Abstract
In this report we describe the development of a standardized three-dimensional (3D) system of the human oral mucosa based on an immortalized human oral keratinocyte cell line (OKF6/TERT-2). The procedure takes approximately 2−3 weeks to complete and includes three main stages: preparation of collagen-embedded fibroblasts, addition of the mucosal component and airlifting of cultures to ensure adequate differentiation/stratification. This procedure results in a multilayer epithelial structure in which layers are organized similarly to the cells in native oral mucosa. Specifically, this model system consists of a stratum basale, having one layer of columnar to round cells, a relatively flattened stratum spinosum and stratum granulosum, and a non-keratinizing stratum corneum. This 3D system resembles the commercially available system based on the cell line TR146 (SkinEthic), with the exception that our model system does not contain dyskeratotic changes and has a submucosal component, and thus better represents the normal human mucosa and submucosa.
INTRODUCTION
Several approaches have been used to study the responses of the human oral mucosa to external stimuli in vitro, including monolayers of epithelial cells isolated from unstimulated saliva, oral squamous cell carcinomas (SCC) or primary gingival keratinocytes1-5. Epithelial cells isolated from unstimulated saliva are terminally differentiated, short-lived cells that generally have lower metabolic capacity than actively growing cells in vivo or in vitro. This may render them hyporesponsive to external stimuli1. In addition, results obtained with SCC cell lines may not always accurately reflect the responses of normal epithelial cells and should be confirmed with multiple cell lines or primary cells. Although primary gingival keratinocyte monolayers can develop desmosomal attachments and express cytokeratins found in stratified epithelia6, they have a variable constitutive and inducible expression of proteins, depending on the donor, as previously reported by our group2-4,7,8 and others9-11. Finally, all of these monolayer systems are additionally limited by the lack of a polarized cell phenotype and the lack of a large number of cell–cell contacts, which affect their function and responses to external stimuli12.
The first 3D systems to be used for experimentation were organ culture systems, which involve removal of small pieces of tissue from humans or animals and their maintenance in vitro for a limited time. These 3D systems are limited by the fact that they are not always readily available owing to ethical considerations, and the fact that each piece of tissue is essentially one experiment, with tremendous variability between them13. In addition, the cell composition of organ cultures cannot be manipulated and it is extremely difficult, if not impossible, to introduce immune cells.
To overcome these limitations, tissue-engineered 3D culture systems of the oral mucosa were developed recently, which provide an organizational complexity that is between the culture of single cell types and organ cultures in vivo. Advantages of this 3D setting include a high degree of differentiation, the potential for histological assessment of the process under study and the potential for monitoring tissue growth or damage, together with the expression of tissue proteins or mRNA in situ14-16.
At least two commercially available oral mucosa models now exist (Skinethic Laboratory and MatTek Corporation), both of which are grown at the air–liquid interface on microporous membranes, in chemically defined medium to form an oral mucosa analogue, with or without keratinization. The Skinethic model was generated using a buccal carcinoma cell line (TR146) and when using carcinoma lines it is generally accepted that results should be confirmed with multiple other cell lines and even then they should be interpreted with caution as they may not always accurately represent normal epithelial cells. Because of the technical complexities of these systems, verification with multiple cell lines may be practically impossible. In addition, most of the commercially available oral systems lack a collagen/fibroblast matrix component. The fibroblast component is not only critical in promoting growth and differentiation of keratinocytes into stratified squamous epithelia, but also ensures the resemblance of the tissue model to the human oral mucosa and submucosa8,17-19.
More recently, a 3D in vitro oral mucosal system was developed using primary gingival epithelial cells and collagen-embedded fibroblasts20,21. This system produces an ortho- and para-keratinized oral mucosa analogue, which closely resembles the human masticatory mucosa. In the process of reproducing this system, we found that large quantities of resected gingival tissues were required to ensure sufficient numbers of primary keratinocytes for seeding, as most of these primary cultures cannot be propagated past the fourth passage in vitro22. Furthermore, cells from different donors differ in their growth rates and lifespan in vitro, resulting in the generation of variable numbers of layers in a 3D setting8.
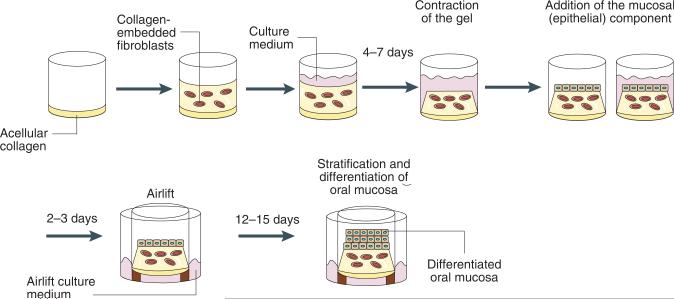
To overcome the limitations of the above 3D systems, we developed a standardized oral mucosa tissue analogue using a “normal” oral epithelial cell line, immortalized by forced expression of telomerase (OKF6/TERT-2)23. The choice of this cell line was based on work from our laboratory and others, which showed that the OKF6/TERT cells resemble primary oral keratinocytes in studies of cytotoxicity or inducible cytokine and beta-defensin expression, and thus are a valuable and reproducible model of normal oral epithelial cells2-4,8,11. The steps involved in this procedure are outlined in Figure 1. The procedure uses the basic guidelines first developed for epidermal systems24 and was further adapted and modified from previously published work in other mucosal tissue systems25,26. Briefly, the procedure consists of three stages: preparation of collagen-embedded fibroblasts, addition of the mucosa (epithelial component) and airlifting of cultures to ensure adequate differentiation/stratification. Because this system also contains a submucosal component, consisting of collagen-embedded fibroblasts, the novelty and additional benefit from this system is that the mucosal–submucosal cell dialogue can be monitored simultaneously, taking into account complex cell–cell contact interactions, as well as interactions that are carried out via secreted or released molecules.
Figure 1.
Outline of the procedure.
Three-dimensional model systems that resemble the oral mucosa in vivo have been used extensively to study host–pathogen interactions8,14,20,21. Our system provides an inexpensive and highly reproducible alternative to commercially available systems in the study of infectious disease pathogenesis. In addition, these models can be used in microbiostatic or microbiocidal assays to study the efficacy of topically applied antimicrobials in an environment resembling the human oral mucosa in vivo (e.g., anti-fungals, anti-bacterials). This system also provides a quick and reproducible alternative to animal testing for safety studies of topically applied pharmacological compounds before clinical trials. For example, this system may be of value for toxicological investigations studying the irritational, inflammatory or tissue-damaging effects of anesthetic pastes, dentifrices, oral rinsing agents, anti-cancer medications, etc. The effects of various agents on oral epithelial morphogenesis or mucosal wound healing can also be studied in this system. Finally, using the basic elements of this technique, epithelium from other human mucosae (esophageal, vaginal, etc.) can be grown for studies pertaining to any mucosal site.
Although the 3D culture methodology of the oral mucosa is a vast improvement over the traditional 3D (i.e., flask monolayers of epithelial cells) culture systems, lack of a vascular component and the resultant inability to introduce cells of the immune system into the submucosal compartment is a significant limitation of these systems. Although immune cells such as lymphocytes27 and dendritic cells28 have been introduced in a 3D setting, a functional capacity of these cells comparable to their in vivo counterparts has not been adequately demonstrated. Therefore, significant challenges remain in the incorporation of other potentially crucial cell types, such as vascular and immune cells, in the submucosal compartment.
MATERIALS
REAGENTS
Keratinocyte-SFM with bovine pituitary extract and epithelial growth factor supplements (KSFM; Invitrogen, cat. no. 17005−042)
DMEM, with 4.5 g l−1 glucose and l-glutamine (Fisher Scientific, cat. no. MT10−013-CV)
Penicillin–streptomycin, 100 IU ml−1–100 μg ml−1 (Fisher Scientific, cat. no. MT30−002-CI)
Ham's F-12 (Invitrogen, cat. no. 11765−054)
PBS, without calcium or magnesium (Fisher Scientific, cat. no. MT21−031-CV)
Fetal bovine serum (FBS; Invitrogen, cat. no. 10437−028), heat-inactivated
Trypsin-EDTA (Fisher Scientific, cat. no. MT25−052-CI)
Bovine type I collagen (Organogenesis Inc.). ▲ CRITICAL other sources of collagen can be used as well (Collagen Type I from rat tail, Sigma, cat. no. C7661 or BD Biosciences, cat. no. 354249). However, the “ready to use” bovine collagen solution from Organogenesis gave the most consistent results.
10× DMEM (Fisher Scientific, cat. no. 50−003-PB)
10% formalin–PBS (v/v; Fisher Scientific, cat. no. SF100−4)
EQUIPMENT
Six-well plates (Fisher Scientific, cat. no. 07−200−83)
100 × 20 mm tissue culture dishes (Fisher Scientific, cat. no. 08−772−22)
50-ml conical tubes (Fisher Scientific, cat. no. 05−538−60)
Millicell culture plate inserts, 30 mm diameter, HA membrane (Millipore, cat. no. PIHA03050)
Sterile stainless steel flat washers, 18 mm outer diameter (Lowe's hardware store)
Sterile forceps (Fisher Scientific, cat. no. 13−812−36)
Sterile spatulas (Fisher Scientific, cat. no. 21−401−5)
Disposable scalpels (Fisher Scientific, cat. no. 08−927−5A)
Histosette II tissue cassettes (Fisher Scientific, cat. no. 15182701C)
Biopsy foam pads, 30 × 25 × 2 mm (Fisher Scientific, cat. no. 22038221)
REAGENT SETUP
Epithelial cell (OKF-6/TERT2 cells23) growth medium
Keratinocyte-SFM (KSFM; Invitrogen, cat. no. 17005−042) supplemented with bovine pituitary extract, 0.2 mg ml−1 human epithelial growth factor (both supplied with the medium by Invitrogen), CaCl2 to final concentration 0.4 mM (from 1,000× stock) and penicillin–streptomycin, 100 IU ml−1–100 μg ml−1.
Fibroblast (3T3 fibroblasts, ATCC) growth medium
DMEM, with 4.5 gl−1 glucose and l-glutamine (Fisher Scientific, cat. no. MT10−013-CV) supplemented with 10% FBS and penicillin–streptomycin, 100 IU ml−1–100 μg ml−1 (Fisher Scientific, cat. no. MT30−002-CI).
Airlift (AL) medium
DMEM (4.5 mg ml−1 glucose) and Ham's F-12 (Invitrogen, cat. no. 11765−054) mixed 3:1, supplemented with 5 μg ml−1 insulin; 0.4 μg ml−1 hydrocortisone; 2 × 10−11 M 3,3′, 5-triiodo-l-thyronine (T3); 1.8 × 10−4 M adenine; 5 μg ml−1 transferrin; 10−10 M cholera toxin; 2 mM l-glutamine; 5% FBS; 100 IU ml−1–100 μg ml−1 penicillin–streptomycin.
10× reconstitution buffer
22 mg ml−1 sodium bicarbonate and 20 mM HEPES in 0.062 N NaOH. Keep aliquots frozen at −20 °C.
10× DMEM
Dissolve 13.48 g of powder in 100 ml of ddH2O (without sodium bicarbonate), filter sterilize, aliquot and store at −20 °C. Note: Precipitate will form. Therefore, before using always take care to have as homogeneous suspension as possible. The precipitate will dissolve after all components of the gel are mixed.
Stock solutions for AL medium
Solution 1 1,000× insulin (5 mg ml−1): Dissolve 50 mg insulin (Sigma-Aldrich, cat. no. I-6634) in 10 ml of 0.005 M HCl, filter sterilize and store aliquots at −20 °C.
Solution 2 1,000× hydrocortisone (0.4 mg ml−1). Dissolve 25 mg hydrocortisone (Sigma-Aldrich, cat. no. H-0888) in 5 ml 100% ethanol (store at −20 °C); dilute 0.5 ml of this solution with 5.75 ml of 1 M HEPES (pH 7), filter sterilize and store aliquots at −20 °C.
Solution 3 1,000× T3 (2 ×] 10−8 M). Dissolve 3.4 mg T3 (Sigma-Aldrich, cat. no. T-6397) in 25 ml 1 M NaOH (store at −20 °C); dilute 0.1 ml of this solution with 9.9 ml PBS; dilute 0.1 ml of this solution with 9.9 ml PBS; filter sterilize and store aliquots at −20 °C.
Solution 4 100× adenine (1.8 × 10−1 M). Dissolve 121.5 mg adenine (Sigma-Aldrich, cat. no. A-2786) in 50 ml 0.05 M HCl, filter sterilize and store aliquots at −20 °C.
Solution 5 1,000× transferrin (5 mg ml−1). Dissolve 50 mg transferrin (Sigma-Aldrich, cat. No. T-1147) in 10 ml PBS, filter sterilize and store aliquots at −20 °C.
Solution 6 1,000× cholera toxin (10−7 M). Reconstitute 1 mg cholera enterotoxin (MP Biomedicals Inc. cat. no. 190329) with 1 ml sterile ddH2O (store at −20 °C); dilute 50 μl of this solution with 5 ml ddH2O, filter sterilize and store aliquots at −20 °C. ! CAUTION Cholera enterotoxin is toxic. Avoid inhalation. Wash thoroughly any area of the body that comes into contact with the toxin. Avoid contact with open wounds.
PROCEDURE
Preparation of collagen-embedded fibroblasts
1| Determine the number of 3D cultures you need to grow. Place Millicell inserts in wells of six-well plates.
| For nine gels, mix on ice: | 1.0 ml 10× DMEM |
| 1.0 ml 10× reconstitution buffer | |
| 1.4 ml FBS | |
| 0.1 ml l-glutamine | |
| 6.5 ml bovine collagen solution |
2| Prepare acellular collagen gels. Each culture requires 1 ml of acellular collagen. Mix components of acellular gel in a 50-ml conical tube on ice in the following order.
▲ CRITICAL STEP Make no more than 30−40 ml of collagen mixture per tube. Collagen solidifies at room temperature; so always keep collagen-containing solutions on ice.
3| Put lids tightly on the tubes and gently invert the tubes repeatedly until the solutions are fully mixed, avoiding bubbles.
4| Using pre-cooled 10-ml pipette, pour 1 ml of the mixture into each insert. Allow gels to solidify in the hood for approximately 30 min.
▲ CRITICAL STEP As the concentration of bovine collagen varies by lot, adjust the volume of bovine collagen in the mix to a final concentration of 0.77 mg ml−1 by changing the volume of FBS. Always make 10% more volume than needed.
5| Prepare suspension of 3T3 fibroblasts in DMEM growth medium. Each culture should contain 3.5−4.5 × 105 3T3 cells. Trypsinize 3T3 fibroblasts. Determine the number of fibroblasts needed and resuspend this number in DMEM–10% FBS growth medium to a concentration of 4 × 106 cells ml−1 (e.g., 6 × 106 3T3 cells in 1.5 ml for 10 gels).
6| Prepare fibroblast-containing collagen gels. Each culture requires 3 ml of fibroblast–collagen gel. Mix components of fibroblast-containing gel in a 50-ml conical tube on ice in the following order.
| For nine gels, mix on ice: | 3.0 ml 10× DMEM |
| 3.0 ml 10× reconstitution buffer | |
| 2.5 ml FBS | |
| 0.3 ml l-glutamine | |
| 20.2 ml bovine collagen |
7| Put lids tightly on the tubes and gently invert the tubes repeatedly until the solutions are fully mixed. Avoid bubbles. ▲ CRITICAL STEP If the solution looks acidic (judged visually by the color of the phenol red in the DMEM), neutralize it by adding a few drops of sterile 1 N NaOH. Mix again by gently inverting the tubes.
8| Add 1 ml of suspension of 3T3 cells, close the tubes and gently mix by inverting. Keep the mixture on ice all the time.
9| Using a pre-cooled pipette, pour 3 ml of the fibroblast–collagen mixture into each insert, above the layer of acellular collagen. Avoid bubbles.
10| Leave the gels in the hood with little or no movement for 1 h. Carefully transfer the plates into a 37 °C, 5% CO2 incubator and leave for another hour. After the gels solidify, add DMEM–10% FBS growth medium: 1.5 ml inside and 1.5 ml outside of the inserts.
Contraction of the fibroblast-containing collagen gels
11| Incubate the gels at 37 °C, 5% CO2 for 4−7 days until they contract. When the gels are contracted away from the sides of the inserts, they form a central raised area approximately 10−14 mm in diameter. Contraction should take place within 7 days. After the gels are contracted, they are ready for use in the next step.
▲ CRITICAL STEP After 2−3 days at 37 °C, release the gels from the walls of the inserts by gently sliding a sterile spatula around the perimeter of the gels.
Addition of the mucosal cell component
12| Using a pipette, aspirate the culture media from the inserts and the wells leaving approximately 1.2 ml in the wells outside the inserts. ▲ CRITICAL STEP Make sure that there is enough medium so that the cultures do not dry, and also not too much so that the medium does not rise into the inserts.
13| Seed oral epithelial cells. Every well insert requires 1.0−1.2 × 106 OKF-6/TERT2 epithelial cells23. Determine how many epithelial cells you need to seed the cultures. Note that oral epithelial cell lines other than OKF-6/TERT2 can be used as well. However, the level of differentiation of the stratified epithelium and degree of dyskeratosis (especially with SCC lines) is variable.
14| Trypsinize OKF-6/TERT2 cells, rinse with PBS and resuspend in KSFM to a concentration of 20 × 106 cells ml−1. Add 50 μl of the suspension to the middle of each insert.
▲ CRITICAL STEP To ensure that epithelial cells form a confluent monolayer on the gels, seed the same amount of epithelial cells into a well of a 12-well plate, to monitor growth.
15| Incubate for 2 h at 37 °C, 5% CO2 until epithelial cells attach to the gels.
16| Gently add 2 ml of KSFM into the inserts and 1.5 ml of DMEM–10% FBS growth medium into the wells outside the inserts. Incubate at 37 °C, 5% CO2.
Growing epithelial cells to confluency
17| Replace medium inside the inserts with fresh KSFM next day, and incubate at 37 °C, 5% CO2 for 2−3 days.
▲ CRITICAL STEP If the monolayer on the 12-well plate does not become confluent after 2−3 days, allow cultures on the gels to grow for a longer time, up to 5−7 days. Feed cultures every 2−3 days.
Airlifting and stratification
18| When the epithelial cells are grown to confluency, transfer the cultures to the air–liquid interface as outlined below.
19| Using sterile forceps, place seven sterile washers (flat side up) in every 100-mm tissue culture dish. Add about 14 ml of AL medium into each dish. Aspirate media from the inserts with the cultures.
20| Using sterile forceps, place inserts on top of the washers, three inserts per dish. Make sure the legs of the inserts stand on the washers.
21| After cultures equilibrate, check media levels to make sure the cultures have contact with the media, but are not covered with the media.
▲ CRITICAL STEP Adjust the amount of medium in the dish to ensure a dry air–liquid interface while maintaining good contact of the base of each culture with the medium. If the medium level is too high, you will achieve a moist interface, which can inhibit full differentiation. If the medium level is too low, the cultures will dry. It is important to remove media from the top of the cultures. Attend to cultures every day to control media level.
22| Incubate cultures at 37 °C, 5% CO2 for 12−15 days. Feed cultures every other day by replacing about 8 ml with fresh AL medium.
Harvesting the 3D cultures for histology
23| Label an appropriate number of processing/embedding cassettes. Place two pieces of biopsy sponges into each cassette and soak the cassettes with the sponges in buffered 10% formalin.
24| Place inserts with the cultures into six-well plates. Add 2 ml of buffered 10% formalin into each well outside the inserts and 1 ml inside the inserts. Take care to not disturb the cultures. Leave for 30 min at room temperature.
25| Aspirate formalin from the inserts. Place insert on a Petri dish and cut the membrane off the walls of the insert using a scalpel. Cut membrane in the middle. Cut a 5-mm-wide strip from the middle of the membrane.
26| Place the strip into a cassette between two sponges. Soak the cassette in buffered 10% formalin.
27| Embed, section and stain. Note that routine histologic processing will not cause detachment of the epithelial layer from the submucosal compartment. However, if desired, mild dispase treatment (0.4% for 1−2 h) will generally result in intact epithelial tissue separation for studies focusing on the epithelial compartment.
▲ CRITICAL STEP The cultures are very soft, slippery and friable. They easily detach from the membrane. While handling the cultures, make sure to not disturb them or touch the top surface (Table 1).
TABLE 1.
Troubleshooting table.
| Problem | Possible reason | Solution |
|---|---|---|
| Collagen gels with embedded fibroblasts did not contract. | Wrong number of fibroblasts added to the gels. | Ensure number of fibroblasts 3.5−4.5 × 105 per gel. |
| Fibroblasts died in the gels because of low pH. | Neutralize the collagen solution with sterile NaOH before adding fibroblasts. | |
| Some gels contracted whereas others did not. | Fibroblasts were poorly mixed in the collagen solution. | Mix fibroblasts with the collagen solution by gently inverting the tubes. |
| Poor epithelial differentiation. | Density of keratinocytes on the gels was too low. | Seed proper number, 1.0−1.2 ×106 oral epithelial cells on the gels. |
| Failure to maintain cultures on air-liquid interface. | Check cultures daily for the medium levels. Aspirate media from inside the inserts. | |
| None, few or disturbed epithelial layers. | Cultures were disturbed while placing into the cassettes or during during histological processing. | While harvesting the cultures, do not disturb them. Make sure the cultures do not move along the sponges in the cassettes. |
TIMING
Steps 2−4: 1 h
Step 5: 15 min
Steps 6−10: 3 h
Step 11: 7 days
Steps 12−16: 3 h
Step 17: 3−4 days
Steps 18−21: varies according to number of 3D cultures
Step 22: 12−15 days
Steps 23−27: varies according to number of 3D cultures
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
ANTICIPATED RESULTS
Histologic characteristics of the three-dimensional model
The morphology of the OKF6–TERT2–fibroblast-based system resembles the commercially available oral mucosal model system based on the cell line TR146 (SkinEthic)15,29, with the exception that our model system does not contain dyskeratotic changes and has a submucosal component and thus better represents the normal oral mucosa and submucosa. The number of viable cell layers in this model system range from 7 to 12 (Fig. 2a). The cells of the viable portion are organized similarly to the cells in native oral mucosa. More specifically, this model system consists of a stratum basale, having one layer of columnar to round cells, a relatively flattened stratum spinosum and stratum granulosum, and a non-keratinizing stratum corneum (Fig. 2a). The cytokeratin expression pattern of OKF cells (originating from the floor of the mouth) grown in a multilayer structure is consistent with stratified, non-keratinizing epithelia (expressing keratins 4 and 19) as shown by the laboratory that developed these cell lines23,25.
Figure 2.
Histologic characterization of the 3D model of oral mucosa. (a) Morphologic characteristics (H&E staining, ×200 magnification). (b) Expression pattern of E-cadherin (immunohistochemical staining, ×200 magnification). (c) Expression pattern of Ki-67 (immunohistochemical staining, ×100 magnification).
E-cadherin is one of the markers found in epithelia that form tight junctions, and is important for the formation and preservation of stratified epithelial barriers30,31. Neoplastic transformation in the oral mucosa involves disruption of cell junctions and limited intracellular granular expression, instead of membrane-associated expression of E-cadherin31. Because we used an immortalized cell line, it was important to confirm the presence of cell membrane-expressed E-cadherin in this system, which is frequently lost by immortalized cells in vitro or in vivo32,33. Asseenin Figure 2b, E-cadherin was strongly expressed at the cell membrane level, consistent with prior reports using primary oral keratinocytes in a 3D setting31. In addition, because 3D systems based on immortalized cells may lose the normal pattern of growth found in stratified epithelia, we assessed cell growth by examining the localization of the Ki-67 proliferation marker31. Unlike other immortalized cell line-based 3D systems, which express Ki-67 throughout all cell layers31, our system showed polarized expression of Ki-67 at the basal/parabasal cell layers only, consistent with normal growth in stratified epithelia (Fig. 2c).
In this model system, cells exposed to air formed a stratified structure within 1 week of coculture with stromal cells, consistent with reports in similar systems containing epidermal keratinocytes18. As we and others have observed, the viability of mucosal epithelial cells remains very high during this coculture period8,34, and the total lifespan of such systems is approximately 5 weeks8,18, which is close to the reported 41−57 days of turnover time (the time necessary to replace all the cells in epithelium) of the oral mucosa in vivo35. Fibroblasts cultured under keratinocyte layers in this manner proliferate slowly and produce fibronectin, which is a major component of the submucosal extracellular matrix, which covers the entire surface of the well within 3−4 weeks17. These cells and their extracellular matrix provide better conditions for growth and differentiation of keratinocytes in vitro compared with artificial gels or membranes17, possibly via release of insulin-like growth factors36.
Use of the 3D model system in the study of host–pathogen interactions
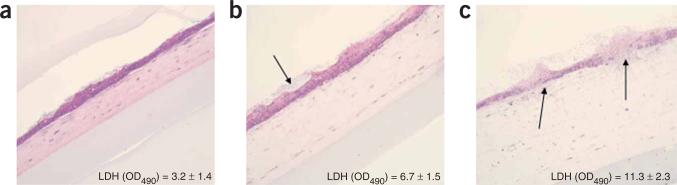
In previous studies8,37, we evaluated tissue damage and the host response to fungal infection using this novel model system and demonstrated its suitability in the study of host–pathogen interactions in the oral mucosa. Extracellular leakage of lactate dehydrogenase is widely used as as a marker of decreased cell viability in response to cytotoxic compounds in 3D cutaneous models38, and was used in these studies to compare resistance of these models to damage by the highly virulent Candida albicans strain SC5314 (Fig. 3). Infection of this 3D tissue model revealed a time-dependent development of a C. albicans soft tissue biofilm with variable levels of tissue invasion and damage, depending on the duration of the infection (Fig. 3b,c). The histopathologic changes we observed in our in-house model are consistent with human oral candidiasis lesions in vivo. These included time-dependent degradation of the uppermost layer of epithelial cells and widening of the intercellular spaces of basal cells (spongiosis; Fig. 3b,c)39. Using several mutant strains of C. albicans, we found that the disruption of different cell layers was proportional to the well-documented virulence of each infecting strain in animal or other models8.
Figure 3.
Application of the 3D model of the oral mucosa in the study of host–pathogen interactions. The organotypic cultures were infected with C. albicans strain SC5314 for (a) 8 h, (b) 14 h or (c) 24 h and tissue damage was assessed by measuring lactate dehydrogenase release into the subnatants. (b) Soft tissue biofilm forming over the epithelial layer (arrow). (c) Fungal invasion (arrows) ofthe tissueswas consistent with tissue damage. (×100 magnification.)
ACKNOWLEDGMENTS
This study was supported by USPHS Research Grant RO1 DE13986 to ADB from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
References
- 1.Steele C, Fidel PL., Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect. Immun. 2002;70:577–583. doi: 10.1128/IAI.70.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dongari-Bagtzoglou AI, Kashleva H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb. Pathogen. 2003;34:169–177. doi: 10.1016/s0882-4010(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 3.Dongari-Bagtzoglou AI, Kashleva H. Granulocyte–macrophage colony-stimulating factor responses of oral epithelial cells to Candida albicans. Oral Microbiol. Immunol. 2003;18:165–170. doi: 10.1034/j.1399-302x.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 4.Dongari-Bagtzoglou AI, Kashleva H, Villar CC. Bioactive interleukin-1α is cytolytically released from Candida albicans-infected oral epithelial cells. Med. Mycol. 2004;42:531–541. doi: 10.1080/1369378042000193194. [DOI] [PubMed] [Google Scholar]
- 5.Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect. Immun. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda D, Watson E. Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell. Dev. Biol. 1990;26:589–95. doi: 10.1007/BF02624208. [DOI] [PubMed] [Google Scholar]
- 7.Dongari-Bagtzoglou A, Wen K, Lamster IB. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol. Immunol. 1999;14:364–370. doi: 10.1034/j.1399-302x.1999.140606.x. [DOI] [PubMed] [Google Scholar]
- 8.Dongari-Bagtzoglou AI, Kashleva H. Development of a novel three-dimensional in vitro model of oral Candida infection. Microb. Pathogen. 2006;40:271–278. doi: 10.1016/j.micpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Cox G, Gauldie J, Jordana M. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am. J. Respir. Cell. Mol .Biol. 1992;7:507–513. doi: 10.1165/ajrcmb/7.5.507. [DOI] [PubMed] [Google Scholar]
- 10.Devalia JL, Bayram H, Abdelaziz MM, Sapsford RJ, Davies RJ. Differences between cytokine release from bronchial epithelial cells of asthmatic patients and non-asthmatic subjects: effect of exposure to diesel exhaust particles. Int. Arch. Allergy Immunol. 1999;118:437–439. doi: 10.1159/000024157. [DOI] [PubMed] [Google Scholar]
- 11.Feucht EC, DeSanti CL, Weinberg A. Selective induction of human beta-defensin mRNAs by Actinobacillus actinomycetemcomitants in primary and immortalized oral epithelial cells. Oral Microbiol. Immunol. 2003;18:359–363. doi: 10.1046/j.0902-0055.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 12.Radyuk SN, Mericko PA, Popova TG, Grene E, Alibek K. In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem. Biophys. Res. Commun. 2003;305:624–632. doi: 10.1016/s0006-291x(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 13.Sacks PG. Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metast. Rev. 1996;15:27–51. doi: 10.1007/BF00049486. [DOI] [PubMed] [Google Scholar]
- 14.Kimball JR, Nittayananta W, Klausner M, Chung WO, Dale BA. Antimicrobial barrier of an in vitro oral epithelial model. Arch. Oral Biol. 2006;51:775–783. doi: 10.1016/j.archoralbio.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J. Invest. Dermatol. 2002;118:652–657. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 16.Korting HC, Patzak U, Schaller M, Maibach HI. A model of human cutaneous candidosis based on reconstructed human epidermis for the light and electron microscopic study of pathogenesis and treatment. J. Infect. 1998;36:259–267. doi: 10.1016/s0163-4453(98)94063-4. [DOI] [PubMed] [Google Scholar]
- 17.Maruguchi T, Maruguchi Y, Suzuki S, Matsuda K, Toda K, Isshiki N. A new skin equivalent: keratinocytes proliferated and differentiated on collagen sponge containing fibroblasts. Plast. Reconstr. Surg. 1994;93:537–544. [PubMed] [Google Scholar]
- 18.Sugihara H, Toda S, Yonemitsu N, Watanabe K. Effects of fat cells on keratinocytes and fibroblasts in a reconstructed rat skin model using collagen gel matrix culture. Br. J. Dermatol. 2001;144:244–253. doi: 10.1046/j.1365-2133.2001.04008.x. [DOI] [PubMed] [Google Scholar]
- 19.Kautsky MB, Fleckman P, Dale BA. Retinoic acid regulates oral epithelial differentiation by two mechanisms. J. Invest. Dermatol. 1995;104:546–553. doi: 10.1111/1523-1747.ep12606058. [DOI] [PubMed] [Google Scholar]
- 20.Mostefaoui Y, Claveau I, Rouabhia M. In vitro analyses of tissue structure and interleukin-1β expression and production by human oral mucosa in response to Candida albicans infections. Cytokine. 2004;25:162–171. doi: 10.1016/j.cyto.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Mostefaoui Y, Bart C, Frenette M, Rouabhia M. Candida albicans and Streptococcus salivarius modulate IL-6, IL-8 and TNFα expression and secretion by engineered human oral mucosa cells. Cell. Microbiol. 2004;6:1085–1096. doi: 10.1111/j.1462-5822.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 22.Southgate J, Williams HK, Trejdosiewicz LK, Hodges GM. Primary culture of human oral epithelial cells. Growth requirements and expression of differentiated characteristics. Lab. Invest. 1987;56:211–223. [PubMed] [Google Scholar]
- 23.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16 INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parenteau N. In: Skin equivalents. in Keratinocyte Methods. Leigh I, Watt F, editors. Cambridge University Press; U.K.: 1994. pp. 45–54. [Google Scholar]
- 25.Schon M, Rheinwald JG. A limited role for retinoic acid and retinoic acid receptors RARa and RARb in regulating Keratin 19 expression and keratinization in oral and epidermal keratinocytes. J. Invest. Dermatol. 1996;107:428–438. doi: 10.1111/1523-1747.ep12363411. [DOI] [PubMed] [Google Scholar]
- 26.Meyers C, Frattini MG, Laimins LA. Tissue culture techniques for the study of human papillomaviruses in stratified epithelia. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press Inc.; 1994. pp. 491–499. [Google Scholar]
- 27.Rouabhia M. In vitro production and transplantation of immunologically active skin equivalents. Lab. Invest. 1996;75:503–517. [PubMed] [Google Scholar]
- 28.Dumont S, Valladeu J, Bechetoille N, Gofflo S, Marechal S, et al. When integrated in a subepithelial mucosal layer equivalent, dentritic cells keep their immature stage and their ability to replicate Type R5 HIV Type 1 strains in the absence of T cell subsets. AIDS Res. Hum. Retroviruses. 2004;20:383–397. doi: 10.1089/088922204323048131. [DOI] [PubMed] [Google Scholar]
- 29.Schaller M, Korting HC, Schafer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 1999;34:169–180. doi: 10.1046/j.1365-2958.1999.01590.x. [DOI] [PubMed] [Google Scholar]
- 30.Castillon N, et al. Polarized expression of cystic fibrosis transmembrane conductance regulator and associated epithelial proteins during the regeneration of human airway surface epithelium in three-dimensional culture. Lab. Invest. 2002;82:989–998. doi: 10.1097/01.lab.0000022221.88025.43. [DOI] [PubMed] [Google Scholar]
- 31.Costea DE, Johannessen AC, Vintermyr OK. Fibroblast control on epithelial differentiation is gradually lost during in vitro tumor progression. Differentiation. 2005;73:134–141. doi: 10.1111/j.1432-0436.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 32.Downer CS, Speight PM. E-cadherin expression in normal, hyperplastic and malignant oral epithelium. Eur. J. Cancer B. Oral Oncol. 1993;29:303–305. doi: 10.1016/0964-1955(93)90053-h. [DOI] [PubMed] [Google Scholar]
- 33.Gasparoni A, Fonzi L, Schneider GB, Wertz PW, Johnson GK, Squier CA. Comparison of differentiation markers between normal and two squamous cell carcinoma cell lines in culture. Arch. Oral Biol. 2004;49:653–664. doi: 10.1016/j.archoralbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Waelti ER, et al. Co-culture of human keratinocytes on post-mitotic human dermal fibroblast feeder cells: production of large amounts of interleukin 6. J. Invest. Dermatol. 1992;98:805–808. doi: 10.1111/1523-1747.ep12499961. [DOI] [PubMed] [Google Scholar]
- 35.Squier CA, Finkelstein MW. Oral mucosa. In: Nanci A, editor. Ten Cate's Oral Histology. 6th Edition Mosby Inc.; Orlando, FL: 2003. [Google Scholar]
- 36.Barreca A, et al. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J. Cell. Physiol. 1992;151:262–268. doi: 10.1002/jcp.1041510207. [DOI] [PubMed] [Google Scholar]
- 37.Villar CC, Kashleva H, Mitchell AP, Dongari-Bagtzoglou AI. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect. Immun. 2005;273:4588–4595. doi: 10.1128/IAI.73.8.4588-4595.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Brugerolle A, et al. Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic) applied to the testing of topical vehicles. Cell Biol. Toxicol. 1999;15:121–135. doi: 10.1023/a:1007577515215. [DOI] [PubMed] [Google Scholar]
- 39.Reichart PA, Philipsen HP, Schmidt-Westhausen A, Samaranayake LP. Pseudomembranous oral candidiasis in HIV infection: ultrastructural findings. J Oral Pathol. Med. 1995;24:276–281. doi: 10.1111/j.1600-0714.1995.tb01182.x. [DOI] [PubMed] [Google Scholar]