Abstract
Differentiation is the process by which tissues/organs take on their final, physiologically functional form. This process is mediated in part by the silencing of embryonic genes and the activation of terminal, differentiation gene products. Mammalian kidney development is initiated when the Wolffian duct branches and invades the overlying metanephric mesenchyme. The newly formed epithelial bud, known as the ureteric bud, will continue to branch ultimately differentiating into the collecting duct system and ureter. Here, we show that Hoxb7-Cre mediated removal of β-catenin from the mouse Wolffian duct epithelium leads to the premature expression of gene products normally associated with the differentiated kidney collecting duct system including the water channel protein, Aquaporin-3 and the tight junction protein isoform, ZO-1α+. Mutant cells fail to maintain expression of some genes associated with embryonic development, including several mediators of branching morphogenesis, which subsequently leads to kidney aplasia or hypoplasia. Reciprocally, expression of a stabilized form of β-catenin appears to block differentiation of the collecting ducts. All of these defects occur in the absence of any effects on the adherens junctions. These data indicate a role for β-catenin in maintaining cells of the Wolffian ducts and the duct derived ureteric bud/collecting duct system in an undifferentiated or precursor state.
Introduction
The metanephric kidney is an essential organ composed primarily of epithelial tubules and blood vessels. In the mouse, metanephric kidney development initiates between embryonic day (e) 10.5 and 11.0 when a caudal portion of the Wolffian duct (WD) branches and invades the dorsally located metanephric mesenchyme (MM). This epithelial branch, known as the ureteric bud (UB), undergoes repeated rounds of branching morphogenesis throughout the embryonic period halting shortly after birth. The UB will give rise to the collecting duct system (CD) and ureter in the mature organ. Defects in ureteric bud branching lead to phenotypes ranging from complete aplasia to hypoplasia and constitute some of the most common causes of renal failure in humans (Shah et al., 2004; Woolf, 2006; Woolf et al., 2004).
The metanephros develops in a centrifugal fashion with the outer-most or cortical epithelia being the youngest and least differentiated while the more internal or medullary epithelia are the oldest and most differentiated. Morphological studies suggest that the more distal/medullary kidney tubules of the mouse metanephros become physiologically active around e15.5 (Woolf and Yuan, 2003). Interestingly, the cortical collecting ducts continue to branch while the medullary collecting ducts are presumably functional in their capacity to concentrate urinary filtrate. This suggests a fine balance between signals that maintain the bud tips in an undifferentiated (precursor) state while allowing the distal epithelium to mature. Presumably, any defects in this balance would have repercussions on the number or ureteric bud tips that form during development and ultimately nephron endowment in an adult kidney. Although recent studies have identified factors that seem to be required for normal proximal/distal patterning and differentiation of the ureter/collecting duct system, the processes regulating precursor cell maintenance and maturity are poorly understood (Airik et al., 2006; Brenner-Anantharam et al., 2007; Yamaguchi et al., 2006).
β–catenin is a multi-functional protein. In one of its roles, it binds to the transmembrane protein E-cadherin and cytoplasmic α–catenin to link the cell-cell adhesion complex known as the adherens junctions (AJ) to the actin cytoskeleton. In flies lacking the β–catenin homolog armadillo (arm), the adherens junction protein E-cadherin fails to localize to the lateral membrane of epithelia (Cox et al., 1996). Mutant epithelia also show defects in apical/basal polarity, presumably as a result of perturbed AJ formation/function (Cox et al., 1996). However, in mice in which β-catenin has been ablated in specific tissues, no defect in AJ formation has been witnessed, presumably due to compensation by the closely related protein, γ-catenin, also known as plakoglobin (Haegel et al., 1995; Murtaugh et al., 2005).
A second function of β-catenin is to act as a transcriptional co-regulator, primarily through interactions with the Lef/Tcf family of transcription factors (Brembeck et al., 2006). In this capacity, β-catenin has been shown to regulate multiple cellular processes including cell proliferation, survival and adhesion. Several studies have demonstrated roles for β-catenin in the maintenance of stem or embryonic precursor cells (DasGupta and Fuchs, 1999; Huelsken et al., 2001; Kielman et al., 2002; Scheller et al., 2006; Zechner et al., 2003). In addition, deregulation of β–catenin signaling has been implicated in various human diseases, especially cancer (Giles et al., 2003).
Recent analyses of β-catenin reporters and transcriptional targets have suggested high levels of transcriptional activity in the developing Wolffian duct and ureteric bud. However, assessing the role of β-catenin in these tissues has been hampered due to early embryonic lethality in null embryos (Haegel et al., 1995; Perantoni, 2003). Here we report that WD/UB/CD specific deletion of β–catenin leads to multiple defects in urogenital development ranging from complete aplasia to duplexed kidneys and cystic/dysplastic kidneys. Analysis of mutant embryos demonstrates that β–catenin is necessary for maintaining cells of the WD/UB in a precursor state. Mutant cells show several signs of premature differentiation including the precocious expression of the terminal tight junction protein isoform, ZO-1α+ and the collecting duct water channel protein, Aquaporin-3, accompanied by the loss of expression of several branching regulators. These data demonstrate an essential role for β–catenin in the formation and normal differentiation of the mammalian urogenital system.
Materials and Methods
Mouse Strains
WD/UB specific deletion of β–catenin was accomplished by crossing mice triply heterozygous for Hoxb7-Cre (Yu et al., 2002), β–catenin (Brault et al., 2001), and the Rosa reporter (Soriano, 1999) to mice homozygous for the conditionally inactive allele of β–catenin (ctnnb1tm2Kem). WD/CD specific activation of β-catenin was accomplished by crossing mice carrying Hoxb7-Cre to mice carrying an allele of β-catenin where exon 3 has been floxed (Harada et al., 1999). Noon on the day of vaginal plug detection was considered E0.5.
Wholemount expression analysis
Rosa reporter activity was assessed as previously described (Soriano, 1999). For in situ hybridization, tissues were post-fixed in 4% paraformaldehyde for 2 hours at 4 degrees prior to hybridization with antisense, digoxygenin labeled mRNA probes. All probes examined have been previously described with the exception of L1-Cam (cut with EcoRI and transcribed with T3 polymerase), Crumbs-3 (EcoRI/T7), lethal giant larvae homolog 2 (SalI, T7) and the Kit ligand (SalI/T7). For mutant analysis, a minimum of six wildtype (heterozygous) and six mutant embryos were analyzed. Hybridized embryos were cleared in 80% glycerol and photographed using a Nikon digital still camera (DXM1200) on a Nikon stereomicroscope (SMZ1500).
Immunohistochemistry
e11.5 and P1 embryos were fixed in 4% paraformaldehyde at 4°C overnight, washed 3 times in PBS, equilibrated in 30% sucrose then imbedded in OCT and flash frozen on dry-ice. Tissue was sectioned at 10uM on a cryostat and sections were stored at −80 degrees C until usage. Thawed sections were boiled for 10 minutes in 10mM Tris, pH 8.0 and 5mM EDTA then incubated with primary antibodies to acetylated-α-tubulin (mouse, Sigma, T 5168), β–catenin (rabbit, Chemicon, AB19022, rabbit, abcam, ab2982 and mouse, Sigma c7207), de-phosphorylated β-catenin (mouse, Upstate Cell Signaling Solutions), γ-catenin (goat, Santa Cruz, Sc-1497), β–tubulin (mouse, Sigma, T4026), Scribble (goat, Santa Cruz,), PCNA (mouse, zymed, 18–0110), Ki-67 (rabbit, Novo-Castra, NCL-Ki67p), Pax2 (rabbit, Covance, PRB-276P), pan-cytokeratin (mouse, Sigma, C2562), E-cadherin (Rat, Zymed, 13–1900 and rabbit, a gift from Rolf Kemler), Laminin (Rabbit, Sigma, L9393), DBA lectin (Sigma), Occludin (Rabbit, Zymed, 71–1500) ZO-1α+ (Rat, Chemicon, Mab1520 [Note; Chemicon no longer makes this antibody. We have tried another version from Hycult Biotechnology but have been unable to get this antibody to work in wax or frozen sections, with and without antigen retrieval.] ), ZO-1 (rabbit, Zymed, Cat.#40–2300), aPKC (rabbit, Santa Cruz, Cat. #:sc-216), Ezrin (rabbit, Upstate Cell Signaling Solutions, Cat.#07–130, 1:100), UT-A, Aqp-2 and 3 (rabbit, provided by Mark Knepper) and Aqp-9 (rabbit, Alpha Diagnostic International, Cat.#AQP91-A, 8ug/ml). All antibodies were diluted 1:500 unless otherwise indicated. The secondary antibodies were Alexafluor 488, 568 and 633 (Molecular Probes). Tissues were mounted in VectaShield with Dapi (Vector labs). For characterization of ectopic buds, only sections caudal of the mesonephric tubules and cranial of the hindlimb bud were analyzed and sections from a minimum of 3 animals were examined. Sections were viewed and photographed on a Zeiss LSM510 Axioplan inverted confocal microscope.
Transmission Electron Microscopy
Thick (1uM), transverse sections were cut of plastic imbedded wildtype and mutant e11.5 Wolffian ducts and stained with toluidine blue. Mutant cells are distinguishable from wildtype histologically by the presence of numerous large vacuoles and decreased apical cytoplasm in mutant cells. When clear ectopic buds were identified in thick sections, thin sections were cut from the adjacent tissue and viewed with a FEI Tecnai G2 Spirit microscope. The above-mentioned histological criteria were used to distinguish wildtype from mutant epithelia.
Organ culture
Organ culture was performed as previously described. Briefly, e10.5 urogenital systems were dissected out and cultured for 48 hours in DMEM with 10% FBS and either 10ug/ml anti-GDNF, 200ng/ml mouse recombinant Dkk1 (both from R &D Systems) or 10ug/ml anti-β–galactosidase or 200ng/ml BSA as the respective negative controls. After 48 hours, tissues were rinsed in PBS and either mRNA was extracted for RT-PCR or the tissues were fixed for β–galactosidase staining, in situ hybridization or antibody staining. GDNF treated issues were hybridized with an antisense probe to Pax-2 or Wnt9b in order to assess the extent of UB branching.
Statistical analysis
To measure basal to apical distance, tissues were stained with anti-laminin and anti-β–catenin antibodies and photographed at the same magnification on a confocal microscope. The cell height was measured using a digital micrometer. Measurements were only made on cells that were clearly continuous with the basement membrane and a line was drawn from (and perpendicular to) the basement membrane to the luminal membrane. In all cases, we measured cells only within approximately 100uM of the hindlimb bud and only on the medial side of the tubule to account for any spatial differences in cell height. A total of 62 mutant and 66 wildtype cells were measured from 4 animals.
To measure proliferation rates, tissues were stained with Dba lectin, β–catenin, Ki67 and Dapi. Only nuclei from Dba lectin positive epithelia were counted. In the case of mutant epithelia, only cells that were clearly identified as b-catenin mutant (by immunohistochemistry) were included in the statistical analysis. For e 11.5, a total of 95 wildtype (6 individual embryos) and 106 mutant cells (6 embryos), for 12.5, 815 wildtype (4 individuals) and 523 mutant (4 individuals) cells, for 14.5, 970 wildtype (5 individuals) and 1630 mutant (7 individuals) cells and for P1, a total of 457 (190 β–catenin +, 267 β–catenin −) and 442 cells from 7 mutant and 5 wildtype individuals, respectively, were counted. P values were calculated using a Student T-test. Values presented are the average and the standard deviation. A similar technique was used to measure apoptosis. Apoptotic cells were identified using TUNEL and an anti-caspase antibody.
Results
In the past several years, a number of groups have engineered mice that report Lef/Tcf (and presumably β-catenin) transcriptional activity (DasGupta and Fuchs, 1999; Iglesias et al., 2007; Jho et al., 2002; Leung et al., 2002; Lustig et al., 2002; Maretto et al., 2003). At least three of these mouse strains report transcriptional activity in the embryonic kidney and in all cases expression is high in the WD and UB (Iglesias et al., 2007; Jho et al., 2002; Leung et al., 2002; Lustig et al., 2002; Maretto et al., 2003).
To examine a potential role for β̃ catenin in the context of the WD/UB/CD system, we took advantage of a conditionally inactive (floxed) allele of β–catenin and a Cre driver line that we previously generated (Hoxb7-Cre) (Brault et al., 2001; Yu et al., 2002). Hoxb7-Cre is expressed in the WD/UB/CD lineage from E9.5 through birth (Yu et al., 2002). Mice carrying the Hoxb7-cre transgene, the ROSA reporter (Soriano, 1999) and one mutant allele of β–catenin (Hoxb7-cre; β–catenin+/−; ROSA) were crossed to mice homozygous for the β–catenin conditional allele (β-cateninc/c) (Brault et al., 2001) to generate Hoxb7-cre; β–catenin−/c embryos, from hereon referred to as β–catenin mutants.
To test the efficiency of Hoxb7-Cre mediated excision, we characterized the expression of β–catenin protein in wildtype and mutant tissue. In wildtype embryonic day (e)11.5 embryos, β–catenin was expressed at high levels on the baso-lateral side of the WD/UB epithelium and at lower levels throughout the cytoplasm (Figure 1A). Nuclear β–catenin was not detected. Although Hoxb7-Cre appears to be active throughout the WD of mutants and wildtype embryos (as visualized by ROSA reporter activity, Figure 2A–F), staining of mutants showed that β–catenin protein was removed in a mosaic fashion in the WD epithelium (figure 1A″ and B″). This appears to be due to differential excision efficiency at the β–catenin locus relative to the ROSA-26 locus. The level of mosaicism varied considerably, with between (approximately) 10% and 100% of the WD cells lacking detectable β–catenin protein at e11.5 (not shown).
Figure 1.

Sagittal sections through e11.5 β–catenin mutant Wolffian ducts. β–catenin staining shows that extruded epithelia can be either wildtype (A) or mutant (B). Ducts have been double stained with antibodies for Pax-2 (single channel red in A′ and B′) and b-catenin (single channel green in A″ and B″).
Figure 2.

Development of the Wolffian duct and ureteric bud at e11.5 and e12.5. Lac-Z stained e11.5 (A, C, E) and e12.5 (B, D, F) Hoxb7-Cre; β–catenin+/c;ROSA+/− (wildtype, A and B) and Hoxb7-Cre; β–catenin−/c;ROSA+/− (mutant, C–F) urogenital systems. Hoxb7-Cre is active in the Wolffian duct (red arrows) and ureteric bud (black arrows) of e11.5–12.5 urogenital systems (A and B). β-catenin mutant collecting ducts show multiple ectopic bud structures branching from the Wolffian duct (arrowheads in C–F) and defective branching of the ureteric buds (black arrows in C–F).
Histological analysis indicated that mosaic Wolffian ducts formed ectopic bud like structures (Figure 1A and B). Surprisingly, there did not appear to be a correlation between the presence and absence of detectable β–catenin and the formation of these ectopic epithelia (Figure 1A and B). When clearly defined, distinct ectopic buds were present, they were either wildtype for β–catenin or mutant, but not mosaic. Ducts that were exclusively composed of mutant cells were relatively normal looking with no ectopic buds (data not shown).
Characterization of ectopic buds in β–catenin mutants
To gain a better understanding of the mechanisms that led to the formation of the ectopic buds, we examined the development of mutant Wolffian ducts and ureteric buds from e10.5 to 12.5.
In wt embryos, a caudal portion of the WD adjacent to the hindlimb begins to bud dorsally around e11.0, invading the overlying metanephric mesenchyme. At e11.5, the bud has invaded the MM and branched once within it forming a T-shaped structure (Figure 2A). Over the next several days, the UB will continue to branch eventually giving rise to the collecting duct system and ureter of the mature kidney (Figure 2B). At e10.5, both wildtype and mutant WDs were smooth and uniform (not shown) although the mutant ducts did not swell at the caudal end in 2/3 embryos. By e11.5, β–catenin mutant embryos displayed several small, ectopic bud-like structures along the entire length of the WD (26/30, Compare Figure 2A with C and E). The ectopic buds were not restricted to the dorsal side of the epithelium but instead protruded from the WD randomly. In some individuals, the more caudal ectopic buds invaded the MM alongside the endogenous UB (Figure 2E), however, in 4/30 instances no ureteric bud had invaded the MM by e11.5 (not shown). At e12.5, ectopic buds were still evident, however those that formed on the ventral side of the WD and those that were significantly anterior of the MM had ceased to grow and at least in some cases appeared to have completely separated from the WD (Compare Figure 2B with 2D and F). In 7/9 cases, the endogenous UB showed greatly reduced branching within the mutant mesenchyme at e12.5 (Figure 2D and F).
Removal of β–catenin from the Wolffian duct/ureteric bud results in multiple defects in the urogenital system
To determine the consequences of β-catenin removal on formation of the urogenital system, we examined the urogenital systems of newborn pups derived from Hoxb7-cre; β–catenin+/−; ROSA and β–catenin c/c intercrosses. Mutants were born in the expected Mendelian ratio, however the majority died within 48 hours of birth. Out of 71 progeny from 10 litters, only two mutant animals survived past post-natal day 5 (approx.18 expected) suggesting early post-natal lethality.
As predicted from our early analysis, comparison of wildtype (Figure 3A) and mutant (Figure 3B, C and E) P1 urogenital systems revealed a range of phenotypes, from extreme hypoplasia/aplasia (17/28, Figure 3C) to dysplastic/cystic kidneys (11/28, Figure 2B and E). In all cases, ureteric bud branching, as determined by the number of lac-Z stained tips, was significantly reduced. In the most severe cases, no ureter or a single unbranched ureter was present (Figure 3C and not shown). The ureters were dilated and tortuous and frequently appeared obstructed (Figure 3B, C and H). Frequently (8/28), more than one ureter (ectopic) was observed accompanied by a duplexed, hypoplastic kidney (Figure 3B).
Figure 3.

β–catenin mutant phenotypes in P1 kidneys. Wholemount Lac-Z stained (A–E) and H and E stained (F–H) P1 kidneys from Hoxb7-Cre; β–catenin+/c;Rosa+/− (wildtype, A, D and F) and Hoxb7-Cre; β–catenin−/c; Rosa+/− (mutant, B, C, E, G and H) pups. B shows a mutant with duplexed kidneys (blue arrows) and ectopic ureters (black arrows). C shows a severely dysplastic kidney with a single ureter (black arrow). D and E show high magnification images of A and B respectively. Note the dilated collecting ducts in E (black arrow). Sections through wildtype (F) and a cystic (G) and severely dysplastic (H) kidney. Note the expanded renal pelvis in G (asterisk).
H and E stained sections revealed that P1 heterozygotes (Figure 3F) had normal appearing kidneys while mutants were cystic and frequently showed a greatly expanded renal pelvis (hydronephrosis, Figure 3G). Dilated/cystic epithelia were visible by e14.5 and were always confined to the UB/CD lineage (Figure 3E and not shown). Cysts frequently had multi-layered polyps extending into the lumen (not shown). In all cases observed, the polyps were made up of wildtype cells adjacent to a mutant epithelium (not shown). In examples of extremely hypoplastic mutants, the ureteric buds were surrounded by condensed, undifferentiated mesenchyme (Figure 3H). Females lacked a uterus and upper vagina while males had an abnormal, dilated epididymis and vas deferens (data not shown).
The variability in β-catenin excision seen in early embryonic stages was still evident at birth with some kidneys containing little or no β-catenin in their collecting ducts while others were highly mosaic (data not shown). The degree of β-catenin excision correlated with the severity of the phenotype with aplastic or severely hypoplastic organs possessing few or no β-catenin positive cells within the ureteric bud derivatives while hypoplastic kidneys showed varying degrees of mosaicism (data not shown).
Molecular Analysis of Ectopic Bud Formation
To address the mechanism behind the β-catenin mutant phenotype, we characterized the epithelia at a molecular level. Formation of duplexed kidneys is thought to be caused by ectopic activation of ureteric bud branching determinants, especially members of the Ret/GDNF signaling axis, within the anterior Wolffian duct, (Basson et al., 2005; Grieshammer et al., 2004; Kume et al., 2000; Towers et al., 1998). Inappropriate activation of the branching pathway within the collecting duct system would also explain the cystic tubules observed in mutants, especially if mosaic loss of β-catenin was causing localized activation of this pathway. To determine if β-catenin removal was affecting the branching pathway, we looked at the expression of a number of regulators of this process in the developing ureteric bud.
At e10.5–11.5, c-ret mRNA is expressed throughout the wildtype Wolffian duct and ureteric bud, with highest levels in the branching tips of the bud (Figure 4A and not shown). Expression analysis revealed that at e10.5, ret mRNA was expressed normally in the WD epithelium (not shown) but by e11.5, expression had become mosaic in the mutant WD (Figure 4B). In 7/11 e11.5 mutant embryos, ret expression looked relatively normal in the UB although in three cases, the UBs did not appear to form ampullary tips (Figure 4B). In the other 4 embryos, ret expression was undetectable and no UB had formed (data not shown). In wildtype embryos at e12.5, ret expression has refined to the tips of the UB (Figure 4C), while in mutants expression was variable. 3/9 embryos had no detectable ret expression in the UB. 4/9 had abnormally branched UBs with expression normal in some epithelia and reduced or absent in other cells (Figure 4D). 2/9 embryos looked normal in regard to branching and ret expression. Immunofluorescent/antisense mRNA co-stainings of e12.5 mutant embryos showed that β–catenin removal from the UB/CD was mosaic and that cells that retained normal levels of c-ret mRNA also had normal levels of β-catenin protein including some well-formed ectopic buds (not shown).
Figure 4.

Molecular characterization of β–catenin mutant Wolffian ducts and ureteric buds. Wholemount in situ hybridization of e11.5 (A, B, E, F, I, J, M, N) and 12.5 (C, D, G, H, K, L, O, P) wildtype (A, C, E, G, I, K, M, O) and mutant (B, D, F, H, J, L, N, P) urogenital systems stained with antisense c-Ret (A–D), Wnt11 (E–H), Pax2 (I–L) and GDNF (M–P). In B, the black arrows indicate the wildtype Wolffian duct while the red arrows indicate areas that show no c-Ret expression. Black arrowheads indicate the wildtype metanephros and the red arrowheads indicate the mutant metanephros (ureteric bud in A– H, metanephric mesenchyme in I–P).
Although the above data suggested that there was a loss of c-ret expressing cells in β-catenin mutants, it was still possible that the branching pathway was activated somewhere downstream of the receptor. Wnt11 has been suggested to be a downstream target of GDNF/Ret signaling (Pepicelli et al., 1997). Normally expression is confined to the tips of the branching ureteric bud and no expression is seen in the wildtype WD at e11.5 or 12.5 (Figure 4E and G and data not shown). Similar to our observations of ret expression, Wnt11 levels in the endogenous UB were reduced or absent in β–catenin mutants at e11.5 and 12.5 (Figure 4F and H). Ventral/lateral branches and ectopic anterior buds, whether they were composed of mutant or wildtype cells, never expressed Wnt11 at detectable levels although in one case, where there was a clearly duplexed kidney at e12.5, the ectopic buds that resided within the MM did express Wnt11 (not shown).
β-catenin mutant cells also failed to express or maintain expression of two additional ureteric bud markers, Emx-2 and Sox-9 (data not shown). Further, formation of the ectopic epithelia did not depend on Ret/GDNF signaling as co-culturing mutant Wolffian ducts with a function blocking GDNF antibody did not affect formation of these structures while it did block formation of the ureteric bud (data not shown).
These data suggested that β-catenin was necessary for the expression of several factors that regulate branching of the UB. In order to address whether this was specific to genes expressed in the bud tips or was a general defect in specification or maintenance of the WD and/or collecting ducts, we examined the expression of several factors whose expression is not restricted to the UB tips.
Pax2 mRNA and protein are expressed in both the WD/UB system and the MM (Dressler et al., 1990). Expression in the WD/UB is not spatially restricted although protein levels are highest at the branching tips. Pax2 transcript and protein expression was examined in β–catenin mutants (Figure 4I–L and Figure 1A and B, respectively). Pax2 transcripts were detected in the Wolffian duct and ectopic ureteric buds and in the MM of β–catenin mutants (Compare Figure 4I and K to J and L). We did not detect mesenchymal expression of Pax2 adjacent to the more anterior or non-dorsal ectopic buds in vivo (Figure 4J and L). However, in 4/6 cases, the MM domain of Pax2 expression was greatly reduced by e12.5 (Figure 4L), most likely due to attenuated branching morphogenesis. Similar results were seen with antibodies to Pax2 although the levels of the protein appeared to be slightly decreased in β–catenin mutant cells compared to wildtype (Figure 1A′ and B′). The decreased expression of Pax2 in the mesenchyme correlated with decreased expression of the Ret ligand, GDNF (Figure 4N and P), a gene previously shown to be a transcriptional target of Pax2 (Brophy et al., 2001). No obvious deficits were seen in the expression of several other general WD markers including Crumbs3, kidney specific cadherin (Cdh16), Lethal giant larvae homolog 2, L1Cam, Hnf1 β and Sprouty1 (not shown) (Basson et al., 2005; Debiec et al., 2002; Gavert et al., 2005).
Proliferation rates are unchanged in early stage β–catenin mutant cells
Previous studies have shown that β-catenin regulates the rate of cell proliferation (He et al., 1998; Shtutman et al., 1999). Local changes in the rates of proliferation could explain the formation of ectopic buds and cysts in mutants. To determine if proliferation rates were affected by loss of β–catenin, we calculated proliferation rates in e11.5, 12.5, 14.5 and 18.5 wildtype and mutant WDs, UBs and Cds. β–catenin mutant cells (i.e. cells not expressing detectable levels of β-catenin protein) within the WD/UB did not show statistically significant differences in the rate of proliferation compared to wildtype at e11.5 (20+/− 9% vs. 16 +/−10% for Ki67, N=6, P=0.551) or 12.5 (8.65 +/− 11.7%, N=19 vs. 8.5 +/− 17.5% N=23, for Ki67, P=0.9). However, at e14.5 and P1, Ki67 staining indicated enhanced rates of cell division in mutant collecting duct cells relative to wildtype (13.2+/−11.0%, N=38 vs. 4.33 +/− 5.12%, N=23, P=.001 for e14.5 and 17.8 +/−.08%, N=7, vs. 1.8 +/−.01%, N=5, P=.001 P1, mutant vs. wildtype respectively).
At P1, some epithelia were still mosaic in their removal of β–catenin. Comparison of the proliferation rates of wildtype and mutant cells within P1 cysts from a mutant animal showed no statistical differences (15.8 +/− 0.1% vs. 17.8 +/−0.1% respectively. N=7. P=0.76) suggesting that the factor(s) leading to increased proliferation at later stages was non-cell autonomous and most likely a secondary consequence of ureter/collecting duct obstruction. TUNEL analysis revealed no statistical differences in cell death between wildtype and mutant WD/CD epithelia at e11.5, 12.5 and 14.5 although there did appear to be increased cell death in the adjacent mesenchyme at e12.5 and 14.5 (not shown). These data suggest that changes in the rates of cell proliferation/survival were not responsible for the ectopic budding phenotype although increased rates of cell proliferation likely contributed to cyst formation.
β-catenin null cells show no signs of adherens junction defects
The above data suggest that ectopic bud formation is not the result of activation of the ureteric bud branching pathway or changes in proliferation rates. A third possibility is that mutant cells are differentially adhesive relative to wildtype cells (i.e. mutant cells are more adhesive to other mutant cells than to wildtype and vice versa). β–catenin has previously been implicated in cell adhesion through its association with E-cadherin at the AJs. In order to determine whether the mutant Wolffian ducts displayed normal AJ formation, we examined the expression of E-cadherin as well as Scribble, a protein whose localization at the AJs requires E-cadherin, in mutant cells (Navarro et al., 2005). Mutant cells expressed both proteins at similar levels and in a similar domain to wildtype cells (Figure 5A″ and B″ and data not shown).
Figure 5.

Adherens junctions are not disrupted in β–catenin mutants. Wildtype (A,C,E) and mutant (B,D,E,F,H) stained for pan-cytokeratin (single channel in A′ and B′), E-cadherin (single channel in A″, B″, C″ and D″) and β–catenin (single channel in A‴, B‴, C‴ and D‴) and plakoglobin (C′ and D′) show mutant epithelia (arrows in B) express E-cadherin and plakoglobin normally. Toluidine blue stained sections (E and F) show histology of mutant extruded epithelia (arrowhead in F) relative to wildtype epithelia (E and arrow in F). Transmission electron microscopy showing the adherens junctions (arrows in G and H) are morphologically undisturbed in mutant epithelia (H) relative to wildtype (G). Magnification in G and H is approx. 60,000X).
As mentioned, in other examples of tissue specific ablation, plakoglobin, has been shown to compensate for mutant β–catenin in adherens junction formation/function (Haegel et al., 1995). To test whether this protein might be compensating for β-catenin in adherens junction formation in the developing kidney, we examined its expression in wildtype and mutant epithelia. Plakoglobin is expressed at low levels on the baso-lateral side of the WD/UB epithelium in wild type and mutant kidneys (Figure 5C′ and D′). Plakoglobin continues to be expressed in mutant cells although protein levels appear to be slightly increased relative to wildtype (Compare figure 5C′ and D′).
Finally, we examined wildtype and mutant epithelia via transmission electron microscopy. 1 uM transverse plastic sections of wildtype and mutant embryos were sectioned and stained with toluidine blue. Mutant sections were examined for the presence of clear ectopic epithelia (Figure 5F). Mutant epithelia were histologically distinct from wildtype due to lighter cytoplasmic staining and the presence of numerous small vacuoles (Arrowhead in Figure 5F). Upon identification of sections containing both a wildtype and mutant epithelia, thin sections were cut from the adjacent tissue and processed for transmission electron microscopy. At the ultra-structural level mutant epithelia possessed adherens junctions similar to wildtype (compare figure 5G and H). These data suggest that formation of ectopic epithelia is not due to defects in AJ formation or maintenance.
Mutant cells show normal apical/basal polarity
Previous studies have suggested that β-catenin regulates apical/basal polarity of epithelia. It seemed possible that defects in this process could lead to differential cell adhesion and the formation of ectopic buds. To address whether epithelial organization was affected by removal of β–catenin, we examined the expression of two apical epithelial markers, atypical protein kinase C (aPKC) and the actin cytoskeleton-associated protein, ezrin, as well as the basal lamina protein, laminin, in wildtype and mutant tissues (Berryman et al., 1993; Horne-Badovinac et al., 2001).
At all stages examined, aPKC, ezrin and laminin expression was indistinguishable between wildtype and β–catenin mutant cells (Figure 6A–G). In fact, completely segregated mutant epithelia expressed apical aPKC and were surrounded by an intact basal lamina as assessed by laminin and periodic acid Schiff’s staining and electron microscopy (Figure 6B, G and not shown). These data suggest that the mutant epithelium is properly organized/polarized.
Figure 6.

β-catenin mutant cells show normal apical/basal polarity. Wildtype (A, C and E) and mutant (B, D, F and G) e11.5 Wolffian ducts triple stained for the apical membrane marker aPKC (red), β-catenin (green) and Dapi (blue) in A and B, the baso-lateral membrane marker E-cadherin (red), the apical cytoskeletal marker Ezrin (green) and β-catenin (not shown) in C and D or the basal lamina marker laminin (red), β-catenin (green) and Dapi (blue) in E–G. β–catenin mutant epithelia are indicated by dotted white lines in B and D. A–D are 1000X. E–G are 630X.
β–catenin mutant cells show signs of premature differentiation
Cell to cell adhesion is largely regulated by the adherens junctions, tight junctions (Tjs) and desmosomes and their associated proteins (Balda and Matter, 2003). As discussed, the AJs were grossly unaffected in mutant cells and we could not detect differences in the expression of several AJ associated proteins and/or mRNAs in mutants including E-, Ksp and N-cadherin and scribble-1 (Figure 5 and not shown). We could not detect the presence of desmosomes in wildtype or mutant WDs at e11.5 by molecular or EM analysis (not shown) suggesting this structure was not playing a role in the observed differential cell adhesion.
The tight junctions consist of a number of transmembrane proteins, the claudins, jams and occludins, that are connected to the actin cytoskeleton by the cytoplasmic zona occludens (ZO) proteins, ZO-1, 2 and 3 (Matter et al., 2005). The ZO-1 protein consists of two isoforms, ZO-1α+ and −, that differ in the presence of an alternatively spliced exon in their C-terminus (Balda and Anderson, 1993; Willott et al., 1992). We initially examined the expression of the α+ isoform with an exon specific antibody as previous work suggested that this protein was expressed in virtually all epithelia, including those of the urogenital system (Kurihara et al., 1992). Surprisingly, our analysis showed ZO-1α+ was not expressed in the wildtype WD or UB through at least e12.5 (Figure 7A, 8A and not shown). At e14.5, ZO-1 α+ was detectable in the distal collecting ducts and ureter but not in the cortical collecting ducts or ureteric bud tips (Figure 8B). At P1, the majority of collecting duct epithelia expressed ZO-1α+ although some UB tips still did not (Figure 8C and not shown). The areas of ZO-1 α+ expression in wildtype embryonic kidneys corresponded to the most differentiated portions of the collecting duct system and the cells that showed low levels of transcriptionally active β-catenin as assessed by low level expression of the β-catenin target gene, Axin2 (Figure 7D and E), and lower cytoplasmic levels of (de-phosphorylated) β-catenin protein (Figure 7F) (Iglesias et al., 2007; Jho et al., 2002).
Figure 7.
ZO-1α+ expression inversely correlates with high levels of β-catenin activity in the developing WD and ureteric buds. Sections of e11.5 (A), 14.5 (B and F) and P1 (C) Wolffian ducts (A) and kidneys (B–C and F) stained with antibodies to the WD/UB/CD marker cytokeratin (blue), ZO-1α− (red) and ZO-1α+ (green) in A–C or a de-phosphorylated beta-catenin antibody (green in F). ZO-1α+ is not expressed in the e11.5 and 12.5 WD and ureteric bud (arrow in A and data not shown). ZO-1α+ expression is detectable in the e14.5 CD (white arrow in B) but not in the proximal tips of the UB (arrowhead in B). The majority of the UB/CD system is ZO-1α+ positive at P1 (arrows in C). ZO-1α+ is expressed in cells that lack high β-catenin transcriptional activity as indicated by the expression of Axin2 mRNA and excluded from cells with the highest levels of active (de-phosphorylated) β-catenin (arrowhead in F). Axin-2 is expressed throughout the e11.5 Wolffian duct (black arrow in D) and ureteric bud (black arrowhead in D) but only in the ureteric bud tips at e14.5 and P1 (black arrowhead in E and not shown).
Figure 8.
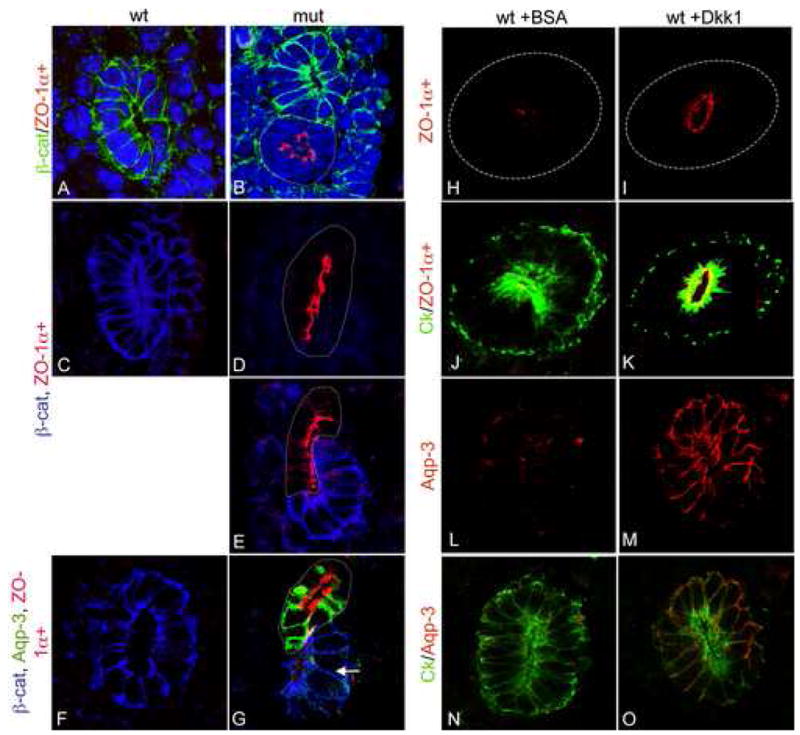
Blocking β–catenin function leads to the premature production of differentiation factors. Transverse sections through the Wolffian ducts of wildtype (A, C, F) or mutant (B, D, E, G) embryos. (A–B) Wolffian ducts triple stained for β–catenin (green), ZO-1α+ (red) and Dapi (blue). Note the expression of ZO-1α+ only in the β–catenin mutant epithelia (arrow in B). (C–E) Wolffian ducts stained for ZO-1α+ (red) and β-catenin (blue). Once again, β-catenin is only in the mutant cells, even when they are still part of an intact but mosaic Wolffian duct (E). F–G, Wolffian ducts stained with, aquaporin-3 (green), ZO-1α+ (red) and β–catenin (blue). Note the expression of Aqp-3 in the β–catenin mutant cells but not in wildtype cells from the same duct (arrow in G). β-catenin mutant cells are outlined with dotted line in all images. H –O show transverse sections through e10.5 Wolffian ducts cultures for 48 hours in the presence of 200ng/ml BSA (H, J, L and N) or recombinant Dkk1 (I, K, M and O) stained with ZO-1α+ (Red in H–K. H and I show single channel.), Aqp-3 (Red in L–O. L and M show single channel.) and cytokeratin (green in J, K, N and O). A–G are 1000x, H and I are 630X.
β–catenin mutant cells expressed ZO-1 α+ prematurely in both the WD and UB at e11.5 and 12.5 (Fig 8B, D, E and G and not shown). To ascertain whether this was a general effect on the tight junction associated proteins, the expression of the alternative isoform of ZO-1 (referred to as ZO-1 α−) and an additional tight junction marker, occludin, was also examined. Both proteins were expressed normally in wildtype and mutant WD and UB/CD at e11.5, 12.5, 14.5 and P1 (data not shown).
The premature expression of ZO-1α+ in β–catenin mutant cells and the loss of expression of factors implicated in precursor cell maintenance (including Sox9) suggested that β-catenin was required to maintain cells of the branching ureteric bud in an undifferentiated or precursor state. Previous studies have shown a role for β-catenin in the maintenance of precursor cells in multiple organ systems as well as in embryonic stem cells (Zechner et al., 2003). To test whether β-catenin was necessary for maintenance of precursors in the WD/UB, we examined the expression of a bona fide marker of the differentiated collecting duct, the water channel protein, Aquaporin-3 (Aqp-3) (Yamamoto et al., 1997). Similar to the case with ZO-1 α+, Aquaporin-3 protein was not detectable in the wildtype WD or UB/CD from e11.5–14.5 (Figure 8F and not shown) but was expressed in β–catenin mutant cells of e11.5 WDs (6/10, Figure 8G).
Importantly, mutant cells that were still part of an intact but mosaic WD expressed ZO-1α+ and Aqp-3 in a cell autonomous fashion and wildtype cells from β-catenin mutant embryos never expressed either protein, even if they were part of an extruded epithelial bud or cyst (not shown). These data suggest that the premature differentiation is due to loss of β-catenin, not epithelial extrusion. To confirm that the premature differentiation was directly caused by a defect in β-catenin signaling, we cultured e10.5 Wolffian ducts for 48 hours in the presence of the canonical Wnt antagonist, Dkk1. Dkk1 treated ducts showed reduced branching of the ureteric bud (not shown) and expressed high levels of ZO-1α+ and Aqp-3 relative to ducts treated with BSA (Figure 8H–O). However, we did find that cultured ducts, in contrast to the situation in vivo, did express low levels of ZO-1α+ and Aqp-3 (Figure 8H and L), suggesting that some factor required to maintain these cells in a fully undifferentiated state is missing from the culture system.
Interestingly, β-catenin mutant cells did not express other markers of the terminally differentiated collecting duct system, including Aquaporin-2, the urea transporter (UTA-1) or the renal outer medullary potassium channel protein (ROMK) or a marker of the terminally differentiated male reproductive tract, Aqp-9, suggesting that mutant cells represented an intermediate step in Wolffian duct/ureteric bud differentiation (not shown).
Overexpression of a stabilized form of β-catenin in the collecting duct system blocks the expression of differentiation products
The loss of β-catenin activity correlates with the expression of differentiation products. We therefore hypothesized that overexpression of β-catenin in the developing collecting duct system would prevent the expression of differentiation markers. To test this hypothesis, mice carrying the Hoxb7-Cre transgene were crossed to mice carrying an allele of β-catenin that has lox-p sites flanking exon 3. Exon 3 carries several serine and threonine residues that are phosphorylated by GSK3β targeting the protein for degradation. Removal of this exon results in expression of a stabilized form of β-catenin.
Mice driving expression of stabilized β-catenin in the collecting ducts die within 24 hours of birth. The majority of mutant pups completely lacked a kidney, although some possessed hypoplastic/cystic kidneys (Figure 9B and data not shown). We examined the expression of ZO-1α+, Aqp-3 and β-catenin in the hypoplastic kidneys. Overexpression of stabilized β-catenin strongly correlated with decreased expression of Aqp-3 throughout the collecting duct system while ZO-1α+ levels appeared to be largely unaffected (Compare Figure 9C and D). We also examined the expression of Aqp-2 in hypoplastic kidneys. Although Aqp-2 was not ectopically expressed in mice lacking β-catenin, we hypothesized that stabilization of β-catenin would block production of many, if not most, differentiation products. Similar to the case with Aqp-3, mice overexpressing β-catenin in their collecting ducts had greatly reduced levels of Aqp-2 (Compare Figure 9E and F). These data suggest that continued expression of β-catenin blocks some aspects of the differentiation process.
Figure 9.

Expression of a stabilized form of β-catenin leads to hypoplasia and a lack of differentiation. Wholemount (A–B) and sections (C–F) of urogenital systems from P1 β-cat ex3fl/+ (A,C,E) or Hoxb7cre; β-cat ex3fl/+ (B,D,F). Sections have been stained with antibodies to Aqp-3 (green), ZO-1α+ (red) and β-catenin (blue) in C–D or Aqp-2 (green), ZO-1α+ (red) and β-catenin (blue) in E–F. Collecting ducts of mice expressing an activated form of β-catenin show greatly decreased levels of Aqp-2 and 3. Arrows in A and B indicate the kidneys.
Discussion
Although a role for β-catenin in kidney development and disease has long been hypothesized, genetic evidence has been lacking. Two recent studies revealed a requirement for this factor in the mesenchymal to epithelial transition that leads to the formation of the renal vesicles (Park et al., 2007; Schmidt-Ott et al., 2007). However, the recent characterization of β-catenin reporters in developing kidneys suggests that this molecule has high transcriptional activity in a distinct cell lineage, the Wolffian duct, and its derivative, the ureteric bud (Iglesias et al., 2007; Jho et al., 2002; Maretto et al., 2003). To determine a role for β-catenin in the development of these tissues, we analyzed mice carrying a Hoxb7-Cre transgene and a floxed allele of β-catenin (catnnbflox). Hoxb7-Cre mediated removal of β–catenin led to multiple defects in the urogenital system including complete aplasia and varying degrees of hypoplasia and cystic dysplasia.
Kidney aplasia/hypoplasia is a relatively common human disorder that is thought to be the result of a failure to initiate the proper developmental programs. Based on several pieces of data, we suggest that these phenotypes in β-catenin mutants are caused by a failure to maintain cells of the ureteric bud tips in a precursor or undifferentiated state. First, β–catenin mutant cells showed several signs of premature differentiation including the precocious expression of ZO-1α+ and Aquaporin-3 and reduced levels of Pax-2 mRNA and protein as well as the loss of several branching markers (e.g. Ret, Wnt11, Emx-2 and Sox-9). General Wolffian duct/ureteric bud/collecting duct markers Lglh2, Crb3, Cadh16, Spry1, Hnf1 β and L1Cam were not affected. The fact that mutant cells maintained expression of generalized epithelial markers and markers of ureteric bud derived collecting duct suggested this was not a defect in cell specification or tissue maintenance. Secondly, expression of a stabilized form of β-catenin in the collecting ducts prevented the expression of several differentiation gene products. Third, there was a tight inverse correlation between high β-catenin activity in wildtype kidneys (as determined by expression of the β-catenin target gene, Axin2) and differentiation of the ureteric bud/collecting duct (as determined by ZO-1α+ and Aquaporin-3 expression) (Iglesias et al., 2007; Jho et al., 2002) and our study). Finally, the premature differentiation that occurred in β-catenin mutant cells occurred in the absence of changes in cell proliferation, suggesting this was not an indirect consequence of decreased rates of cell proliferation.
Wolffian ducts from Hoxb7-Cre; catnnb−/flox embryos formed ectopic epithelial buds that led, in some cases, to multiplexed kidneys. Interestingly, due to mosaic ablation of β-catenin, these ectopic epithelia could be composed of either wildtype or mutant cells but they were never mosaic. Initially we assumed that these ectopic structures were ectopic ureteric buds and that removal of β-catenin caused hyperactivation of the ureteric bud branching pathway. However, molecular analysis suggested this was not the case. Ureteric bud branching is a complex process regulated by both positive and negative effectors (Costantini and Shakya, 2006). In all models of ectopic ureteric bud formation examined to date, the GDNF/Ret pathway appears to be ectopically activated (Basson et al., 2005; Grieshammer et al., 2004; Kume et al., 2000; Shakya et al., 2005). However, we saw no expansion of Gdnf mRNA expression in the mesenchyme of mutants. In fact, mutant epithelial cells appeared to express greatly reduced levels of mRNA for the GDNF co-receptor, Ret. Further, the majority of ectopic epithelia did not express the Ret target gene Wnt11 suggesting that the Ret signaling pathway was not active in these cells. Finally, the observation that culturing mutant ducts in the presence of a function-blocking antibody to GDNF did not affect formation of the ectopic epithelia provided additional support that this phenotype occurred independent of Ret/GDNF signaling. However, it is important to note that in all cases where ectopic buds gave rise to duplexed kidneys, the collecting duct epithelium of the ectopic organ was genetically wildtype or mosaic, suggesting that it was derived from a wildtype ectopic bud (N=3). Although we found several clear cases where β-catenin mutant epithelia invaded the MM, these mutant cells were not capable of undergoing normal branching morphogenesis, presumably due to the loss of ret expression.
The data discussed above raise the question of how or why mutant Wolffian ducts form ectopic buds? Insight into this question came from the observation that Wolffian ducts that were composed largely or exclusively of mutant cells had a rough appearance but did not form ectopic epithelia or even an endogenous ureteric bud (Figure 8 not shown). These data suggested that the formation of the ectopic epithelia was dependent on the presence of both wildtype and mutant cells in the same duct. We suggest the following explanation of these results. Ectopic epithelial buds are actually cells that are being extruded from the Wolffian duct. Extrusion is the result of differential adhesiveness between wildtype and mutant cells (i.e. wildtype cells are more adhesive to other wildtype cells than to mutant cells and vice versa). Epithelial extrusion occurs when cells of the two opposite genotypes (β–catenin + or −) abut. Differential adhesiveness causes the cells of each genotype to co-segregate with other cells of the same genotype but to segregate away from cells of the alternate genotype. If the majority of cells in a duct are wildtype, then mutant cells are extruded and vice versa. If all cells are mutant (or wildtype), no cells are extruded and therefore no ectopic buds form. If the extruded cells are wildtype and they invade the MM, they can support normal growth of the kidney. If more than one wildtype population is extruded in the vicinity of the MM, they can each branch leading to multiplexed kidneys. However, if cells adjacent to the MM are mutant or lose β-catenin shortly after invasion, branching ceases, at least in part due to loss of ret expression, resulting in aplastic or hypoplastic organs. As the mutant ectopic epithelia do not express markers of the ureteric bud but do express markers of the differentiated collecting duct, they should not be called ectopic ureteric buds.
We suggest that all of the phenotypes discussed above can be explained by the observed premature differentiation that occurs in mutant cells. Relative differences in adhesive strength between differentiated (β-catenin −) and undifferentiated (β-catenin +) cells (and the subsequent epithelial extrusion) may be the result of the differential expression of ZO-1 isoforms. Previous studies have shown that ZO-1α+ is the last component incorporated into the developing tight junction in the mouse blastocyst and that expression of this protein correlates with a differentiated, rigid epithelium (Balda and Anderson, 1993; Fleming et al., 2000; Kurihara et al., 1992; Sheth et al., 1997; Willott et al., 1992). Premature expression of ZO-1α+ in the mutant Wolffian ducts and ureteric bud/collecting duct system presumably would cause these cells to be more adhesive to other mutant/ZO-1α+-expressing cells than to wildtype, undifferentiated cells. It will be interesting to determine whether premature expression of ZO-1α+ alone is sufficient to lead to differential cell adhesion and whether production of this isoform can be used as a generalized readout for loss of β-catenin signaling in other epithelial tissues. As alternative splicing and inclusion or exclusion of the α+ exon determines the production of the two ZO-1 isoforms, premature expression of the α+ isoform cannot be a direct response to loss of β-catenin. However, β–catenin might directly regulate the expression of a splicing factor that acts on ZO-1 pre-mRNA.
Differential adhesiveness, along with the loss of factors necessary for ureteric bud branching (such as Ret), would prevent further branching. We cannot rule out the possibility that expression of some ureteric bud tip markers is directly regulated by β-catenin. For instance, previous studies have suggested that c-Ret is a target of canonical Wnt signaling in PC12 cells (Zheng et al., 1996), however we do not see expansion of c-Ret expression in the Hoxb7-Cre;catnbexon3flox kidneys suggesting this is unlikely (data not shown). Nor can we completely rule out the possibility that the differential adhesiveness between wildtype and mutant cell is unrelated to the premature differentiation and is instead the result of an additional role for β-catenin in mediating cell adhesion. Although previous studies have shown that, in addition to its signaling role, β-catenin also plays a role in adherens junction formation, TEM and molecular analysis of Hoxb7Cre;catnb−/flox Wolffian ducts and kidneys suggested that this structure was intact in mutant cells. This is most likely due to functional compensation by plakoglobin, a protein that is closely related to β–catenin, and strongly expressed in mutant cells. However, we cannot rule out the possibility that β-catenin plays a more subtle role, directly or indirectly, in regulating the relative adhesive states of cells and that this is unrelated to the premature differentiation phenotype.
Our data suggest that high levels of β-catenin signaling are necessary to maintain the ureteric bud fate, at least in part by keeping these cells in an undifferentiated/precursor state. However, whether the differentiation of the entire collecting duct system requires ablation of β-catenin activity or merely a decrease remains an open question. The fact that some transgenic β-catenin reporters show expression in the distal collecting duct suggests that lower levels of β-catenin activity may be present in this cell type (Iglesias et al., 2007; Maretto et al., 2003). Interestingly, although all β-catenin mutant cells expressed ZO-1α+, not all mutant Wolffian duct cells expressed Aqp-3. It seems plausible that these two markers are differentially responsive to β-catenin levels and it is tempting to speculate that different levels of β-catenin may play roles in the cortical/medullary patterning of the collecting ducts with the highest levels necessary for precursor cell maintenance.
In summary, we have shown that Wolffian duct/ureteric bud derived cells expressing high levels of transcriptionally active β-catenin represent undifferentiated precursor cells. Reduction of β–catenin activity, either as a result of normal inactivation or genetic ablation, results in activation of the differentiation program while expression of a signaling active version of the protein blocks differentiation. We suggest that β-catenin levels mediate the balance between differentiated and undifferentiated epithelia that occurs during normal development and branching of the collecting duct system and that disruption of this balance and the secondary effects it has on other cellular processes, such as cell adhesion, most likely contributes to the progression of various diseases of epithelial tissues including kidney aplasia and cystic hypoplasia.
Acknowledgments
The authors would like to thank Ondine Cleaver, Peter Igarashi, Roy Zent and Courtney Karner for reading and commenting on this manuscript, Mark Knepper, Fangming Lin, Michel Baum, Chou-Long Huang and Jean-Paul Borg for providing us with antibodies, Makoto Taketo and Jon Graff for providing us with mice and Bryan Adams for technical support and Cormac and Ella for non-technical support. This work was supported by grants from the Polycystic Kidney Research Foundation, the Irene Simons Kidney Cancer Foundation, the University of Alabama, Birmingham Recessive Polycystic Kidney Disease Core Center (NIH 5 P30 DK074038-02) and a Gottschalk Young Investigator Award from the American Society for Nephrology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–74. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Anderson JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–24. doi: 10.1152/ajpcell.1993.264.4.C918. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. Epithelial cell adhesion and the regulation of gene expression. Trends Cell Biol. 2003;13:310–8. doi: 10.1016/s0962-8924(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–39. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105 ( Pt 4):1025–43. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–9. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–75. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–56. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–27. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol. 1996;134:133–48. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Debiec H, Kutsche M, Schachner M, Ronco P. Abnormal renal phenotype in L1 knockout mice: a novel cause of CAKUT. Nephrol Dial Transplant. 2002;17(Suppl 9):42–4. doi: 10.1093/ndt/17.suppl_9.42. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–95. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Papenbrock T, Fesenko I, Hausen P, Sheth B. Assembly of tight junctions during early vertebrate development. Semin Cell Dev Biol. 2000;11:291–9. doi: 10.1006/scdb.2000.0179. [DOI] [PubMed] [Google Scholar]
- Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, Ben-Ze’ev A. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–42. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–17. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol. 2007 doi: 10.1152/ajprenal.00416.2006. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–95. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Farquhar MG. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1992;89:7075–9. doi: 10.1073/pnas.89.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–65. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–8. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Law AC, Dor Y, Melton DA. Beta-catenin is essential for pancreatic acinar but not islet development. Development. 2005;132:4663–74. doi: 10.1242/dev.02063. [DOI] [PubMed] [Google Scholar]
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, Birnbaum D, Borg JP. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–9. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–9. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol. 1997;192:193–8. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- Perantoni AO. Renal development: perspectives on a Wnt-dependent process. Semin Cell Dev Biol. 2003;14:201–8. doi: 10.1016/s1084-9521(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–47. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Masckauchan TN, Chen X, Hirsh BJ, Sarkar A, Yang J, Paragas N, Wallace VA, Dufort D, Pavlidis P, Jagla B, Kitajewski J, Barasch J. {beta}-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development. 2007;134:3177–90. doi: 10.1242/dev.006544. [DOI] [PubMed] [Google Scholar]
- Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development. 2004;131:1449–62. doi: 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, Burke R, D’Agati V, Costantini F. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. doi: 10.1016/j.ydbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–37. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Towers PR, Woolf AS, Hardman P. Glial cell line-derived neurotrophic factor stimulates ureteric bud outgrowth and enhances survival of ureteric bud cells in vitro. Exp Nephrol. 1998;6:337–51. doi: 10.1159/000020541. [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992;262:C1119–24. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- Woolf AS. Renal hypoplasia and dysplasia: starting to put the puzzle together. J Am Soc Nephrol. 2006;17:2647–9. doi: 10.1681/ASN.2006080841. [DOI] [PubMed] [Google Scholar]
- Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004;15:998–1007. doi: 10.1097/01.asn.0000113778.06598.6f. [DOI] [PubMed] [Google Scholar]
- Woolf AS, Yuan HT. Development of Kidney Blood Vessels. In: Peter ASW, Vize D, Bard Johnathan BL, editors. The Kidney: From Normal Development to Congenital Disease. Vol. 1. Academic Press; Boston: 2003. pp. 251–266. [Google Scholar]
- Yamaguchi Y, Yonemura S, Takada S. Grainyhead-related transcription factor is required for duct maturation in the salivary gland and the kidney of the mouse. Development. 2006;133:4737–48. doi: 10.1242/dev.02658. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, Fujinaka H, Marumo F, Kihara I. Expression of AQP family in rat kidneys during development and maturation. Am J Physiol. 1997;272:F198–204. doi: 10.1152/ajprenal.1997.272.2.F198. [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–12. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–18. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zheng S, Ramachandran B, Haigh JR, Palos TP, Steger K, Howard BD. The induction of ret by Wnt-1 in PC12 cells is atypically dependent on continual Wnt-1 expression. Oncogene. 1996;12:555–62. [PubMed] [Google Scholar]



