Abstract
Dopamine D4 receptor (D4R) knockout mice (D4R−/−) provide for unique neurochemical studies designed to understand D4R contributions to dopamine (DA) regulation. In this study, post-mortem brain tissue content of DA did not differ between D4R+/+ and D4R−/− mice in the striatum (Str) or nucleus accumbens core (NAc). However, there was a significant decrease (82%) in the content of 3,4-dihydoxyphenylacetic acid (DOPAC), a major metabolite of DA, in the NAc of D4R−/− mice. Microdialysis studies performed in a region of brain spanning the dorsal Str and NAc showed lower baseline levels of DA and a significant reduction in KCl-evoked overflow of DA in the D4R−/− mice. Baseline extracellular levels of DOPAC and homovanillic acid were also significantly lower in the D4R−/− mice. In vivo chronoamperometric recordings of KCl-evoked release of DA also showed decreased release of DA in the Str and NAc of the D4R−/− mice. These studies demonstrate a role of D4Rs in pre-synaptic DA regulation and support the hypothesis that alterations in D4Rs may lead to diminished DA function.
Keywords: dopamine receptors, d-amphetamine, dopamine, ADHD, chronoamperometry, striatum, nucleus accumbens core
1. Introduction
ADHD is one of the most common psychiatric disorders in children and adolescents and is characterized by inattentiveness, restlessness, impulsiveness, forgetfulness, and disorganization (Solanto et al., 2001; American Psychiatric Association, 1994). Stimulant treatment for ADHD patients and current ADHD animal models (like the spontaneously hypertensive rats) have led some to theorize that there may be dopamine (DA) hypofunction in ADHD (Sagvolden et al., 2005a; Russell et al., 2005). Recently, the DA D4 receptor (D4R) has received much attention as a possible contributor to the pathophysiology of ADHD. In both human and animal studies, the D4R has been implicated in novelty seeking, hyperactivity, and impaired behavioral inhibition (Benjamin et al., 1996; Ebstein et al., 1996; Zhang et al., 2001; Falzone et al., 2002; Zhang et al., 2002; Avale et al., 2004), all characteristics of ADHD (Swanson et al., 1998). One human variant, the DRD4.7 allele, has been shown to have increased frequency in children and family members with ADHD as compared to the more common population variant of DRD4.4 (Faraone et al., 2001). Expressed DRD4.7 protein displays different signaling characteristics including a reduced affinity for DA (about half as potent) resulting in decreased activity of adenylate cyclase (Van Tol et al., 1992; Asghari et al., 1995). Another study found a higher rate of errors in transcription and decreased RNA stability that resulted in lower plasma membrane expression of the DRD4.7 (D’Souza et al., 2004). Thus, the DRD4.7 may contribute to decreased D4R surface expression and receptor function in subjects with ADHD.
It has been difficult to study the neurochemical effects of reduced D4Rs because commercially available ligands for the D4R are nonspecific (Hai-Bin et al., 2005). An alternative approach to understanding the contribution of D4Rs to aspects of ADHD is the utilization of genetically engineered mice that lack functional D4Rs, thus exhibiting no surface expression and function of the D4R, which may be somewhat analogous to the DRD4.7 in humans. Although not designed as a model for ADHD, D4R deficient mice have been shown to exhibit different locomotor and novelty seeking responses in various behavioral paradigms. D4R−/− mice exhibit low levels of exploration in novel situations (Dulawa et al., 1999). In approach/avoidance conflict paradigms, D4R−/− mice are more hesitant to travel into an open arm of a maze or lighted area in comparison to their wildtype counterparts (Falzone et al., 2002). It is thought that both anxiety and low novelty seeking are implicated in the intensity of avoidance behavior in these mice (Falzone et al., 2002). Baseline locomotor response is not altered in D4R−/− mice, but in a study by Kruzich et al. (2004), a 1 mg/kg dose of d-amphetamine caused equivalent changes in locomotion in the D4R−/− mice as compared to the D4R+/+ animals, while a 3 mg/kg dose of d-amphetamine resulted in significant increases in locomotor activity in D4R−/− mice. D4R−/− mice have also been shown to be supersensitive to ethanol and higher doses of psychomotor stimulants when compared to D4R+/+ mice (Rubinstein et al., 1997). Hyperactivity in D4R−/− animals can also be induced with a 6-hydroxydopamine lesion (Avale et al., 2004). Thus, the D4R−/− animals exhibit many behavioral changes consistent with alterations in synaptic DA function.
In these studies, we sought to directly investigate the effects of D4R deletion on regulation of DA in the Str and NAc through neurochemical studies in D4R−/− and D4R+/+ mice. First, we used high performance liquid chromatography coupled to electrochemical detection to determine brain tissue content of DA and its major metabolites in both genotypes. Secondly, we used microdialysis methods to measure baseline sample levels of DA, DA metabolites and both potassium- (KCl) and d-amphetamine-induced release of DA in the Str/NAc of these animals. Finally, in vivo chronoamperometric studies were performed to measure KCl-evoked release of DA in the Str and NAc of these animals. These studies indicate that reduction of D4Rs can result in pre-synaptic alterations in DA neurotransmission.
2. Materials and methods
2.1. Animals
Male mice (3–6 months) descended from the original F2 hybrids of DA D4R−/− mice (129/SvEv × C57BL/6J; Rubinstein et al., 1997) were derived by backcrossing the heterozygous (D4R+/−) mouse line for 10 generations (N10). In all experiments, the D4R−/− mice were compared to litter-matched D4R+/+ animals. For details concerning mutagenesis and generation of these animals, consult Rubinstein et al. (1997). Mice were group-housed (2–4 per cage) with unlimited access to food and water. Mice were maintained on a twelve hour light/dark cycle (lights on at 0600 hrs). Protocols for animal use were approved by the Institutional Animal Care and Use Committee (IACUC), which is Association for Assessment and Accreditation of Laboratory Animal Care International approved, and all procedures were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2. Whole Brain Studies of DA and DA metabolite content
The Str and NAc were removed from the brain of adult, N10, litter-matched D4R−/− and D4R+/+ mice (n=5 per group). The tissue was weighed, processed in pH 4.1 buffer, and DA and its metabolites were separated by reverse-phase high pressure liquid chromatography and measured by electrochemical detection (HPLC-EC) using dihydroxybenzylamine (DHBA) as an internal standard (Glaser et al., 2005). The DA turnover ratio was determined by the cumulative amounts of 3,4-dihydoxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) divided by the total DA content [(DOPAC+HVA)/DA]. Changes in DA turnover are indicative of changes in DA release, uptake and degradation and were described as ratios (Thiffault et al., 2000). Whole tissue content was expressed in units of ng/g wet weight of tissue.
2.3. Surgeries for Microdialysis and Chronoamperometric Recordings
Mice were anesthetized using intraperitoneal (i.p.) injections of 12.5% urethane solution (1.25 g/kg) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) fitted with a Cunningham™and Neonatal Rat Adaptor (Stoelting Co., Wood Dale, IL). A circulating heating pad (Gaymar Industries, Inc., Orchard Park, NY) coupled to a rectal temperature probe (Yellow Spring Instrument Co., Yellow Springs, OH) was used to maintain body temperature at 37°C. The skull overlying the medial cortex was removed bilaterally for recordings in Str and NAc. For chronoamperometric recordings, an additional hole, remote from the site of surgery, was drilled for the Ag/AgCl reference electrode.
2.4. Microdialysis Studies of Baseline Levels of DA and Evoked Release of DA
D4R−/− (n=4) and D4R+/+ mice (n=3) were anesthetized and prepared for surgery as described above. Prior to the experiment, CMA/11 microdialysis probes (2 mm membrane length, 0.24 mm outer diameter) were tested for probe recovery of a standard solution and showed ~10% recovery of DA, DOPAC and HVA. These pre-tested probes were lowered into the rat brain to sample both the Str and NAc (Fig. 1.; AP: +1.3, ML: ±1.5, DV: −5.0; Franklin and Paxinos, 1997). Isotonic artificial cerebral spinal fluid (aCSF; 123 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 25 mM NaHCO3, 1 mM NaH2PO4• H2O, 5.9 mM Glucose; pH 7.2–7.4) was perfused through the probes at a flow rate of 1 μl/min. Following the collection of three 20-minute baseline samples, reverse microdialysis of KCl was performed with isotonic 100 mM KCl (in an altered aCSF buffer: 26 mM NaCl, 100 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 25 mM NaHCO3, 1 mM NaH2PO4•H2O, 5.9 mM Glucose; pH 7.2–7.4) for 20 minutes, analogous to Hebert et al. (1996). After one hour of perfusion with aCSF, a 20-minute stimulation with reverse microdialysis of 25 μM d-amphetamine in aCSF was performed. The dose of d-amphetamine used in this study is supported by a similar experiment in C57BL\6J mice where 25 μM d-amphetamine was projected to produce a measurable amount of DA overflow in the nucleus accumbens (Auclair et al., 2002). Each dialysis sample (~20 μl/20 min) was analyzed using HPLC-EC. Samples were separated using a Keystone Scientific reverse phase column (zC18 Nucleosil) with a citrate/acetate mobile phase (pH 4.0 – 4.1) delivered at a flow rate of 2.0 ml/min (Stanford et al., 2001). An ESA Coulochem II detector was used with detector 1 set at +350 mV and detector 2 set at −250 mV. No attempt was made to use dialysis samples for absolute extracellular DA or DA metabolite levels. Data were analyzed by comparing D4R−/− to D4R+/+ samples to determine changes in DA levels and metabolites, baseline DA levels, and the response to KCl or d-amphetamine.
Fig. 1.
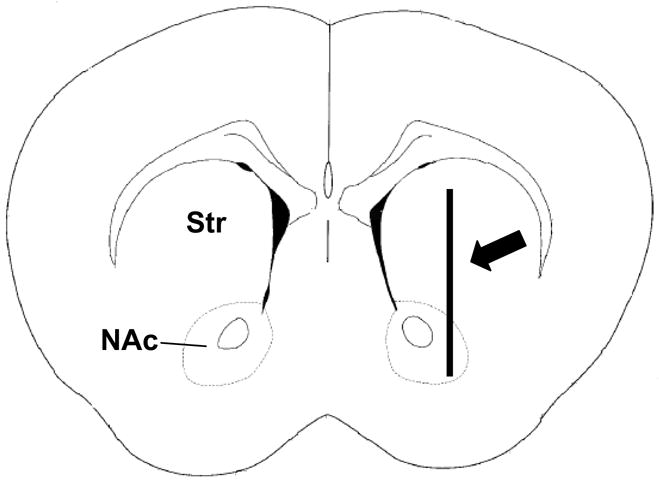
Figure showing microdialysis probe placement within the mouse Str and NAc. The 2 mm dialysis probe spanned from the dorsal Str to the NAc (modified from Franklin and Paxinos, 1997).
2.5 Chronoamperometric Recordings of KCl-Evoked Release of DA
High-speed chronoamperometric measurements were performed using the FAST-12 system (Quanteon, L.L.C., Nicholasville, KY). Briefly, square wave potentials (applied, +0.55 V; resting, 0.0 V; versus Ag/AgCl reference) were applied to Nafion-coated carbon microelectrodes for 100 ms and repeated at a frequency of 5 Hz. Resulting oxidation and reduction currents were continually recorded and averaged to 1 Hz. In all recordings, the ratio of reduction to oxidation current (redox ratio) was used to confirm the detection of DA (Gerhardt and Hoffman, 2001; Glaser et al., 2005).
2.5.1. Carbon Fiber Electrode Preparation and In Vitro Calibration
Single carbon fiber electrodes (SF1A; 30 μm o.d. X 100 μm length; Quanteon, L.L.C., Lexington, KY) were used for recordings. They were coated with Nafion® prior to in vivo studies (Aldrich Chemical Co., Milwaukee, WI) and were calibrated in vitro to determine selectivity, sensitivity, limit of detection, and redox ratio (Gerhardt et al., 1984; Gerhardt and Hoffman, 2001). The selectivity for DA of all electrodes used in these experiments was ≥450:1 for DA versus DOPAC. The detection limit for the measurements of DA averaged 38.0 ± 4.7 nM. The average redox ratio was 0.720 ± 0.03 (mean ± SEM), which is indicative for DA (Gerhardt et al., 1984; Hebert and Gerhardt 1998). The calibrated single carbon fiber electrode was then affixed to a micropipette (12–15 μm o.d.) positioned 230–260 μm from the carbon fiber electrode tip using Kerr Brand sticky wax. The micropipette was filled with filtered isotonic KCl solution (120 mM KCl, 29 mM NaCl, 2.5 mM CaCl2•2H2O) using a 4 inch pulled needle and 1 cc syringe. The pH was adjusted to 7.2–7.4.
2.5.2. In vivo Experimental Protocol
D4R+/+ and D4R−/− (n=4, 3–6 months old) mice were anesthetized and prepared according to the surgical procedures described above (section 2.3.). The electrode–micropipette assembly was positioned into the brain according to the following stereotaxic coordinates where all anterior–posterior (AP) measures were from bregma, medial–lateral (ML) measures were from midline and dorsal–ventral (DV) measures were from dura: +1.2 mm AP; +/−1.2 mm ML; +2.5–+4.5 mm DV (Franklin and Paxinos, 1997).
2.5.3. KCl-Induced Release of DA
KCl was locally applied by pressure ejection (5–25 psi for 1 s) into the Str and NAc. The volume of KCl delivered (10–150 nl) was measured by determining the amount of fluid ejected from the micropipette using a dissection microscope fitted with an eyepiece reticule that was calibrated so that 1 mm of movement was equivalent to ~250 nl of fluid ejected (Cass et al., 1992; Friedemann and Gerhardt, 1992). Experiments were initiated with the insertion of a micropipette-microelectrode assembly into a stereotactically selected region of the Str or NAc. Once a steady-state signal was achieved, the effects of a single local application of KCl solution on DA release and uptake kinetics were determined (Gerhardt et al., 1985; Gerhardt et al., 1986; Gerhardt et al., 1987; Gerhardt and Palmer, 1987; Luthman et al., 1993; Cass et al., 1993a). Data regarding chronoamperometric recordings were volume matched between 75 and 150 nl prior to data analysis to investigate the same amount of KCl stimulation in the D4R−/− and D4R+/+ animals. Parameters included for analysis were amplitude, amplitude/nanoliter, rise time (Tr), time of 80% decay (T80), and uptake rate (the amount of DA cleared per second). DA uptake data were fit to a first order decay rate, which is calculated by taking the slope (following maximum amplitude) of the linear regression of the natural log of the recorded data over time (this is the rate constant and is referred to as k−1; units = seconds−1) and by multiplying the rate constant by the maximum DA amplitude (uptake rate = μM/s; Hascup et al., 2006).
2.6. Drugs
Urethane and d-amphetamine sulfate were obtained from Sigma (St. Louis, MO). Dopamine, HVA, and DOPAC were obtained from Aldrich (Milwaukee, WI).
2.7. Histology
Brains were removed and processed for histological evaluation of microelectrode recording tracts. Only data from histologically confirmed placements of microelectrodes into the Str and NAc were used for final data analysis. No data recordings were rejected in these studies based on poor stereotactic placement of the microelectrodes or microdialysis probes.
2.8. Data Analysis
For whole tissue content studies, data were analyzed using an unpaired, 2-tailed Student’s t-test. An ANOVA with Bonferroni’s post hoc tests was used to analyze the reverse microdialysis studies of both KCl- and d-amphetamine-evoked DA overflow in the D4R−/− and D4R+/+ animals. Baseline levels of DA, DOPAC, and HVA between genotypes at individual points were analyzed an unpaired, 2-tailed, Student’s t-test. Electrochemical measures of the amplitudes of KCl-evoked DA release signals, rise times (Tr), 80% decay times (T80), and rate of DA uptake were analyzed using an unpaired, 2-tailed Student’s t-tests. In all experiments, statistical significance was defined as p<0.05.
3. Results
3.1. Whole Tissue Content of DA and DA Metabolites in D4R−/− and D4R+/+ mice
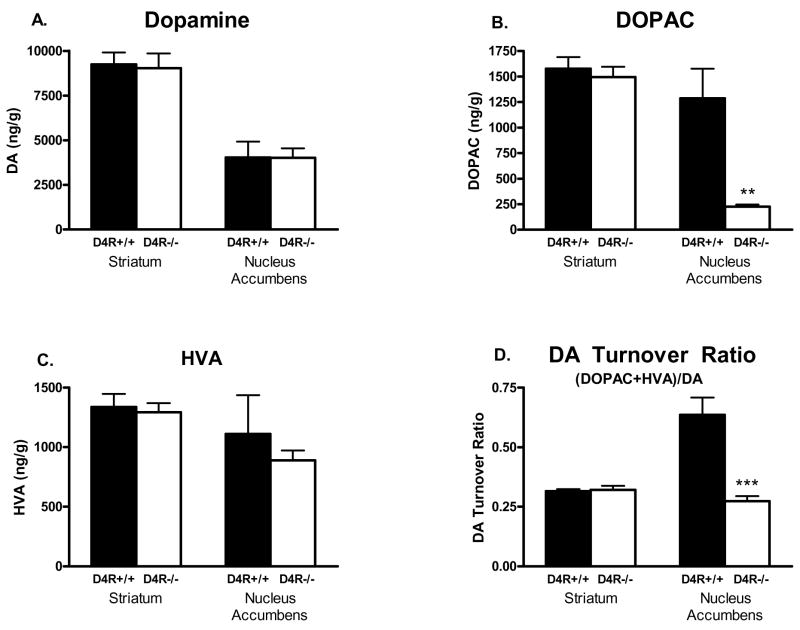
Whole brain tissue analysis was used to establish if the total tissue content of DA and DA metabolites in the Str and NAc were changed as a result of D4R deletion. Whole tissue DA content was very similar between genotypes (see Fig. 2) in the Str (D4R+/+ 9248 ± 662 ng/g vs. D4R−/− 9032 ± 820 ng/g; n=5 animals per group) and the NAc (D4R+/+ 4028 ± 894 ng/g vs. D4R−/− 4009 ± 536 ng/g). In addition, the whole tissue content of the major DA metabolite, DOPAC, was similar between genotypes in the Str (D4R+/+ 1577 ± 114 ng/g vs. D4R−/− 1495 ± 101 ng/g). However, of particular note was a dramatic 82% reduction seen in DOPAC tissue content in the NAc of the D4R−/− mice (D4R−/− 226 ± 22 ng/g vs. D4R+/+ 1,286 ± 292 ng/g; p<0.001). By contrast whole tissue content of the DA metabolite, HVA, was similar between genotypes in both the Str (D4R+/+ 1337 ± 110 ng/g vs. D4R−/− 1293 ± 78 ng/g) and NAc (D4R+/+ 1110 ± 326 ng/g vs. D4R−/− 889 ± 83 ng/g). The DA turnover ratios, defined as [(DOPAC + HVA)/DA], were significantly lower in the NAc of D4R−/− mice in comparison to D4R+/+ mice (D4R−/− 0.27 ± 0.02 vs. D4R+/+ 0.64 ± 0.07; p<0.05; see Fig. 2), which was likely due to the lower DOPAC content seen in the NAc of the D4R−/− mice. Thus, analysis of DA and DA metabolites showed no differences in Str but a significant 82% decrease of DOPAC in the NAc of D4R−/− mice.
Fig. 2.
Bar graphs showing whole tissue content of DA and DA metabolites in the Str and NAc of D4R−/− and D4R+/+ mice. DA content in the Str and NAc was unchanged, but DOPAC content was greatly decreased in the NAc of D4R−/− mice (**p<0.01; *** p<0.0001). Data are expressed in ng/gram wet weight of tissue. The error bars represent S.E.M.
3.2. Microdialysis studies of DA Release in Str and NAc of D4R−/− and D4R+/+ mice
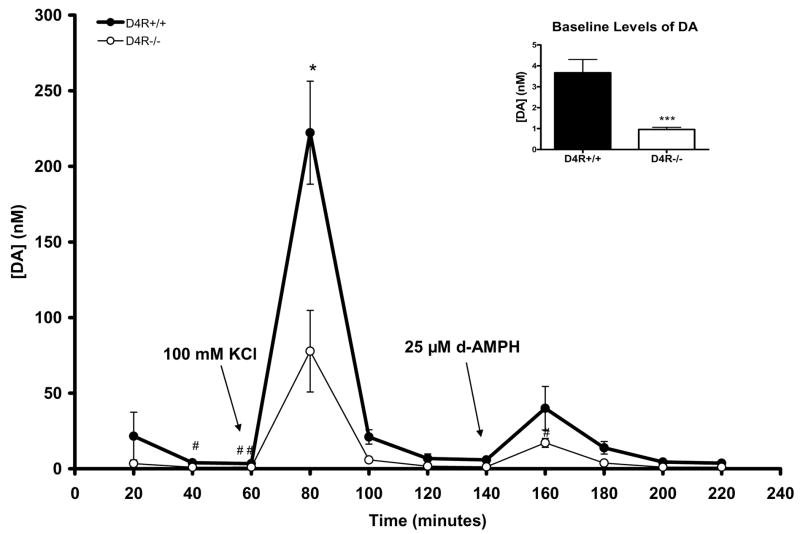
Using intracranial microdialysis, we compared baseline levels of DA and DA metabolites, and stimulus-evoked overflow of DA in the Str/NAc of D4R+/+ and D4R−/− mice. Of particular note in these studies is that the data represent measures with the dialysis tip of the probe spanning from the dorsal Str to the NAc (Str/NAc; see Fig. 1). Baseline levels of DA were significantly lower by ~74% in D4R−/− mice in comparison to the D4R+/+ animals (D4R−/− 0.96 ± 0.11 nM vs. D4R+/+ 3.67 ± 0.64 nM; p<0.001) (see also inset of Fig. 3). Reverse microdialysis stimulation with KCl caused a significant increase in DA levels in both groups (p<0.001). However, evoked release of DA by KCl in the Str/NAc was significantly lower by ~65% in D4R−/− mice compared to the D4R+/+ mice (D4R−/− 77.7 ± 27.0 nM vs. D4R+/+ 222.2 ± 34.0 nM p<0.05; See Fig. 3). Reverse microdialysis of aCSF with 25 μM d-amphetamine (d-AMPH) provided results that were inconclusive. Although d-AMPH caused significant DA overflow in the D4R−/− mice (n=4; p<0.01), the overflow was not significant in the D4R+/+ mice (n=3; p=0.07). There was no difference in DA overflow between D4R+/+ and D4R−/− following stimulation with 25 μM d-AMPH (Fig. 3), despite a trend for increased release in the D4R+/+ mice. The lack of conclusive results may reflect the small sample size, large variability, or need for increased concentrations of d-amphetamine. Taken together, the data support large reductions in basal DA and KCl-evoked DA in the D4R−/− mice.
Fig. 3.
Graph showing effects of reverse microdialysis with KCl or d-AMPH stimulation on extracellular levels of DA in the Str/NAc of D4R−/− and D4R+/+ mice. KCl-evoked DA release was significantly decreased in the D4R−/− mice in comparison to D4R+/+ animals. At individual time points of 40 and 60 minutes, baseline DA levels were significantly lower in the D4R−/− mice (#p<0.05 and ##p<0.01); the inset shows that the combined baseline DA levels in the D4R−/− mice are significantly lower than those in the D4R+/+ mice (*p< 0.05, ***p< 0.001). The error bars represent S.E.M.
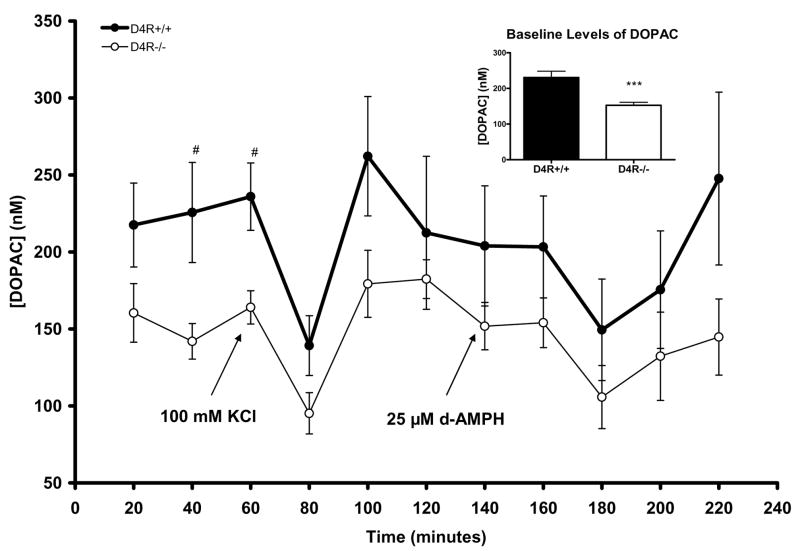
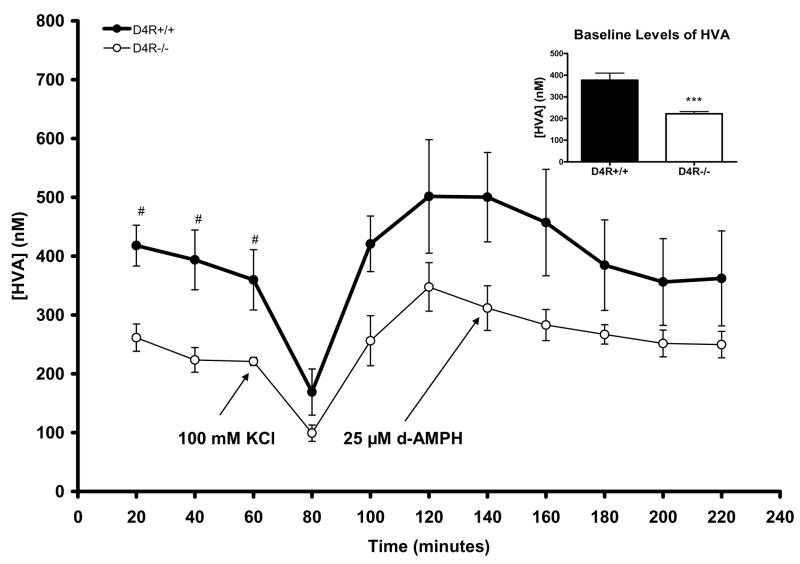
Aside from DA, we also studied the major extracellular metabolites of DA in the Str/NAc by microdialysis. Baseline extracellular levels of DOPAC were significantly lower in the D4R−/− mice by ~34% compared to levels measured in D4R+/+ mice (D4R−/− 153.0 ± 8.4 nM vs. D4R+/+ 230.8 ± 17.7 nM; p<0.001; see inset to Fig. 4). Specifically, baseline DOPAC levels were lower in D4R−/− mice at the individual time marks of 40 and 60 minutes (p<0.05 for both; Fig. 4). DOPAC levels significantly decreased from baseline in both genotypes after application of KCl but not d-AMPH (D4R+/+ p<.05; D4R−/− p<0.01) (Fig. 4). Extracellular levels of DOPAC after KCl or d-AMPH stimulation were not significantly different between the genotypes. The D4R−/− mice also showed ~40% lower baseline sample levels of HVA (D4R−/− 222.3 ± 10.2 nM vs. D4R+/+ 376.7 ± 33.1 nM; p<0.001; see Fig. 5), while changes of HVA measures due to KCl- or d-AMPH applications did not differ between the genotypes (Fig. 5). Thus, baseline levels of both DOPAC and HVA were significantly lower in the D4R−/− animals.
Fig. 4.
Graph showing extracellular measures of DOPAC resulting from reverse microdialysis with KCl or d-AMPH stimulation in the Str/NAc of D4R−/− and D4R+/+ mice. Although DOPAC was significantly decreased in both genotypes as a result of KCl treatment, there were no significant differences in the amount of DOPAC reduction between genotypes. At individual time points of 40 and 60 minutes, baseline levels of DOPAC were significantly lower in D4R−/− mice (#p<0.05); the inset shows that combined baseline DOPAC levels were significantly lower in the D4R−/− mice (*p< 0.05, ***p< 0.001). The error bars represent S.E.M.
Fig. 5.
Graph showing extracellular measures of HVA resulting from reverse microdialysis with KCl or d-AMPH stimulation in the Str/NAc of D4R−/− and D4R+/+ mice. Although HVA significantly decreased in both genotypes as a result of KCl treatment, there were no significant differences in the amount of HVA reduction between genotypes. At the individual time points of 20, 40, and 60 minutes, baseline levels of HVA were significantly lower in D4R−/− mice (#p<0.05); the inset shows that combined baseline HVA levels were significantly lower in the D4R−/− mice (***p< 0.001). The error bars represent S.E.M.
3.3. In Vivo Chronoamperometric Measures of DA release in D4R−/− and D4R+/+ mice
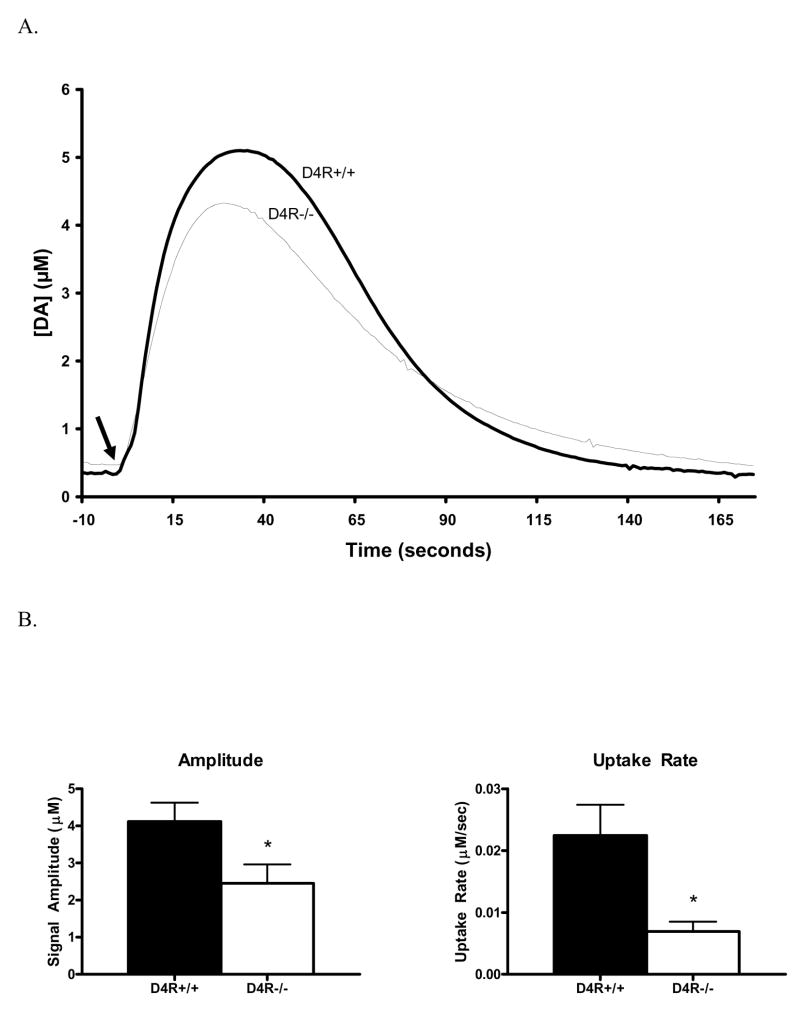
We used high-speed chronoamperometry to study second-by-second release of DA in the Str and NAc of the D4R−/− and D4R+/+ mice. This technology has excellent time resolution, high sensitivity, and good selectivity in order to efficiently measure potential changes in DA release kinetics as a result of D4R deletion. Local application of KCl produced robust DA overflow in the Str and NAc of D4R−/− and D4R+/+ mice. However, the D4R−/− mice were seen to release ~38% less DA than the D4R+/+ mice (D4R−/− 2.58 ± 0.51 μM vs. D4R+/+ 4.17 ± 0.50 μM; n=4 animals per group; p< 0.05; Fig. 6B). Traces of KCl-evoked DA signals are shown in Figure 6A. The average amount of DA released per nanoliter of KCl applied was also significantly reduced by ~43% in the D4R−/− animals (D4R−/− 0.024 ± 0.0050 μM DA/nl KCl vs. D4R+/+ 0.042 ± 0.0055 μM DA/nl KCl; p< 0.05). These data are consistent with microdialysis data demonstrating a lower KCl-evoked DA overflow. The kinetic parameters of the KCl-evoked DA signals were also compared between the two genotypes. The rise time (D4R+/+ 25.0 ± 2.0 s vs. D4R−/− 22.5 ± 2.5 s) and 80% decay time (D4R+/+ 68.4 ± 4.2 s vs. D4R−/− 67.7 ± 5.3 s; respectively) of the signals were both unchanged. However, the uptake rate was significantly slower in D4R−/− mice (D4R−/− 0.006 ± 0.002 μM/s vs. D4R+/+ 0.022 ± 0.005 μM/s; p<0.05) (Fig. 6B). Previous studies suggest this observed difference in uptake may be due in part to the lower amplitude of the D4R−/− signals, but changes in DAT function and number are also a possibility (Hebert et al., 1996; Zahniser et al., 1999). Taken together, the chronoamperometric studies support that KCl-evoked release of DA in the Str and NAc are significantly reduced in the D4R−/− mice.
Fig. 6.
Graphs showing KCl-evoked DA release measured by in vivo chronoamperometric recordings in the Str and NAc of D4R−/− and D4R+/+ mice. A) Graph A shows traces of KCl-evoked DA release in the D4R−/− mice compared with D4R+/+ animals. KCl was locally applied by pressure ejection at the arrow to induce DA release. B) The bar graph in B (left side) shows volume matched KCl-evoked DA release data from the Str and NAc of D4R−/− and D4R+/+ mice. A significantly lower amplitude of DA release was seen to be produced by the same volume of KCl solution in the D4R−/− mice as compared to the D4R+/+ mice (*p<0.05). The bar graph in B (right side) shows that there was a significant decrease in the rate of DA uptake in the D4R−/− mice (*p<0.05). The error bars represent S.E.M.
4. Discussion
In these studies we tested the hypothesis that the D4R is involved in DA regulation by comparing measures of DA neurotransmission in D4R−/− and litter-matched D4R+/+ animals. Whole tissue content of DA and DA metabolites indicated that DA was unchanged, yet DOPAC content and the DA turnover ratios were significantly lower in the NAc of D4R−/− mice. Microdialysis studies showed significantly decreased baseline levels of DA, DOPAC and HVA as well as lower levels of KCl-evoked DA release in the Str/Nac of D4R−/− animals. Chronoamperometric measures also showed a similar decrease in KCl-evoked DA release in the Str and NAc. Taken together, these data support a role for D4Rs in the regulation of basal DA levels, DA metabolism and release of DA in the ventral striatum and NAc.
In the analysis of the brain tissue samples we saw ~82% lower content of DOPAC only in the NAc of D4R−/− mice as compared to D4R+/+ animals. By contrast, these microdialysis studies showed a ~32% decrease in baseline DOPAC levels in the D4R−/− mice. It is also interesting that we saw ~ 40% lower baseline HVA level in D4R−/− mice, while the whole tissue content showed no significant differences in HVA. The apparent discrepancy between the microdialysis data and the whole tissue content data is most likely due to the fact that the microdialysis probe spanned between Str and the NAc (see Fig. 1). Also, the microdialysis studies only sample extracellular levels while the tissue studies measure both intracellular and extracellular compartments. It should also be noted that we used an anesthetic during microdialysis studies and no anesthesia was used prior to whole tissue collection. Although DA levels are not as likely to be affected by urethane anesthesia (Sabeti et al., 2003), regulatory neurotransmitters like glutamate are reduced (Rutherford et al., 2006), which may affect the regulation of DA metabolism. These discrepancies are possible explanations for why we saw differences in our tissue studies in comparison to microdialysis measures. However, the tissue data support that DA regulation may be most affected in the ventral Str and NAc of D4R−/− mice.
The microdialysis data indicated significantly lower baseline levels of DA (and DA metabolites) as well as lower KCl-evoked release of DA. These data were collected in the Str/NAc. The current developmental theories converge on the hypothesis surrounding hypofuctionality of DA in the etiology of ADHD (Sagvolden et al., 1998; Sagvolden et al., 2005b; Grace 2001; Solanto et al., 2001; Costellanos et al., 2002; Biedermann and Faraone 2002). Hypofunction of DA in the limbic circuit is believed to affect ADHD behavior by decreasing reinforcement and extinction behaviors resulting in ADHD characteristics like impulsiveness, hyperactivity in novel situations, inattentiveness and failure to inhibit response (Sagvolden et al., 2005b). Furthermore, DA hypofunction in the nigrostriatal pathway is hypothesized to result in alterations in coordination and learning habits (Sagvolden et al., 2005b). Our data indicate that dysfunction in the D4Rs could contribute to this hypofunctional DA scenario. However, the mechanism(s) by which the D4R could regulate these states is not understood and needs to be more fully investigated.
In the chronoamperometric studies we saw a significant reduction in the amplitude of KCl-evoked DA release in the D4R−/− mice, which complemented the microdialysis findings. In addition, we saw a decrease in DA uptake rate. Take together, these data could also be explained as an increase in the surface expression of the DA transporter (DAT) on the surface of DA nerve fibers (Cass et al., 1993b). However, if DATs were increased in surface expression, we may expect to see similar or greater amounts of DA metabolites in our microdialysis studies. Extracellular levels of both DOPAC and HVA were significantly decreased in the D4R−/− mice supporting the hypothesis that these levels are related to lower DA release in the D4R−/− animals. Take together, these data support the hypothesis that DAT is not likely affected by D4R deletion but further studies are needed to determine surface expression and whole tissue protein levels of DAT in these animals.
The changes in DA release patterns measured in these experiments indicate alterations in presynaptic DA regulation. Although there is support for the presynaptic localization of D4R in the Str and NAc of glutamate nerve endings, very little data support that presynaptic D4Rs are located on DA projections from the ventral tegmental area or substantia nigra pars compacta (Svingos et al., 2000; Berger et al., 2001; Rubinstein et al., 2001; Rivera et al., 2002). Possible explanations for D4R deletion affecting a presynaptic mechanism is that retrograde transport of either arachidonic acid (AA) or nitric oxide (NO) may be affected, which can cause changes in DA neurotransmission and metabolism. Arachidonic acid is modulated by D4Rs and has been shown to change presynaptic DA release (in CHO cell lines) (Chio et al., 1994; Nilsson et al., 1998; L’hirondel et al., 1999). Deletion of D4R would result in less AA release. West, Galloway and Grace (2002) have shown that NO signaling pathways can also modulate DA at the level of the terminal in a retrograde fashion and is thought to be involved in basal ganglia regulation, although how D4R deletion would affect NO transport is unknown. Thus, alterations in the presynaptic regulation of DA may be related to alterations in postsynaptic second messengers, which need to be investigated in these animals.
The D4R has been localized to postsynaptic neurons in the prefrontal cortex, ventral pallidum, Str, nucleus accumbens, thalamus, amygdala, and cerebellum (mice only) (Noain et al., 2006; Cooper et al., 2003; Mrzljak et al., 1996; Ariano et al., 1997; Tarazi et al., 2004; Gan et al., 2004). Of relevance to our current data is the presence of D4R on glutamatergic neurons in the PFC that project to the ventral Str and NAc and thus may mediate the firing of GABAergic medium spiny neurons (MSNs) (Mrzljak et al., 1996). MSNs project to the globus pallidus (GP) and then to the thalamus, thus regulating excitatory feedback on pyramidal cells in the PFC (Noain et al., 2006; Mrzljak et al., 1996). Alterations in Glu firing may have presynaptic effects on DA neurotransmission and postsynaptic effects on medium spiny neuron (MSN) firing. Further studies are needed to determine the resulting effects of the loss of D4R function on neuronal circuitry in these animals.
One caveat of transgenic mice is that compensatory effects on non-DA systems may be contributing to the neurochemical effects that we measured. The D4R−/− mice have been found to have increased expression of DA D1 receptors (D1R) (+42%) and NMDA receptors (+40%) within the Str. Likewise, increased D1R (+39%) and NMDA (+31%) receptors in the NAc were also seen in D4R−/− mice in comparison to D4R+/+ animals. There were no changes in the amounts of DA D2 receptors (D2R), AMPA, or kainate receptors between genotypes (Gan et al., 2004). By contrast, another study showed that D4R−/− mice expressed a 9-fold increase of D2Rs in the high affinity state in comparison to D2Rs in the low affinity state while the D2R protein levels remained similar in both genotypes (Seeman et al., 2005). It has been shown that high affinity D2Rs tend to be autoreceptors (Cooper et al., 2003). The compensatory effects on DA neurotransmission resulting from increased levels of D1, NMDA, and D2-high affinity receptors could be contributing to the findings reported in this paper and need to be further investigated.
In summary, we have investigated DA neurotransmission in mice genetically altered to lack D4R expression. We have demonstrated decreases in baseline levels of DA, DOPAC and HVA, KCl-evoked DA release, but no alterations in whole tissue content of DA. However, a highly significant reduction in DA metabolism, through reductions in DOPAC was observed in the NAc. These studies support the role of D4Rs in both pre- and post-synaptic DA regulation and suggest that alterations in D4Rs may lead to diminished DA function.
Acknowledgments
These studies were supported by USPHS grants DA07262, DA12062, DA017186, MH066393, and MH067497.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–65. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res. 1997;752(1–2):26–34. doi: 10.1016/s0006-8993(96)01422-9. [DOI] [PubMed] [Google Scholar]
- Auclair A, Cotecchia S, Glowinski J, Tassin JP. D-amphetamine fails to increase extracellular dopamine levels in mice lacking alpha 1b-adrenergic receptors: relationship between functional and nonfunctional dopamine release. J Neurosci. 2002;22(21):9150–4. doi: 10.1523/JNEUROSCI.22-21-09150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:718–26. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Current concepts on the neurobiology of Attention-Deficit/Hyperactivity Disorder. J Atten Disord. 2002;6(Suppl 1):S7–16. doi: 10.1177/070674370200601s03. Review. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet. 1996;12:81–4. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Berger MA, Defagot MC, Villar MJ, Antonelli MC. D4 dopamine and metabotropic glutamate receptors in cerebral cortex and striatum in rat brain. Neurochem Res. 2001;26(4):345–52. doi: 10.1023/a:1010990812840. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. Reduced clearance of exogenous dopamine in rat nucleus accumbens, but not in dorsal striatum, following cocaine challenge in rats withdrawn from repeated cocaine administration. J Neurochem. 1993a;61:273–83. doi: 10.1111/j.1471-4159.1993.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Zahniser NR, Flach KA, Gerhardt GA. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J Neurochem. 1993b;61:2269–78. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem. 1992;59:259–66. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chio CL, Drong RF, Riley DT, Gill GS, Slightom JL, Huff RM. D4 dopamine receptor-mediated signaling events determined in transfected Chinese hamster ovary cells. J Biol Chem. 1994;269(16):11813–9. [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 8. Oxford University Press; New York: 2003. pp. 225–70. [Google Scholar]
- D’Souza UM, Russ C, Tahir E, Mill J, McGuffin P, Asherson PJ, Craig IW. Functional effects of a tandem duplication polymorphism in the 5’lanking region of the DRD4 gene. Biol Psychiatry. 2004;56(9):691–7. doi: 10.1016/j.biopsych.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–56. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Falzone TL, Gelman DM, Young JI, Grandy DK, Low MJ, Rubinstein M. Absence of dopamine D4 receptors results in enhanced reactivity to unconditioned, but not conditioned, fear. Eur J Neurosci. 2002;15:158–64. doi: 10.1046/j.0953-816x.2001.01842.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1052–57. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Gan L, Falzone TL, Zhang K, Rubinstein M, Baldessarini RJ, Tarazi FI. Enhanced expression of dopamine D(1) and glutamate NMDA receptors in dopamine D(4) receptor knockout mice. J Mol Neurosci. 2004;22(3):167–78. doi: 10.1385/JMN:22:3:167. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Hoffman AF. of recording media composition on the responses of Nafion®-coated carbon fiber microelectrodes measured using high-speed chronoamperometry. J Neurosci Methods. 2001;109:13–21. doi: 10.1016/s0165-0270(01)00396-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Palmer MR. Characterization of the techniques of pressure ejection and microiontophoresis using in vivo electrochemistry. J Neurosci Methods. 1987;22:147–59. doi: 10.1016/0165-0270(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Rose GM, Hoffer BJ. In vivo electrochemical demonstration of potassium-evoked monoamine release from rat cerebellum. Brain Res. 1987;413:327–35. doi: 10.1016/0006-8993(87)91024-9. [DOI] [PubMed] [Google Scholar]
- Gerhardt GrA, Rose GM, Hoffer BJ. Release of monoamines from striatum of rat and mouse evoked by local application of potassium: evaluation of a new in vivo electrochemical technique. J Neurochem. 1986;46:842–50. doi: 10.1111/j.1471-4159.1986.tb13048.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt G, Rose G, Stromberg I, Conboy G, Olson L, Jonsson G, Hoffer B. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse: an in vivo electrochemical study. J Pharmacol Exp Ther. 1985;235:259–65. [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. Nafion®-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984;290:390–5. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- Glaser PE, Thomas TC, Joyce BM, Castellanos FX, Gerhardt GA. Differential effects of amphetamine isomers on dopamine release in the rat striatum and nucleus accumbens core. Psychopharmacology (Berl) 2005;178(2–3):250–8. doi: 10.1007/s00213-004-2012-6. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant actions on dopamine and limbic srystem function: Relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant drugs and ADHD: Basic and clinical neuroscience. Vol. 5. Oxford University Press; New York: 2001. pp. 134–157. [Google Scholar]
- Hai-Bin T, Duan-Zhi Y, Lan Z, Li-Hua W, Chun-Fu Z, Ming-Wei W, Chun-Ying W, Yong-Xian W. Dopamine D(4) receptor antagonist 3-(4-[(18)F]fluorobenzyl)-8-methoxy-1,2,3,4-tetrahydrochromeno[3,4-c]pyridin-5-one([(18)F]FMTP): radiosynthesis and in vivo characterization in rats. Appl Radiat Isot. 2005;63(3):333–42. doi: 10.1016/j.apradiso.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister JJ, Gerhardt GA. Second-by-second measures of l-glutamate and other neurotransmitters using microelectrode arrays. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Vol. 19. CRC Press; FL: 2006. pp. 407–50. [Google Scholar]
- Hebert MA, van Horne CG, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279(3):1181–90. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Research. 1998;1(797):42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Suchland KL, Grandy DK. Dopamine D4 receptor-deficient mice, congenic on the C57BL/6J background, are hypersensitive to amphetamine. Synapse. 2004;53:131–39. doi: 10.1002/syn.20043. [DOI] [PubMed] [Google Scholar]
- L’hirondel M, Cheramy A, Artaud F, Godeheu G, Glowinski J. Contribution of endogenously formed arachidonic acid in the presynaptic facilitatory effects of NMDA and carbachol on dopamine release in the mouse striatum. Eur J Neurosci. 1999;11(4):1292–300. doi: 10.1046/j.1460-9568.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- Luthman J, Friedemann MN, Hoffer BJ, Gerrhardt GA. In vivo electrochemical measurements of exogenous dopamine clearance in normal and neonatal 6-hydroxydopamine-treated rat striatum. Exp Neurol. 1993;122:273–82. doi: 10.1006/exnr.1993.1127. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381(6579):245–8. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M, Rubinstein M. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci. 2006;24(9):2429–38. doi: 10.1111/j.1460-9568.2006.05148.x. [DOI] [PubMed] [Google Scholar]
- Nilsson CL, Hellstrand M, Ekman A, Eriksson E. Direct dopamine D2-receptor-mediated modulation of arachidonic acid release in transfected CHO cells withorut the concomitant administration of a Ca2+-mobilizing agent. Br J Pharmacol. 1998;124:1651–58. doi: 10.1038/sj.bjp.0702025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Cuellar B, Giron FJ, Grandy DK, de la CA, Moratalla R. Dopamine D4 receptors are heterogeneously distributed in the striosomes/matrix compartments of the striatum. J Neurochem. 2002;80:219–29. doi: 10.1046/j.0022-3042.2001.00702.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Cepeda C, Hurst RS, Flores-Hernandez J, Ariano MA, Falzone TL, Kozell LB, Meshul CK, Bunzow JR, Low MJ, Levine MS, Grandy DK. Dopamine D4 receptor-deficient mice display cortical hyperexcitability. J Neurosci. 2001;21:3756–63. doi: 10.1523/JNEUROSCI.21-11-03756.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;15:1–9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Johnson KW, Gerhardt GA. Second-by-second enzyme-based microelectrode recordings of basal L-glutamate in the prefrontal cortex of awake rats. In: Di Chiara G, Carboni E, Valentini V, Acquas E, Bassareo V, Candoni C, editors. Monitoring Molecules in Neuroscience: 11th International Conference on In Vivo Methods; Villasimius-Cagliari, Italy. May, 2006; pp. 334–336. [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Chloral hydrate and ethanol, but not urethane, alter the clearance of exogenous dopamine recorded by chronoamperometry in striatum of unrestrained rats. Neurosci Lett. 2003;343(1):9–12. doi: 10.1016/s0304-3940(03)00301-x. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger DF. Altered reinforcement mechanisms in Attention-Deficit/Hyperactivity Disorder. Behav Brain Res. 1998;94:61–71. [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005a;57(11):1239–47. doi: 10.1016/j.biopsych.2005.02.002. Review. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005b;28(3):397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A. 2005;102(9):3513–8. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Arnsten AFT, Castellanos FX. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press; New York: 2001. [Google Scholar]
- Stanford JA, Currier TD, Purdom MS, Gerhardt GA. Nomifensine reveals age-related changes in K(+)-evoked striatal DA overflow in F344 rats. Neurobiol Aging. 2001;22(3):495–502. doi: 10.1016/s0197-4580(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Periasamy S, Pickel VM. Presynaptic dopamine D4 receptor localization in the rat nucleus accumbens shell. Synapse. 2000;36:222–32. doi: 10.1002/(SICI)1098-2396(20000601)36:3<222::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Swanson J, Castellanos FX, Murias M, LaHoste G, Kennedy J. Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Curr Opin Neurobiol. 1998;8:263–71. doi: 10.1016/s0959-4388(98)80150-5. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Dopamine D4 receptors: beyond schizophrenia. J Recept Signal Transduct Res. 2004;24(3):131–47. doi: 10.1081/rrs-200032076. Review. [DOI] [PubMed] [Google Scholar]
- Thiffault CJ, Langston W, Di Monte DA. striatal dopamine turnover following acute administration of rotenone to mice. Brain Research. 2000;885(2):283–88. doi: 10.1016/s0006-8993(00)02960-7. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–52. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- West AR, Galloway MP, Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse. 2002;44(4):227–45. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289(1):266–77. [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Baldessarini RJ. Role of dopamine D(4) receptors in motor hyperactivity induced by neonatal 6-hydroxydopamine lesions in rats. Neuropsychopharmacology. 2001;25:624–32. doi: 10.1016/S0893-133X(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Davids E, Baldessarini RJ. Plasticity of dopamine D4 receptors in rat forebrain: temporal association with motor hyperactivity following neonatal 6-hydroxydopamine lesioning. Neuropsychopharmacology. 2002;26:625–33. doi: 10.1016/S0893-133X(01)00404-3. [DOI] [PubMed] [Google Scholar]