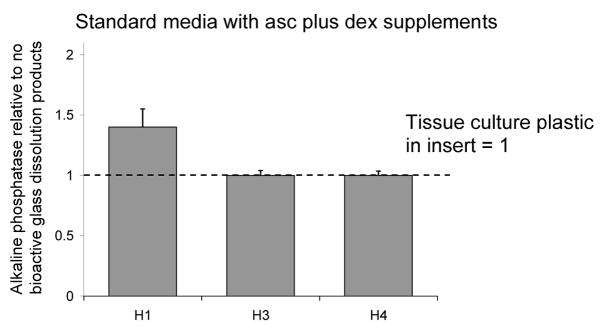
Rat MSCs show a significant effect of 45S5 bioactive glass dissolution products on alkaline phosphatase levels [6]. In contrast, as with the substrate effect, cells from different human donors behaved in a variable manner in response to bioactive glass dissolution products. In one sample (H1) alkaline phosphatase activity was modestly higher in cells exposed to 45S5 dissolution products, but in two other donors there was no effect of dissolution products in the medium (Fig. 5).
FIGURE 5.
Effect of glass dissolution products on alkaline phosphatase activity of human MSCs from 3 patients. There were no consistent effects of dissolution products compared with having plastic disks in well inserts. Values are mean ± SD of alkaline phosphatase/MTS unit relative to controls (=1).
4. Discussion
Alkaline phosphatase activity as measured by reduction of p-nitophenol phosphate is a common marker of bone cell differentiation. Multiple studies have suggested that alkaline phosphatase activity is enhanced when cells are grown on bioactive glass in both animal-derived cells [3], human osteoblasts [32] and rat MSCs [21, 6, 33]. It is known that bioactive glass has positive effects on bone apposition in both animal studies [11, 12, 16, 20] and clinical trials [13, 14]. A hypothesis for increased bone formation around bioactive glass implants is that either attachment to the bioactive glass or exposure to its dissolution products enhances osteoblastic differentiation from osteogenic precursors which arise from mesenchymal stem cells. This hypothesis was supported by our finding that alkaline phosphatase activity was enhanced in rat MSCs seeded on 45S5 bioactive glass [6], as well as other studies with rat marrow derived cells on various compositions of bioactive glass [21, 33].
In the current experiments we examined the ability of 45S5 bioactive glass to enhance alkaline phosphatase activity in human MSCs. We obtained highly variable effects of bioactive glass between MSCs derived from four human donors with no consistent effects. While the control surface in these experiments is tissue culture plastic and therefore a idealized cell culture surface (not a bioinert surface) it is interesting to note that rat MSCs consistently produce higher alkaline phosphatase when grown on bioactive glass compared to tissue culture plastic whereas human MSCs do not. In addition, human MSCs grown on tissue culture plastic but exposed to the dissolution products of bioactive glass showed no consistent increase in alkaline phosphatase activity over controls without bioactive glass exposure, despite the reports from our laboratory and others that rat MSCs [6] mouse embryonic stem cells [24] and human mature osteoblasts [8] show solution mediated effects of bioactive glasses.
Interestingly, unexpected differences are not unique to the bulk bioactive glass substrates discussed in this paper. Experiments in our laboratory on poly(lactic-co-glycolic acid) (PLGA) foams containing bioactive glass particles showed that rat cells grown on, or in the conditioned medium of, foams including 45S5 bioactive glass particles exhibited higher alkaline phosphatase activity, than in PLGA foams with no inclusions. However, this effect was not seen in human cells, under the same conditions [7]. Yang et al [16] showed that poly(DL-lactic acid) (PDLLA) foams containing bioactive glass particles were much more effective in increasing alkaline phosphatase activity when they contained only 5 wt% glass compared with 40% wt% glass. A PLGA scaffold coated with 45S5 bioactive glass had no effect on alkaline phosphatase activity or osteocalcin production by human MSCs compared with uncoated scaffolds at 1, 2 or 4 weeks of culture [17]. While it is difficult to compare our results with those of others who have used different composites, pre-treatment and characterization techniques, it is interesting to note that these papers all have in common a low or no alkaline phosphatase response by human MSCs to bioactive glasses.
The apparent lack of response of human MSCs to bioactive glass is unlikely to be mediated by the media composition, as a change in medium composition known to markedly increase the human MSC alkaline phosphatase response to BMP-2 [26] had no significant effect on the lack of response to bioactive glass (Fig. 5b). In this study we have only examined the alkaline phosphatase response to bioactive glass, because it is a commonly used marker repeatedly found to increase on bone cell differentiation. It is possible that other markers of differentiation could increase independently of alkaline phosphatase in human MSCs. However, we believe this is unlikely because our studies with rat cells showed that mRNA for alkaline phosphatase and other gene level responses (mRNA for osteopontin, osteocalcin and bone sialoprotein) were regulated in exactly the same way in response to 45S5/PLGA composite [7]. In addition, another study of bioactive glass coated PLGA showed that levels of osteocalcin responded in the same way as the levels of alkaline phosphatase in response to the bioactive glass coating [17].
Leach et al [17] have suggested that the effects of bioactive glass in vivo may be unrelated to effects on MSCs, proposing that the enhanced bone growth associated with implantation of bioactive glass or bioactive glass-coated materials was a secondary effect of increased vasculogenesis. The basis for their suggestion was the observation that 45S5 coated scaffolds that did not affect alkaline phosphatase activity of human MSCs in vitro, still elicited a marked increase in bone formation in vivo, which was associated with a positive affect on blood vessel recruitment. While we did not test vasculogenesis, our data supports the suggestion that bioactive glass supports bone formation by a more complex mechanism than direct stimulation of MSC differentiation.
It is possible that when testing the effect of bioactive glass on bone cells in vitro a mixed cell population needs to be present to better represent the in vivo environment. For example osteoclastic differentiation and activity (as assayed by tartrate-resistant acidic phosphatase staining) can also increase when osteoclast precursors derived from bone marrow are seeded on bioactive glass [26] [34]. Since osteoclasts and osteoblasts modulate the differentiation of each other, osteoclasts may need to be present for an effect on differentiation to be seen in human cells.
5. Conclusions
The hypothesis that bioactive glass stimulates bone formation through direct effects on MSC differentiation is not supported by our data on MSCs from five different human donors. While human MSCs proliferated and differentiated as well on bioactive glass as on tissue culture plastic, there was no enhanced production of the bone differentiation marker alkaline phosphatase. This is in contrast to the previously reported osteogenic effects of 45S5 bioactive glass on rat MSCs. While our results do not contradict the usefulness of bioactive glass as an implant material, where a complex mixture of cells and growth factors is present, a more thorough characterization of human MSC responses to bioactive glass in vitro is necessary in order to understand how bioactive glass might be used as a scaffold for in vivo tissue engineering.
Acknowledgments
We are grateful to Wyeth/Genetics Institute for providing rhBMP-2. We would like to thank Geeta Bhargave for technical assistance, and Drs. David L. Diefenderfer and Jonathan Garino for providing human bone marrow cells. The cell culture experiments were devised and carried out in the laboratory of Phoebe S. Leboy at the Department of Biochemistry, University of Pennsylvania. We would like to thank Professor Leboy for her guidance and expertise throughout the project and helpful contributions to the manuscript. This work was supported by NIH grant: RO1 DE13800.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schepers E, Declercq M, Ducheyne P, Kempeneers R. Bioactive glass particulate material as a filler for bone-lesions. J Oral Rehab. 1991;18(5):439–52. doi: 10.1111/j.1365-2842.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 2.Huygh A, Schepers E, Barbier L, Ducheyne P. Microchemical transformation of bioactive glass particles of narrow size range, a 0–24 months study. J Mater Sci Mater Med. 2002;13(3):315–20. doi: 10.1023/a:1014023304148. [DOI] [PubMed] [Google Scholar]
- 3.Loty C, Sautier JM, Tan MT, Oboeuf M, Jallot E, Boulekbache H, et al. Bioactive glass stimulates in vitro osteoblast differentiation and creates a favorable template for bone tissue formation. J Bone Miner Res. 2001;16(2):231–9. doi: 10.1359/jbmr.2001.16.2.231. [DOI] [PubMed] [Google Scholar]
- 4.Xynos ID, Hukkanen MVJ, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass® 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67(4):321–9. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 5.Ducheyne P, Qiu Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20(23–24):2287–303. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 6.Radin S, Reilly G, Bhargave G, Leboy P, Ducheyne P. Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res. 2005;73A(1):21–9. doi: 10.1002/jbm.a.30241. [DOI] [PubMed] [Google Scholar]
- 7.Yao J, Radin S, Reilly G, Leboy P, Ducheyne P. Solution-mediated effect of bioactive glass in poly (lactic-co-glycolic acid)-bioactive glass composites on osteogenesis of marrow stromal cells. J Biomed Mater Res. 2005;75A(4):794–801. doi: 10.1002/jbm.a.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J Biomed Mater Res. 2001;55(2):151–7. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Hench LL, Paschall HA. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J Biomed Mater Res. 1973;7(3):25–42. doi: 10.1002/jbm.820070304. [DOI] [PubMed] [Google Scholar]
- 10.Hench LL. Bioceramics. J Am Ceram Soc. 1998;81(7):1705–28. [Google Scholar]
- 11.Schepers E, Ducheyne P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1–24 month experiment with several materials and particle sizes and size ranges. J Oral Rehab. 1997;24(3):171–81. [PubMed] [Google Scholar]
- 12.Marcolongo M, Ducheyne P, Garino J, Schepers E. Bioactive glass fiber/polymeric composites bond to bone tissue. J Biomed Mater Res. 1998;39(1):161–70. doi: 10.1002/(sici)1097-4636(199801)39:1<161::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Kuru B, Yilmaz S, Argin K, Noyan U. Enamel matrix derivative alone or in combination with a bioactive glass in wide intrabony defects. Clin Oral Invest. 2006;10(3):227–34. doi: 10.1007/s00784-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 14.Trombelli L, Heitz-Mayfield LJA, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002;29:117–35. doi: 10.1034/j.1600-051x.29.s3.7.x. [DOI] [PubMed] [Google Scholar]
- 15.Schepers E, Ducheyne P, Babier L, Schepers S. Bioactive glass particles of limited size range: A new material for the repair of bone defects. Impl Dent. 1993;2:151–6. doi: 10.1097/00008505-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Yang XBB, Webb D, Blaker J, Boccaccini AR, Maquet V, Cooper C, et al. Evaluation of human bone marrow stromal cell growth on biodegradable polymer/Bioglass® composites. Biochem Biophys Res Comm. 2006;342(4):1098–107. doi: 10.1016/j.bbrc.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of vegf-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249–55. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue-engineering applications: Current status and future prospects. Tissue Eng. 2005;11(5–6):787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 19.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. [PubMed] [Google Scholar]
- 20.Livingston T, Ducheyne P, Garino J. In vivo evaluation of a bioactive scaffold for bone tissue engineering. J Biomed Mater Res. 2002;62A(1):1–13. doi: 10.1002/jbm.10157. [DOI] [PubMed] [Google Scholar]
- 21.Laczka-Osyczka A, Laczka M, Kasugai S, Ohya K. Behavior of bone marrow cells cultured on three different coatings of gel-derived bioactive glass-ceramics at early stages of cell differentiation. J Biomed Mater Res. 1998;42(3):433–42. doi: 10.1002/(sici)1097-4636(19981205)42:3<433::aid-jbm13>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Amaral M, Costa MA, Lopes MA, Silva RF, Santos JD, Fernandes MH. Si3N4-bioglass composites stimulate the proliferation of MG63 osteoblast-like cells and support the osteogenic differentiation of human bone marrow cells. Biomaterials. 2002;23(24):4897–906. doi: 10.1016/s0142-9612(02)00249-1. [DOI] [PubMed] [Google Scholar]
- 23.Marion NW, Liang W, Reilly GC, Day DE, Rahaman MN, Mao JJ. Borate glass supports the in vitro osteogenic differentiation of human mesenchymal stem cells. Mechanics Of Advanced Materials And Structures. 2005;12(3):239–46. [Google Scholar]
- 24.Bielby RC, Pryce RS, Hench LL, Polak JM. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with ionic dissolution products of 58S bioactive sol-gel glass. Tissue Eng. 2005;11(3–4):479–88. doi: 10.1089/ten.2005.11.479. [DOI] [PubMed] [Google Scholar]
- 25.Diefenderfer DL, Osyczka AM, Reilly GC, Leboy PS. BMP responsiveness in human mesenchymal stem cells. Conn Tissue Res. 2003;44:305–11. [PubMed] [Google Scholar]
- 26.Osyczka AM, Leboy PS. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology. 2005;146(8):3428–37. doi: 10.1210/en.2005-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Ghannam A, Ducheyne P, Shapiro IM. Bioactive material template for in-vitro synthesis of bone. J Biomed Mater Res. 1995;29(3):359–70. doi: 10.1002/jbm.820290311. [DOI] [PubMed] [Google Scholar]
- 28.El-Ghannam A, Ducheyne P, Shapiro I. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. J Orthop Res. 1999;17(3):340–5. doi: 10.1002/jor.1100170307. [DOI] [PubMed] [Google Scholar]
- 29.Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone-marrow stromal cell-cultures by dexamethasone and BMP-2. Devel Biol. 1994;161(1):218–28. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 30.Noshi T, Yoshikawa T, Dohi Y, Ikeuchi M, Horiuchi K, Ichijima K, et al. Recombinant human bone morphogenetic protein-2 potentiates the in vivo osteogenic ability of marrow/hydroxyapatite composites. Artificial Organs. 2001;25(3):201–8. doi: 10.1046/j.1525-1594.2001.025003201.x. [DOI] [PubMed] [Google Scholar]
- 31.Santos EM, Radin S, Shenker BJ, Shapiro IM, Ducheyne P. Si-Ca-P xerogels and bone morphogenetic protein act synergistically on rat stromal marrow cell differentiation in vitro. J Biomed Mater Res. 1998;41(1):87–94. doi: 10.1002/(sici)1097-4636(199807)41:1<87::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Bosetti M, Zanardi L, Hench L, Cannas M. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. J Biomed Mater Res. 2003;64A(1):189–95. doi: 10.1002/jbm.a.10415. [DOI] [PubMed] [Google Scholar]
- 33.Bosetti M, Cannas M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials. 2005;26(18):3873–9. doi: 10.1016/j.biomaterials.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 34.Karpov M, Laczka M, Leboy PS, Osyczka AM. Sol-gel bioactive glasses promote both osteoblast and osteoclast differentiation of cultured human bone marrow cells. J Biomed Mater Res. doi: 10.1002/jbm.a.31386. Part A: In Press. [DOI] [PubMed] [Google Scholar]