Abstract
Objectives
This study sought to determine the effects of endogenous tetrahydrobiopterin (BH4) bioavailability on endothelial nitric oxide synthase (eNOS) coupling, nitric oxide (NO) bioavailability, and vascular superoxide production in patients with coronary artery disease (CAD).
Background
GTP-cyclohydrolase I, encoded by the GCH1 gene, is the rate-limiting enzyme in the biosynthesis of BH4, an eNOS cofactor important for maintaining enzymatic coupling. We examined the associations between haplotypes of the GCH1 gene, GCH1 expression and biopterin levels, and the effects on endothelial function and vascular superoxide production.
Methods
Blood samples and segments of internal mammary arteries and saphenous veins were obtained from patients with CAD undergoing coronary artery bypass grafting (n = 347). The GCH1 haplotypes were defined by 3 polymorphisms: rs8007267G<A, rs3783641A<T, and rs10483639C<G (X haplotype: A, T, G; O haplotype: any other combination). Vascular superoxide (± the eNOS inhibitor NG-nitro-L-arginine methyl ester [L-NAME]) was measured by lucigenin-enhanced chemiluminescence, whereas the vasorelaxations of saphenous veins to acetylcholine were evaluated ex vivo.
Results
Haplotype frequencies were OO 70.6%, XO 27.4%, and XX 2.0%. The X haplotype was associated with significantly lower vascular GCH1 messenger ribonucleic acid expression and substantial reductions in both plasma and vascular BH4 levels. In X haplotype carriers both vascular superoxide and L-NAME–inhibitable superoxide were significantly increased, and were associated with reduced vasorelaxations to acetylcholine.
Conclusions
GCH1 gene expression, modulated by a particular GCH1 haplotype, is a major determinant of BH4 bioavailability both in plasma and in the vascular wall in patients with CAD. Genetic variation in GCH1 underlies important differences in endogenous BH4 availability and is a determinant of eNOS coupling, vascular redox state, and endothelial function in human vascular disease.
Key Words: tetrahydrobiopterin, endothelial nitric oxide synthase, GTP-cyclohydrolase I, superoxide, haplotype, atherosclerosis
Maintenance of endothelial function is a critical aspect of vascular homeostasis. Loss of normal endothelial production of nitric oxide (NO) is an early and characteristic feature of vascular disease states and plays a role in disease pathogenesis (1). Endothelial nitric oxide synthase (eNOS) is regulated by the cofactor tetrahydrobiopterin (BH4) (2). Reduced BH4 availability in disease states seems to be an important aspect of impaired eNOS activity and increased vascular superoxide production (1,2). In humans with vascular disease states, acute administration of BH4 can improve endothelial function (3,4), and treatments such as folates or vitamin C, which improve endothelial function, may exert some of their effects by increasing BH4 availability (5–9). However, the extent to which alterations in endogenous BH4 availability, rather than pharmacologic effects of BH4, might play a direct role in modulating endothelial function in humans in vivo remains unclear.
GTP cyclohydrolase I (GTPCH) is the rate-limiting enzyme in the biosynthesis of BH4 (10), and is a major determinant of BH4 levels, through transcriptional regulation of GCH1 expression. In some experimental models of vascular disease, reduced BH4 is associated with reduced GTPCH protein levels, suggesting reduced BH4 biosynthesis (11). However, BH4 oxidation by free radicals such as peroxynitrite (ONOO−), generating dihydrobiopterin (BH2) and biopterin (B), may cause loss of BH4 without changes in biopterin synthesis (5,7,12). The relative importance of synthesis versus oxidation of BH4 in atherosclerosis is complex because both local and systemic inflammation up-regulate GCH1 expression (13) but also increase ONOO− production, potentially resulting in greater BH4 oxidation (14).
In genetic mouse models, constitutive reduction in GCH1 expression causes BH4 deficiency, resulting in abnormal eNOS regulation (14–16). Conversely, transgenic overexpression of GTPCH in the vascular endothelium is sufficient to augment vascular BH4 levels (17), improve endothelial function in models of vascular disease (14), and rescue the vascular effects of genetic BH4 deficiency (16). Thus, in experimental models, genetic alteration of endogenous BH4 biosynthesis has provided useful insights into the role of BH4 in vascular disease pathogenesis. In contrast, it is unknown whether alterations in endogenous BH4 synthesis in humans are sufficient to alter systemic or vascular BH4 availability, or have any impact on vascular function.
We have recently identified a novel haplotype of the GCH1 gene that is associated with significantly lower GTPCH activity in stimulated leukocytes (18,19). This haplotype is defined by 3 single nucleotide polymorphisms: rs8007267G>A in the putative promoter region, rs3783641A>T in intron 1 and rs10483639C>G in the 3′ untranslated region of the GCH1 gene (18,19).
In this study we examined the functional effects of this GCH1 haplotype on the levels of BH4 and total biopterins both in plasma and in vessels obtained from patients with coronary artery disease (CAD). Furthermore, we investigated how intrinsic genetic variation in vascular BH4 biosynthesis alters regulation of eNOS function in the human vasculature through effects on superoxide production and NO-mediated endothelial function.
Methods
Study subjects
We studied 347 patients with CAD undergoing elective coronary artery bypass grafting (CABG) at the John Radcliffe Hospital, Oxford, United Kingdom. Exclusion criteria were any inflammatory, infective, liver, or renal disease; malignancy; acute coronary event during the last 2 months; or clinically overt heart failure. Patients receiving nonsteroidal anti-inflammatory drugs, dietary supplements of folic acid, or antioxidant vitamins were also excluded. Demographic characteristics of the patients are presented in Table 1. The study was approved by the local research ethics committee, and each patient gave written informed consent.
Table 1.
Demographic Characteristics of Patients
| Number of patients | 347 |
| Male/female | 292/55 |
| Age (yrs) | 65.42 ± 0.49 |
| Saphenous veins (n) | 289 |
| Internal mammary arteries (n) | 166 |
| Risk factors | |
| Hypertension (%) | 72.7 |
| Hypercholesterolemia (%) | 71.6 |
| Smokers (current/ex) (%) | 8.6/69.8 |
| Diabetes mellitus (%) | 27.5 |
| Family history for coronary artery disease (%) | 63.3 |
| Body mass index (kg/m2) | 28.04 ± 0.34 |
| Triglycerides (mmol/l) | 1.65 ± 0.09 |
| Cholesterol (mmol/l) | 4.07 ± 0.08 |
| High-density lipoprotein (mmol/l) | 1.10 ± 0.02 |
| C-reactive protein (mg/l) | 2.48 ± 0.70 |
| Plasma biopterin levels | |
| Plasma tetrahydrobiopterin (nmol/l) | 20.46 (10.13–41.86) |
| Plasma dihydrobiopterin (nmol/l) | 15.19 (11.77–19.41) |
| Plasma biopterin (nmol/l) | 4.09 (2.83–6.98) |
| Plasma total biopterins (nmol/l) | 46.11 (30.04–71.33) |
| BH4/tBio ratio | 0.49 ± 0.018 |
| Medication (%) | |
| Statins | 90.0 |
| ACEI/ARB | 66.0 |
| Calcium-channel blockers | 40.8 |
| Beta-blocker | 74.6 |
| Aspirin | 87.1 |
Values expressed as mean ± SEM or median (25th to 75th percentile) unless otherwise noted.
ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blockers; BH4 = tetrahydrobiopterin; tBio = total biopterins.
Plasma and tissue samples
Blood samples were obtained immediately before surgery, after overnight fasting. Samples were centrifuged at 2,500 rpm for 10 min, and serum or plasma was stored at −80°C until assayed. Samples of saphenous vein (SV) and internal mammary artery (IMA) were obtained at the time of CABG surgery, as we have previously described (5,20). Paired vessel segments were snap-frozen and stored at −80°C for measurement of biopterin content, or were transferred to the laboratory for functional studies within 30 min, in ice cold Krebs-Henseleit buffer (5,20).
Deoxyribonucleic acid (DNA) extraction and genotyping
Genomic DNA was extracted from 2 ml of whole blood using standard methods (QIAamp DNA blood Midi kit, Qiagen, Germantown, Maryland). In addition, DNA samples were available from 741 unrelated subjects of Caucasian ethnicity (mostly medical students) who had consented into genotyping and served as control subjects for allelic frequencies in the present study.
The DNA samples were screened for that particular functional GCH1 haplotype identified as being associated with decreased GCH1 expression and BH4 production in a previous study (21). The complete haplotype consists of 15 DNA positions of the GCH1 gene. However, as described elsewhere (19), the diagnosis of the particular GCH1 haplotype of present interest is possible with 100% sensitivity and specificity by screening for just 3 GCH1 single-nucleotide polymorphisms that span the entire DNA range of the haplotype: c. −9610G>A (dbSNP rs8007267G>A) in the 5′ untranslated region, c.343+8900A>T (dbSNP rs3783641A>T) in intron 1, and c.*4279 (dbSNP rs10483639C>G) in the 3′ untranslated region. Thus, for diagnosis purposes the haplotype of interest reduces to rs8007267A/rs3783641T/rs10483639G. The GCH1 haplotype of interest was identified by means of validated Pyrosequencing screening assays (Pyrosequencer PSQ95, Uppsala, Sweden) diagnosing the 3 selected sodium nitroprussides (SNPs) (19). Two different types of positive control samples were implemented into all Pyrosequencing screening analyses. One positive control set contained DNA samples from carriers of the haplotype independently diagnosed for all 15 GCH1 DNA positions by the 5′-exonuclease method (22). The second set of positive controls consisted of DNA samples homozygous or heterozygous for the frequent or rare alleles at the 3 SNP positions at the GCH1 DNA, diagnosed externally by means of conventional sequencing (AGOWA GmbH, Berlin, Germany) (18,19).
Ribonucleic acid (RNA) isolation and real-time quantitative polymerase chain reaction (RT qPCR)
Snap-frozen vascular rings (IMA and SV) were initially lysed in Trizol reagent (Tri-Reagent, Sigma, St. Louis, Missouri), followed by RNA purification from the aqueous phase using the RNeasy Micro kit (Qiagen, Stanford, California). Ribonucleic acid was converted into complementary DNA (Superscript II reverse transcriptase, Invitrogen, Carlsbad, California), then subjected to qPCR using the TaqMan system (Applied Biosystems, Foster City, California; Assay ID GCH = Hs00609198_m1, Assay ID GAPDH = Hs02758991_g1) and analyzed on an iCyclerIQ (Biorad, Hercules, California). Relative expression was calculated using the 2−ΔΔCT method.
Determination of plasma and vascular biopterin levels
BH4, BH2, and biopterin levels in plasma or vessel tissue lysates were each determined separately from the same sample, by high-performance liquid chromatography followed by serial electrochemical and fluorescent detection, as previously described (23). Total biopterins were quantified by summing BH4, BH2, and B. Biopterin levels were expressed as pmol/g of tissue for vessels and nmol/l for plasma.
Determination of vascular superoxide production
Vascular superoxide production was measured in paired segments of SV using lucigenin-enhanced chemiluminescence as previously described (5,24). Vessels were opened longitudinally to expose the endothelial surface and equilibrated for 20 min in oxygenated (95% O2/5% CO2) Krebs-4-(2-hydroxyethyl)-1-piperazine-ethane-sulfonic acid buffer (pH = 7.4) at 37°C. Lucigenin-enhanced chemiluminescence was measured using low-concentration lucigenin (5 μmol/l) (25) because higher concentrations of lucigenin (up to 250 μmol/l) favor redox cycling (24). As a measure of eNOS coupling we determined NOS-derived superoxide production, which was estimated as the difference in superoxide production after 20 min incubation with the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (100 μmol/l).
Vasomotor studies
Endothelium-dependent and -independent dilatations were assessed in SV obtained at the time of CABG, using isometric tension studies (5,26). Four rings from each vessel were pre-contracted with phenylephrine (3 × 10−6 μmol/l), then endothelium-dependent relaxations were quantified using acetylcholine (ACh, 10−9 to 10−5 μmol/l). Finally, relaxations to the endothelium-independent NO donor SNP (10−10 to 10−6 μmol/l) were evaluated in the presence of L-NAME (100 μmol/l) as we have previously described (5,26).
Determination of oxidized low-density lipoprotein (ox-LDL) and C-reactive protein (CRP) levels
Serum levels of ox-LDL were measured by enzyme-linked immunosorbent assay using commercially available kit (Mercodia, Sweden). Serum levels of CRP were measured by immunonephelometry using a high-sensitivity method (Dade Behring Marburg GmbH, Marburg, Germany).
Statistical analysis
GCH1 haploblocks and linkage disequilibrium among the 3 SNPs with parameters D′ and r2 (27,28) were analyzed using the solid spine of LD algorithm implemented in Haploview (29). Allelic frequencies of the patient cohorts were compared with those observed in a random sample of 741 healthy unrelated control subjects of Caucasian ethnicity (mainly medical students of the University of Frankfurt am Main, Germany) by means of the Fisher exact test. On the basis of the observed allelic frequency, the expected number of homozygous and heterozygous carriers of the respective SNP was calculated using the Hardy-Weinberg equation as p2 + 2pq + q2 = 1, where p and q are defined as the probabilities of occurrence for the dominant and mutated alleles, respectively (30). The correspondence between the observed numbers of homozygous and heterozygous individuals and the numbers expected on the basis of the Hardy-Weinberg equilibrium, indicating that the study sample corresponded to a random sample of subjects, was assessed using the chi-square goodness of fit test. Allelic frequencies of the cohort of 347 patients were compared with those observed in a random sample of 741 healthy unrelated control subjects of Caucasian ethnicity (mainly medical students of the University of Frankfurt am Main, Germany) by means of the Fisher exact test. Between these cohorts, the number of noncarriers, homozygous carriers, and heterozygous carriers of the particular GCH1 haplotype were compared using chi-square statistics.
All variables were tested for normal distribution using the Kolmokorov-Smirnov test. Normally distributed variables are presented as mean ± SEM, whereas nonnormally distributed variables were log-transformed for analysis and are presented as median [25th to 75th percentiles] and range. Comparisons of baseline and demographic characteristics among the 3 genotypes were performed using one-way analysis of variance for multiple comparisons, whereas an unpaired t test was used to compare variables between 2 groups (for recessive models).
Univariate analysis was performed by calculation of the Pearson coefficient. Multivariate analysis was used to examine the effect of the genotype on vascular/plasma biopterins, vascular superoxide, L-NAME–induced change of vascular superoxide, or maximum relaxations to ACh as dependent variables. As independent variables we used GCH1 genotype, and those clinical characteristics (age, gender, diabetes mellitus, smoking, dyslipidemia, hypertension, body mass index, and medications) that showed an association with the dependent variable in univariate analysis at the level of 15%. A backward elimination procedure was applied in all models, having p > 0.1 as the threshold to remove a variable from the model. All p values were 2-tailed, and a value of p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, Illinois).
Results
We defined GCH1 haplotypes in 347 patients with CAD and 741 control subjects. In CAD patients, the 3 SNPs used to define the haplotypes were in linkage disequilibrium. Specifically, values of D′ and r2 were 95 and 77 for rs8007267 and rs3783641, 87 and 69 for rs3783641 and rs10483639, and 86 and 5 for rs8007267 and rs10483639, respectively. The 3 SNPs were located within 1 single haploblock (29). The numbers of 245 noncarriers (OO) and 95 heterozygous (XO) and 7 homozygous (XX) carriers of the GCH1 X haplotype was in accordance with the Hardy-Weinberg law (chi-square goodness of fit test: p = 0.81). Hardy-Weinberg equilibrium also applied to the control subjects (OO, n = 543; XO, n = 178; XX, n = 20; chi-square goodness of fit test: p = 0.51). The allelic frequencies of the GCH1 X haplotype of 15.7 and 14.7 in patients and healthy control subjects, respectively, did not differ between groups (Fisher exact test: p = 0.56). Moreover, the numbers of noncarriers and heterozygous and homozygous carriers of the GCH1 X haplotype were similar in patients and control subjects (chi-square test: p = 0.42).
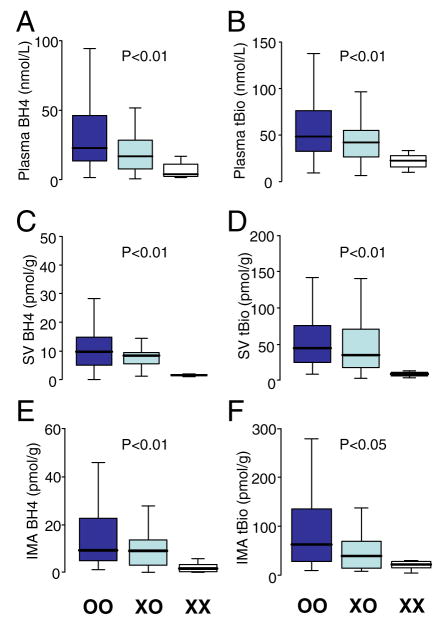
We first examined the effect of GCH1 haplotype on the levels of plasma biopterins. Patients with the X haplotype had significantly lower plasma levels of both BH4 and total biopterins than patients homozygous for the common O haplotype; median plasma BH4 levels in patients with XX genotype were reduced by approximately 80% compared with OO patients. Indeed, plasma BH4 and total biopterin levels were reduced in a striking allele-dependent manner from OO, XO, and XX genotypes (Fig. 1). Because vascular biopterin levels are likely more important for eNOS regulation, and could plausibly be differentially regulated from plasma biopterin levels, we next sought to examine the effect of GCH1 haplotype on vascular biopterins, in both SV and IMA. The presence of the X haplotype was associated with significantly lower levels of vascular BH4 and total biopterins in both SV and IMA (Fig. 1). These findings suggest a direct effect of this GCH1 haplotype on biopterin synthesis.
Figure 1. Effect of GCH1 Haplotype on Plasma/Vascular BH4.
The presence of the haplotype (XO or XX) was associated with significantly lower levels of plasma tetrahydrobiopterin (BH4) (A) and total biopterins (tBio) (B). A similar effect was also observed in saphenous veins (SV) (C and D) and internal mammary arteries (IMA) (E and F).
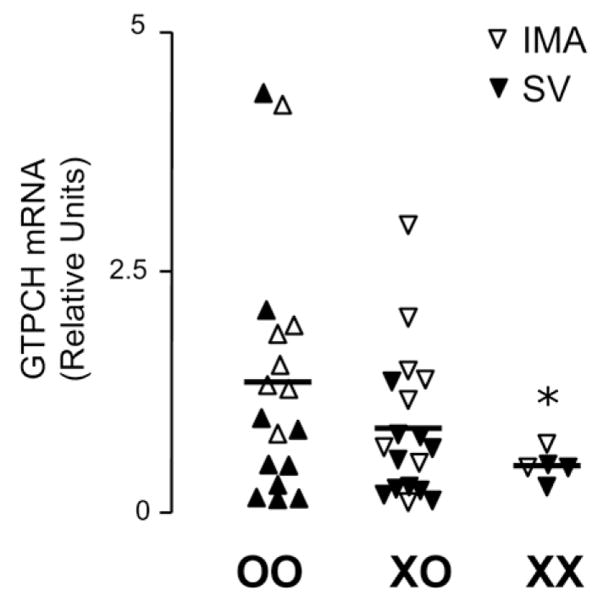
To investigate whether the effect of the GCH1 haplotype on biopterin levels was mediated by changes in GCH1 gene expression, we performed RT-PCR to quantify GCH1 messenger (m)RNA in samples of SV and IMA from patients with different GCH1 genotypes. We observed that the XX genotype was associated with significantly reduced vascular GCH1 expression compared with the OO genotype (Fig. 2). Furthermore, vascular GCH1 mRNA levels across all genotypes were significantly correlated with both plasma (r = 0.394, p = 0.010) and vascular (r = 0.336, p = 0.042) BH4 levels.
Figure 2. Effect of GCH1 Haplotype on Vascular GTPCH mRNA Levels.
The presence of the XX genotype was associated with significantly lower messenger ribonucleic acid (mRNA) levels of GTPCH compared with OO genotype. Shown are the data derived from 23 saphenous veins (SVs) and 17 internal mammary arteries (IMAs). *p < 0.05 versus OO.
We next sought to investigate how these genetically determined differences in biopterin levels might influence NO-mediated endothelial function and vascular superoxide production. Because homozygosity for the X haplotype is relatively uncommon (2% of our population), we used a recessive model, comparing patients with or without X haplotype. In support of the recessive model, patients with at least 1 X haplotype (i.e., XO or XX genotypes) had significantly lower BH4 and total biopterins than OO patients, in both plasma (16.6 [5.2 to 28.3] and 41.2 [23.4 to 54.9] nmol/l vs. 21.57 [12.1 to 45.24] and 48.3 [32.6 to 76.4] nmol/l, p <0.05 for both) and SV (7.1 [2.2 to 9.3] and 32 [22 to 93] nmol/l vs. 11.0 [5.7 to 17.8] and 43 [24 to 76] nmol/g, p < 0.05 for both).
Vascular superoxide production was significantly increased in X haplotype carriers (Fig. 3). To explore the specific contribution of eNOS coupling, we quantified the difference in vascular superoxide production after NOS inhibition by L-NAME. Incubation of vessels with L-NAME inhibited superoxide production to a significantly greater extent in X haplotype carriers than in OO homozygotes (Fig. 3). Increased oxidative stress in X haplotype carriers was also supported by the observation of lower BH4:total biopterin ratio, and increased circulating levels of oxidized LDL (Fig. 3).
Figure 3. Effect of GCH1 Haplotype on Vascular Superoxide, Oxidative Stress, and Endothelial Function.
The presence of the X haplotype was associated with higher total superoxide (O 2−) production (A) and greater L-NAME-inhibitable delta[O2−] (B) in human saphenous veins. The X haplotype was also associated with significantly lower plasma BH4:tBio ratio (C) and higher plasma levels of oxidized low-density lipoprotein (ox-LDL) (D). In addition, the X haplotype was also associated with lower maximum vasorelaxations in response to acetylcholine (ACh) (E) in saphenous veins, although it had no impact on the vasomotor responses to sodium nitroprusside (SNP) (F).
To investigate how this GCH1 haplotype and the associated differences in biopterin levels modulate NO-mediated endothelial function, we quantified the vasomotor responses of vessel segments to ACh in an organ bath system. Vasorelaxation responses to ACh were significantly reduced in X haplotype carriers than in OO homozygotes, whereas endothelium-independent vasomotor responses to the direct NO donor, SNP, were identical between genotypes (Fig. 3).
Multivariate analysis
Because BH4 levels and endothelial function may potentially be altered by many factors, we used multivariate analysis to further investigate the relationships between the GCH1 haplotype and biopterin levels, taking into account other clinical and demographic factors. Presence of the X haplotype was an independent predictor of reduced BH4 levels in both plasma and vascular tissue (plasma: β [SE] −11.764 [4.722], p = 0.014; SV: β [SE] −3.06 [1.47], p = 0.039). Furthermore, presence of the X haplotype was independently associated with increased vascular superoxide production (SV: β [SE] 1.196 [0.573], p = 0.04) and with reduced vasorelaxations to ACh (SV: β [SE] −5.304 [2.355], p = 0.026). Of the other additional demographic and clinical factors, diabetes mellitus was independently associated with increased vascular superoxide production (β [SE] 1.520 [0.619], p = 0.016) and with reduced ACh vasorelaxations (β [SE] −5.442 [2.313], p = 0.020), and hypercholesterolemia with reduced ACh vasorelaxations (β [SE] −7.703 [2.236], p = 0.001). Female gender was independently associated with reduced ACh vasorelaxations (β [SE] −7.646 [2.741], p = 0.006) and with reduced BH4 levels (β [SE] −5.24 [2.25], p = 0.022).
Discussion
In this study we have identified a striking effect of a novel haplotype of the GCH1 gene on both plasma and vascular BH4 levels in patients with CAD, mediated through altered GCH1 expression. In turn, we have shown that these genetically determined differences in BH4 availability are independent and strong predictors of key features of endothelial function, including aspects of eNOS coupling such as vascular superoxide production and NO-mediated vasorelaxation.
Tetrahydrobiopterin is an essential cofactor for eNOS function in the vascular endothelium, with increasingly recognized roles in the pathogenesis of vascular disease states through effects on eNOS coupling (1) in both the vascular wall and in circulating endothelial progenitor cells (31). However, little is known about the pathophysiologic control of endothelial BH4 levels in humans. In disease states such as atherosclerosis (32), diabetes mellitus (17,31), hypertension (12), or insulin resistance (33,34), vascular BH4 deficiency seems to be mediated, at least in part, by the increased intracellular oxidation of BH4 to BH2 and B by reactive oxygen species (such as ONOO−) (7,32). However, other studies suggest that changes in BH4 biosynthesis, through regulation of the rate-limiting enzyme GTPCH (35), may also be an important mechanism regulating vascular BH4 bioavailability (34,36). The GCH1 gene, encoding GTPCH, is expressed in several cell types, such as macrophages (37), endothelial cells (38), and others. However, it has been unclear to what extent altered GCH1 expression in the human vascular wall is related to differences in vascular BH4 levels, or to aspects of endothelial function in subjects with vascular disease. We have now used the genetic strategy of Mendelian randomization in humans to investigate the functional effects of intrinsic differences in vascular GCH1 expression.
The importance of GCH1 expression and GTPCH enzymatic activity in regulating BH4 availability is well established. Loss-of-function mutations of GCH1 cause severe BH4 deficiency, resulting in diseases such as dopa-responsive dystonia (OMIM 128230) and variant phenylketonuria (OMIM 233910), because of dysfunction of the BH4-dependent enzymes tyrosine hydroxylase and phenylalanine hydroxylase. Experimental mouse models with alterations in systemic or vascular-specific GCH1 expression (10,16) have shown that GTPCH is a key regulator of vascular BH4 levels in vivo. For example, the hph-1 mouse has an ENU mutant allele that maps to the GCH1 locus, but without mutations in the either the GCH1 coding sequence or minimal promoter (10). The hph-1 allele results in reduced GCH1 expression and reduced BH4 levels, an intermediate phenotype in heterozygote animals and functional defects in both neurotransmitter and NO synthesis (16).
We have recently shown that a specific GCH1 haplotype (whose presence we define as X and absence as O) confers significant reduction in the induction of GCH1 expression and biopterin levels in leukocytes after a forskolin or lipopolysaccharide challenge (18,19). Furthermore, this GCH1 haplotype is associated with changes in pain perception and the severity of postoperative pain, likely because of differences in GCH1 expression after nerve injury (18,19). In addition, systemic inflammation seems to be a key regulator of biopterin synthesis in patients with CAD because plasma biopterins are correlated with plasma CRP (39). We have now shown that the presence of the X haplotype is associated with lower levels of both plasma BH4 and total biopterins (which is the sum of BH4, BH2, and B, reflecting the overall biosynthetic activity of GTPCH) in patients with multivessel CAD. These patients represent a model of moderate inflammatory stimulation (mean CRP 2.2 mg/l, with range from 0.12 to 15.5 mg/l), consistent with the effect of the haplotype on the regulation of GCH1 expression in white blood cells in response to lipopolysaccharide exposure. This effect was observed not only in the uncommon XX genotype (2% of our population), but also in the X haplotype carriers (XO genotype; 27.4% of the population), suggesting that the X haplotype may be an important and common factor in regulating circulating biopterin levels in patients with CAD. Importantly, we observed that the effect of the X haplotype on plasma BH4 was paralleled by an effect on vascular BH4, and on GCH1 mRNA levels in vascular tissue. It seems most likely that the functional SNP or SNPs in the GCH1 X haplotype block will have effects on GCH1 expression, for example through effects on transcription, or on GCH1 mRNA stability and translation. However, we have no evidence that any of the SNPs used to define the GCH1 X haplotype have any direct functional effects. Indeed, the SNPs chosen to screen the GCH1 gene were not selected on the basis of possible functional importance. Further recent evidence suggests that another SNP, located in the 3′ UTR of the GCH1 gene, is also associated with reduced biopterin-dependent effects (40). Whether this SNP is part of the same or another haploblock as the X haplotype identified in our studies remains to be seen. Further studies will be required to ascertain which component SNP or SNPs confer the functional effects on GCH1 expression and how such an effect might be mediated.
Our observations suggest that GCH1 haplotype plays a potentially important role in regulating vascular BH4 bio-availability in CAD. More generally, our results highlight the central role of GTPCH, and GCH1 gene expression in particular, as key regulators of biopterin availability in humans, and in the functional responses of the vascular endothelium in vascular disease states. In the vascular endothelium, BH4 mediates coupling of oxygen reduction to heme-catalyzed L-arginine oxidation to form NO and L-citrulline (2). Therefore, BH4 deficiency is proposed to lead to eNOS uncoupling, resulting in decreased NO bioavailability and increased production of superoxide radicals from the uncoupled enzymatic form (41). A BH4 deficiency is associated with eNOS uncoupling in experimental models (17), resulting in increased superoxide production and decreased NO bioavailability in the vascular wall (41). Indirect evidence from previous clinical studies suggested that increased BH4 bioavailability is associated with improved endothelial function and eNOS coupling (5,6). However, the impact of endogenous BH4 levels on vascular function in patients with vascular disease has remained uncertain.
The identification of a GCH1 haplotype with a major effect on endogenous BH4 levels in patients with CAD provided us with a novel means to test the functional effect on BH4 in the vascular wall. Indeed, we observed that the presence of the X haplotype was associated with both higher vascular superoxide and a greater L-NAME inhibitable fraction of superoxide, representing superoxide derived from uncoupled eNOS. This finding supports the hypothesis that vascular BH4 regulates eNOS coupling in vessels from patients with CAD. The impact of the X haplotype on vascular redox state was also reflected in the systemic levels of ox-LDL and the BH4/total biopterin ratio, both systemic biomarkers of the overall oxidative stress status. To further evaluate the impact of the X haplotype on eNOS function, we examined its effect on the vasomotor responses of the same vessels to acetylcholine, observing that the X haplotype was associated with reduced vasorelaxations to acetylcholine, implying that it has a direct impact on NO bioavailability and endothelial function in these vessels. Importantly, we observed that the effects of this haplotype on vascular BH4, superoxide production, eNOS coupling, and endothelial function were independent of the presence of other risk factors for atherosclerosis, suggesting that this haplotype may prove to be an independent risk factor for atherosclerosis.
Study limitations
Some limitations of the present study need to be acknowledged. We were unable to evaluate the effect of GCH1 haplotypes in vessels from healthy individuals because these measurements require surgical material (segments of SV and IMA grafts used in CABG) that is not available from healthy individuals. We evaluated endothelial function in isolated SV segments, rather than arterial segments, because of the limited quantity of arterial tissue available from IMA grafts. However, we have used SV in the past as a reliable model system for the study of human vascular endothelium (5,6,20). Finally, our use of lucigenin-enhanced chemiluminescence provides only a measure of overall vascular superoxide generation rather than identifying the specific cell types involved.
Conclusions
In conclusion, we have shown that a novel haplotype of the GCH1 gene is a major determinant of BH4 bioavailability in both plasma and vascular endothelium in patients with CAD. Our findings show that GCH1 expression is a key regulator of vascular biopterin levels, and in turn an important determinant of vascular oxidative stress and endothelial function. Genetic differences in BH4 availability could have potentially important effects on vascular disease pathogenesis, and provide a means to test the effects of BH4 on clinical outcomes and cardiovascular risk, a question that will need to be addressed in large case-control and prospective follow-up studies.
Acknowledgments
This study was supported by the Marie Curie Intra-European Fellowship, within the 6th European Community Framework Programme (Dr. Antoniades). This work was also supported by the National Institute of Health Research Oxford Biomedical Research Centre Programme, and by grants from the British Heart Foundation (RG/02/006 to Dr. Channon, FS/03/105/16340 to Dr. Shirodaria). Drs. Costigan and Woolf were supported by the National Institutes of Health.
Abbreviations and Acronyms
- ACh
acetylcholine
- BH4
tetrahydrobiopterin
- BH2
dihydrobiopterin
- B
biopterin
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CRP
C-reactive protein
- eNOS
endothelial nitric oxide synthase
- GTPCH
GTP-cyclohydrolase I
- IMA
internal mammary artery
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- O2−
superoxide radical
- ONOO−
peroxynitrite
- Ox-LDL
oxidized low-density lipoprotein
- RT-qPCR
real-time quantitative polymerase chain reaction
- SNP
sodium nitroprusside
- SV
saphenous vein
References
- 1.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 2.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med. 2004;14:323–7. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Stroes E, Kastelein J, Cosentino F, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41– 6. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitzer T, Brockhoff C, Mayer B, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 5.Antoniades C, Shirodaria C, Warrick N, et al. 5-methyl-tetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and eNOS coupling. Circulation. 2006;114:1193–201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 6.Shirodaria C, Antoniades C, Lee J, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115:2262–70. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 7.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–54. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 8.Stroes ES, van Faassen EE, Yo M, et al. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86:1129–34. doi: 10.1161/01.res.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 9.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, Rabelink TJ. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–41. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]
- 10.Khoo JP, Nicoli T, Alp NJ, Fullerton J, Flint J, Channon KM. Congenic mapping and genotyping of the tetrahydrobiopterin-deficient hph-1 mouse. Mol Genet Metab. 2004;82:251–4. doi: 10.1016/j.ymgme.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Meininger CJ, Marinos RS, Hatakeyama K, et al. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349:353–6. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang A, Zhang YY, Chen K, Hatakeyama K, Keaney JF., Jr Cytokine-stimulated GTP cyclohydrolase I expression in endothelial cells requires coordinated activation of nuclear factor-kappaB and Stat1/Stat3. Circ Res. 2005;96:164–71. doi: 10.1161/01.RES.0000153669.24827.DF. [DOI] [PubMed] [Google Scholar]
- 14.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–50. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 15.Cosentino F, Barker JE, Brand MP, et al. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 16.Khoo JP, Zhao L, Alp NJ, et al. A pivotal role for tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–33. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 17.Alp NJ, Mussa S, Khoo J, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–35. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–77. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 19.Lotsch J, Belfer I, Kirchhof A, et al. Reliable screening for a pain-protective haplotype in the GTP cyclohydrolase 1 gene (GCH1) through the use of 3 or fewer single nucleotide polymorphisms. Clin Chem. 2007;53:1010–5. doi: 10.1373/clinchem.2006.082883. [DOI] [PubMed] [Google Scholar]
- 20.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–62. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 21.Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–77. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 22.Shi L, Norling LA, Lau AS, Krejci S, Laney AJ, Xu Y. Real time quantitative PCR as a method to evaluate simian virus 40 removal during pharmaceutical protein purification. Biologicals. 1999;27:253–62. doi: 10.1006/biol.1999.0213. [DOI] [PubMed] [Google Scholar]
- 23.Heales S, Hyland K. Determination of quinonoid dihydrobiopterin by high-performance liquid chromatography and electrochemical detection. J Chromatogr. 1989;494:77–85. doi: 10.1016/s0378-4347(00)82658-4. [DOI] [PubMed] [Google Scholar]
- 24.Skatchkov MP, Sperling D, Hink U, et al. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999;254:319–24. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- 25.Guzik TJ, West NEJ, Black E, et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:85–90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 26.Channon KM, Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol. 2002;53:515–24. [PubMed] [Google Scholar]
- 27.Lewontin RC. The interaction of selection and linkage. II Optimum models. Genetics. 1964;50:757–82. doi: 10.1093/genetics/50.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaut BS, Long AD. The lowdown on linkage disequilibrium. Plant Cell. 2003;15:1502–6. doi: 10.1105/tpc.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 30.Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28:49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
- 31.Thum T, Fraccarollo D, Schultheiss M, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–74. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 32.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki K, Nishio Y, Okamura T, et al. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res. 2000;87:566–73. doi: 10.1161/01.res.87.7.566. [DOI] [PubMed] [Google Scholar]
- 34.Shinozaki K, Hirayama A, Nishio Y, et al. Coronary endothelial dysfunction in the insulin-resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J Am Coll Cardiol. 2001;38:1821–8. doi: 10.1016/s0735-1097(01)01659-x. [DOI] [PubMed] [Google Scholar]
- 35.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Tetrahydrobiopterin-dependent formation of nitrite and nitrate in murine fibroblasts. J Exp Med. 1990;172:1599–607. doi: 10.1084/jem.172.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- 37.Werner ER, Werner-Felmayer G, Fuchs D, et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990;265:3189–92. [PubMed] [Google Scholar]
- 38.Linscheid P, Schaffner A, Blau N, Schoedon G. Regulation of 6-pyruvoyltetrahydropterin synthase activity and messenger RNA abundance in human vascular endothelial cells. Circulation. 1998;98:1703–6. doi: 10.1161/01.cir.98.17.1703. [DOI] [PubMed] [Google Scholar]
- 39.Antoniades C, Shirodaria C, Crabtree M, et al. Altered plasma vs. vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function and inflammation. Circulation. 2007;116:2851–9. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Rao F, Zhang K, et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–71. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]