Summary
Recent studies report that B cells acquire large lymph-borne antigens and immune complexes directly from subcapsular sinus macrophages while small antigens appear to diffuse directly into the B cell follicles. To directly compare entrance of large and small Ags into the follicles, we used multiphoton intravital microscopy to track drainage of Ag into the peripheral lymph nodes and subsequent encounter by B cells and follicular dendritic cells in the underlying follicles. We find that a system of conduits extends into the follicles and mediates delivery of small antigens to cognate B cells and follicular dendritic cells. The follicular conduits provide an efficient and rapid mechanism for delivery of small antigens to B cells, which directly contact the CXCL13 chemokine-enriched conduits. By contrast, large antigens were bound by subcapsular sinus macrophages and subsequently transferred to follicular B cells as previously reported.
Introduction
Lymph nodes (LNs) are strategically positioned and functionally partitioned to facilitate the initiation of immune responses to foreign antigens (Ags). Ags drain into the node via the afferent lymph, or are delivered by dendritic cells (DC) (Kissenpfennig et al., 2005). A series of cellular interactions in anatomically distinct compartments of the LN then governs the development of both a cellular and humoral immune response aimed at the elimination of the eliciting Ag (von Andrian and Mempel, 2003). While T cells rely on processed Ag presented in the context of MHC molecules, B cells recognize Ag in its native, unprocessed form via their immunoglobulin (Ig) receptor (Germain, 1994; Rajewsky, 1996). The anatomical site in which B cells first encounter Ag depends on the route of infection, i.e. intravenous (i.v.), mucosal or subcutaneous (s.c.). Naïve B cells circulate through the secondary lymphoid organs including the spleen and peripheral LNs to increase the likelihood of Ag encounter (Allen et al., 2007; Hauser et al., 2007; Okada and Cyster, 2006; Schwickert et al., 2007; von Andrian and Mempel, 2003). Their accumulation in the follicular region is controlled by chemokines such as CXCL13, which is secreted by specialized stromal cells including follicular dendritic cells (FDC) and follicular stromal cells (FSC) (Allen and Cyster, 2008; Gunn et al., 1998). On encounter with Ag, B cells are activated and migrate to the T-B cell border where they interact with cognate T cells (Garside et al., 1998; Okada et al., 2005). Given their central location, FDC provide an efficient site for retention of Ag that enters via the blood or lymph. Ag is retained chiefly by complement- (CD21/CD35) and Fc- (FcγRIIB) receptors (Carroll, 2004; Nimmerjahn and Ravetch, 2006; Tew et al., 1990). Moreover, FDC foster development of the germinal center reaction once B cells are activated (Cyster et al., 2000; Kosco and Gray, 1992). This specialized environment in which B cells undergo expansion, class switch recombination and somatic hypermutation, promotes differentiation of the Ag specific B cells into memory or plasma cells (MacLennan, 1994; Kelsoe, 1995; Rajewsky, 1996; Victoratos et al., 2006).
In the spleen, marginal zone B cells transport Ag into the follicles (Cinamon et al., 2008; Ferguson et al., 2004; Youd et al., 2002; Guinamard et al., 2000; Pozdnyakova et al., 2003). However, LNs lack an equivalent B cell subset. Earlier studies reported transport of Ag into the LN follicles via a subset of subcapsular sinus (SCS) macrophages identified as binding a fusion protein of the N-terminal cysteine rich (CR) domain of the mannose receptor fused to the Fc region of human IgG (CRFc+) (Berney et al., 1999; Martinez-Pomares et al., 1996). In addition, non-phagocytic cells of dendritic appearance have been implicated in Ag transport (Szakal et al., 1983). An alternative mechanism is a system of conduits that permeates both LNs and spleen, yet these are generally considered to mainly supply the T cell areas of the LN (Gretz et al., 2000; Okada and Cyster, 2006; Sixt et al., 2005). The recent finding that lymph-borne Ags are differentially taken up and subsequently processed according to size suggests a more selective process. Large lymph-borne Ags are bound by SCS macrophages which line the floor of the LN (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2007) and made available to the underlying B cell compartment. Thus, cognate B cells survey the SCS for specific Ag while immune complexes (ICs) are taken-up by follicular (FO) B cells via complement receptors CD21/CD35 (Phan et al., 2007). By contrast, small Ags (less than 70 kDa) either enter conduits leading to the paracortical (T cell) area (Sixt et al., 2005) or may diffuse via gaps in the SCS floor into the follicles where they are taken up by cognate B cells (Pape et al., 2007).
To directly compare transport of large and small Ag into the B cell follicles in vivo, we have used a combination of multiphoton intravital microscopy (MP-IVM) (Lindquist et al., 2004; Mempel et al., 2004; Miller et al., 2002; Shakhar et al., 2005), electron microscopy, confocal analyses of cryosections (Carrasco and Batista, 2007; Gretz et al., 2000; Pape et al., 2007; Sixt et al., 2005), and flow cytometry analyses (FACS) of peripheral LNs (Phan et al., 2007). As reported earlier, large Ags are transported into B cell areas via FO B cells in a complement-dependent manner. By contrast, a novel network of follicular (FO) conduits was identified that mediates rapid transport of small soluble protein Ags from the SCS into the B cell follicular region. Moreover, the conduits provide a source of B cell chemokine (CXCL13), which is secreted by FSC. Together, the results suggest a pathway for channelling small Ags and chemokines into the B cell area.
Results
Differential transport of large and small Ags
To compare directly the uptake of large and small Ags by FDC, mice were injected s.c. in the hind footpad with a small volume of soluble ICs prepared from fluorescently labelled KLH (large, monomer 450 kDa) or turkey egg lysozyme (TEL) (small, 14 kDa). On cryosections of draining popliteal LNs (pLNs) we identified uptake of TEL on FDC 6 hrs post-injection (Figure 1A, right panel). By contrast, negligible levels of the larger KLH Ag were observed at this time (Figure 1A, left panel). However, by 24 hrs post-injection, similar levels of TEL and KLH had accumulated on FDC (results not shown). Similar results were obtained with mice actively immunized by a previous injection of TEL or passively immunized with specific rabbit polyclonal antisera (results not shown). Since IC-formation in situ upon injection of free Ag more closely mimics the physiological process, we passively immunized mice in subsequent experiments by intraperitoneal (i.p) injection of antiserum several hours before s.c. injection of Ag. A similar approach was reported by Phan et al to track uptake and transport of large Ag into the B cell follicles (Phan et al., 2007).
Figure 1.
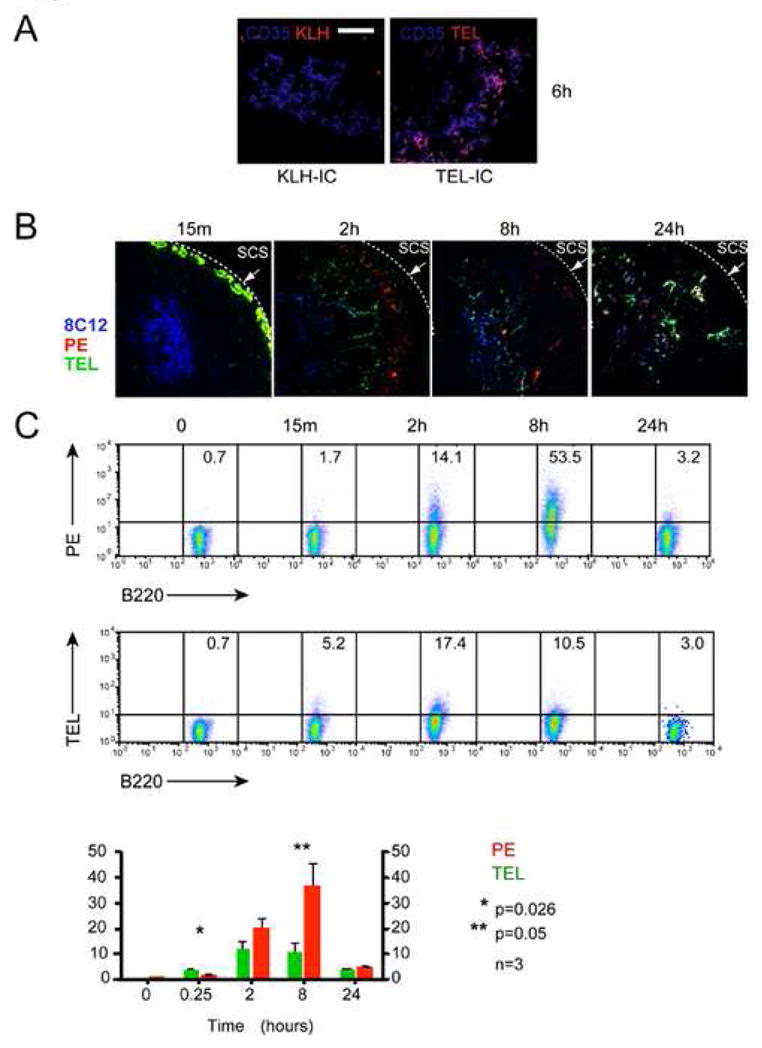
Uptake of Ag on FDC in peripheral LNs is size dependent. (A) Confocal fluorescence micrographs of histological sections of pLNs show delay of KLH deposition on FDC (Blue, Cy5-anti-CD35) relative to TEL, 6 hrs after injection of IC. Scale bar represents 50 μm. Images are representative of at least 3 independent experiments. (B, C) Immunized mice were left untreated (t=0) or injected with a mixture of 10 μg TEL Alexa 488 and 10 μg B-PE s.c. in the hindflanks. (B)The draining inguinal LNs were removed at various times and the deposition of IC on FDC was analyzed by confocal microscopy using a monoclonal antibody specific for CD35 to label FDCs. (C) In vivo uptake of IC containing small or large Ags by polyclonal B cells was analyzed by FACS. The percentages of B220+ cells that have acquired TEL-IC or PE-IC are indicated and are represented in the graph as the mean values ± SEM from at least three LNs. CD45.1+ cells were added during processing of inguinal LNs from CD45.2+ recipient mice to control for ex vivo capture of IC. Statistical significance was determined using a one-tailed, paired Student’s t-test.
To examine more closely the kinetics of uptake of large and small lymph-borne Ags by FDC, passively immunized mice were injected s.c. in the hind flanks with an equal molar mixture of large (Phycoerytherin, PE, ~240 kDa) and small (TEL, ~12–14 kDa) Ags. Draining inguinal LNs were harvested at various times for analysis. Both Ags were present in the SCS sinus by 15 min and small Ag was first observed on FDC at 2 hrs post-injection, whereas PE Ag was not observed on FDC until at least 8 hrs, consistent with Phan et al (Phan et al., 2007) (Figure 1B). By 24 hrs, both Ags were observed on FDCs, consistent with our previous finding using KLH and TEL in the pLN. Thus, small Ags are taken-up by FDC more rapidly than large ones in both the pLNs and inguinal LNs, although the process is slower in the latter probably due to less efficient drainage from the injection site. Small Ag (TEL) injected in non-immune mice did not detectably bind to FDC and was not retained in the draining LN (Figure S1).
IC composed of large Ags such as PE are transported by naïve B cells from the SCS into the follicles and transferred to FDC in a complement receptor-dependent manner (Phan et al., 2007). To evaluate the relative efficiency of FO B cell transport of large and small Ags, single cell suspensions prepared from inguinal LNs at varying times following s.c. injection were analyzed by FACS. PE was found on FO B cells as early as 2 hrs, peaked at 8 hrs then decreased at 24 hrs post-injection (Figure 1C). Uptake was dependent on expression of CD21/CD35 on FO B cells as characterization of Cr2 mixed chimeric mice identified significantly greater levels of PE Ag on Cr2+ than Cr2− B cells, consistent with the report by Phan et al. (Phan et al., 2007)(Figure S2). By contrast, B cell uptake of small Ag was less pronounced, with maximal uptake at 2 hrs and subsequent decrease (Figure 1C). Thus, B cell transport of small Ags is not as efficient as with large Ags, yet the former is identified on FDC within a shorter time period (Figure 1B & C). This suggests that small Ags are transported to FDC by both B cell-dependent and -independent mechanisms.
Conduits mediate small Ag transport into follicles
Previous studies on Ag transport in secondary lymphoid organs have identified a system of conduits that originate from the SCS and permeate the interfollicular zone and paracortical T cell area in LNs (Gretz et al., 2000; Sixt et al., 2005). These conduits consist of a core of collagen fibres ensheathed by a basement membrane and a layer of fibroblastic reticular cells (FRC), forming an inner tubular space accessible to small molecules with a hydrodynamic radius of about 4–5 nm or an approximate molecular weight of 70 kDa (Gretz et al., 2000; Sixt et al., 2005; Nolte et al., 2003). Since DC can sample Ags from conduits, these were first proposed to have an important function in facilitating the presentation of lymph-borne Ags to T cells by conduit-associated DC (Sixt et al., 2005). In addition, Qi et al. found that DC could also present intact Ag to B cells when these transit the T zone after entering the LN via high endothelial venules (Qi et al., 2006).
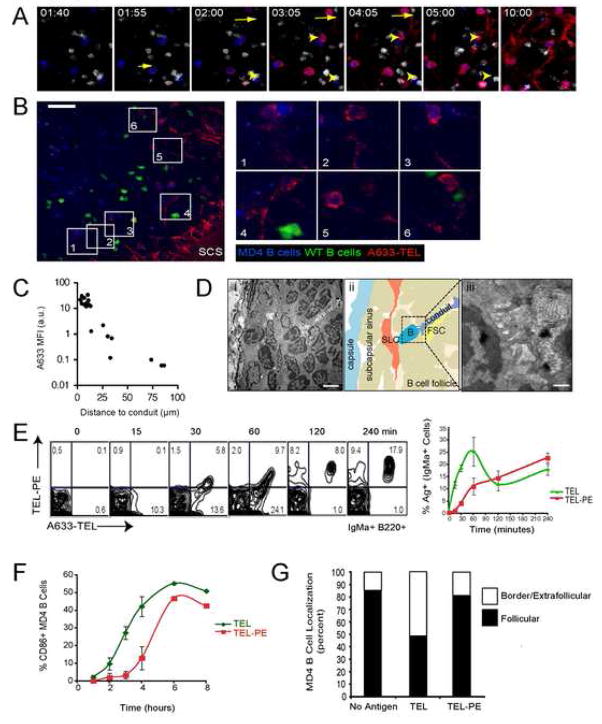
The reticular collagen fibre network (which under the illumination conditions required for multiphoton fluorescence excitation becomes visible due to second harmonic generation) within B cell follicles is less abundant than that in T cell areas (Gretz et al., 2000). To determine whether collagen fibres in B follicles are also associated with conduits and if these participate in channelling Ag directly into the B cell follicle, non-immune mice were injected s.c. in the hind footpad with a mixture of TEL (12–14 kDa) conjugated either to the large molecular weight (~240 kDa) fluorochrome PE (“large TEL”, TEL-PE) or to the small molecule (~1.2 kDa) dye Alexa Fluor 633 (“small TEL”, A633-TEL) and the pLN imaged by MP-IVM directly thereafter. In addition, to track entry of Ags into the follicles, recipient mice were seeded with fluorescently labelled naïve polyclonal primary B cells and with BCR transgenic MD4 B cells that recognize TEL with high affinity. Notably, small TEL was first observed within the SCS and then started to fill FO conduits in a centripetal direction within less than two minutes following injection (Figure 2A and B and Movie S1). Importantly, the small Ag became detectable in the interstitial space and on MD4 B cells only with several minutes delay (Figure 2C), suggesting that conduits did not accumulate the Ag from an interstitial pool, but conversely that conduits delivered the Ag to the interstitium. By contrast, the large TEL conjugate was excluded from the conduits, as expected, based on its size (Figure 2A and B). The absence of large TEL-PE conjugates from the follicular conduits is consistent with observations of exclusion of proteins of approximate size over 70 kDa (Gretz et al., 2000; Sixt et al., 2005). We noted, though, at a time-point when the concentration of small TEL had reached its maximum in the conduits, that large TEL began to slowly penetrate the follicle from the SCS in an interstitial pattern, independent of conduit filling (Figure 2A, B, and D). We speculate that this delayed entry of the large Ag occurs through a distinct mechanism, possibly by diffusion (Pape et al., 2007), or by binding to non-Ag-specific B cells after shuttling of the Ag across the floor of the SCS (Phan et al., 2007).
Figure 2.
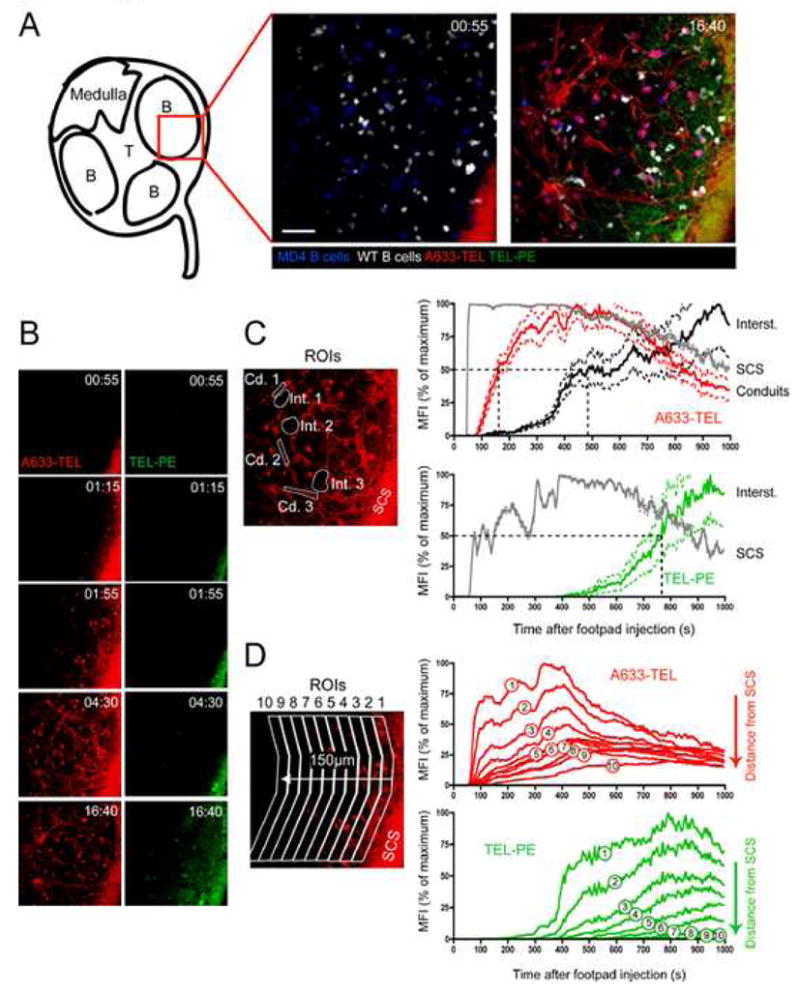
Small Ags are rapidly delivered to LN follicles through FO conduits. A633-TEL (red, containing 50 ng TEL) and TEL-PE (green, containing 5 μg TEL) were injected into the footpads of mice and their entry into the draining LN monitored by MP-IVM (See also Movie S1). (A) Intravital micrographs depicting the distribution of the small (A633-TEL, red) and large (TEL-PE, green) Ag in a B follicle early (55 sec., left panel) or late (16 min., 40 sec, right panel) after footpad injection. (WT B = white; MD4 B = blue) Scale bar = 30 μm. (B) Micrographs from selected time-points depicting either A633-TEL or TEL-PE. Time in minutes and seconds. (C) Regions of interest (ROIs) were defined to measure the mean fluorescence intensity (MFI; individually normalized, averaged MFIs ± SEM vs. time after TEL injection) of A633-TEL and TEL-PE in the subcapsular sinus (SCS), conduits (Cd. 1–3), and in the interstitial space (Int. 1–3). Dotted lines indicate half-maximal values for TEL and TEL-PE in conduits and interstitium. (D) ROIs with increasing distance from the SCS were defined and the normalized MFIs of A633-TEL (top panel) and TEL-PE (bottom panel) plotted against time after injection. This experiment was repeated twice with similar results.
To examine further the size exclusion of FO conduits, mice were injected s.c. in the footpad with fluorescently labelled KLH (~450 kDa) and dextrans (of either 40 or 150 kDa molecular weight). Confocal analysis identified localization of these Ags in the SCS within minutes of injection but only the small, 40 kDa dextran also entered the conduits (Figure S3 and results not shown). Thus, FO conduits appear to have a similar size exclusion limit as those characterized in the T cell area (Gretz et al., 2000).
Conduits are structurally similar in the T and B cell areas
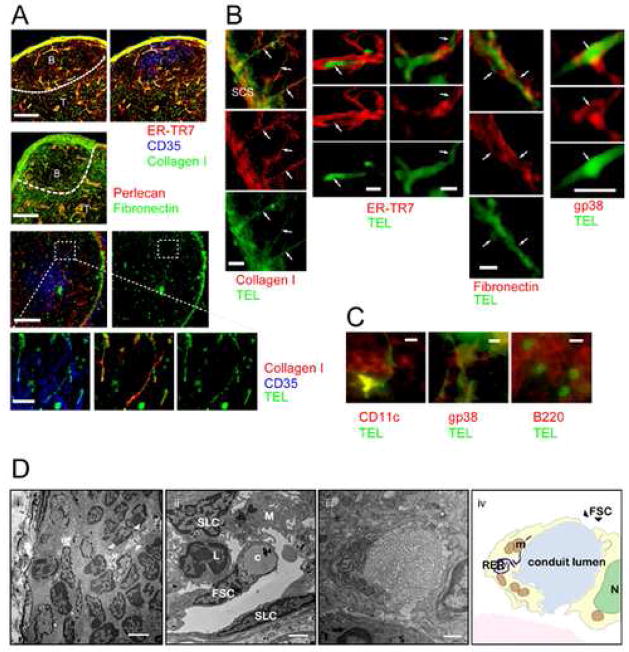
Conduits within the T cell area are characterized by a type I collagen core ensheathed by a fibronectin-containing basement membrane. They are derived from fibroblastic reticular cells (FRC) and are identified by antibodies to ER-TR7 and gp38 (Katakai et al., 2004). To characterize further the conduits in the B cell follicles, naïve mice were injected s.c. in the footpad with labelled TEL and cryosections of the in situ fixed draining pLNs analyzed immunohistochemically. The B cell areas (marked by their central clusters of CD35+ FDC) contained network of conduits positive for type I collagen, fibronectin, perlecan and ER-TR7, which was, however, less dense than that of T cell areas (Gretz et al., 2000; Sixt et al., 2005) (Figure 3A). Importantly, TEL co-localized with conduits, supporting the MP-IVM results that small Ag drains from the SCS into the follicles via conduits (Figure 3B & C). Analysis at higher magnification revealed that TEL is flanked by markers of the conduit-forming FRC, such as ER-TR7 and gp38, as well as by fibronectin (Figure 3B & C). Moreover, TEL was found in association with CD11c+ DC and B220+ B cells (Figure 3C). These results combined with the observation of size exclusion support the interpretation that small Ags enter the follicles via the FO conduits.
Figure 3.
Characterization of the FO conduits network by immunohistology and EM. (A) Imaging of thick cryosections (40 μm) stained for collagen I (green) and ER-TR7 (red) (top panels) or fibronectin (green) and perlecan (red) (middle panels) in B cell follicles. Scale bars are 100 μm. Hatched lines indicate the T-B border. Bottom panels: Imaging of thick cryosections after s.c. injection of Ag (TEL, green; collagen I, red and CD35, blue). Hatched box identifies area magnified in lower panels. Scale bars are 100 μm and 20 μm respectively. (B) Immunofluoresence on thin cryosections (10 μm) of LN after s.c. injection of Ag (TEL, green). Left column: collagen I, (red). Scale bar is 20 μm. 2nd and 3rd column: ER-TR7 (red), 4th column: fibronectin (red,) and 5th column: gp38 (red). Scale bars are 5 μm. (C) Ag transported via conduits is acquired by T cell area dendritic cells (CD11c, left panel, red), FSC (gp38, middle panel, red) and B cells (B220, right panel, red). Scale bars are 5 μm. (D) Ultrastructural characterization of FO conduits. (i) Transmission EM of the SCS and the B cell follicle. The white arrowheads indicate part of a conduit in the B cell follicle. Scale bar is 1 μm. (ii) Electron micrograph of a conduit (c) in the SCS area. FSC, SLC and M represent follicular stromal cell, sinus lining cell and macrophage, respectively. Scale bar is 2 μm. (iii) Electron micrograph and schematic drawing (iv) of a transverse section of a FSC located in the follicular area of the LN. The high metabolic activity of these cells is indicated by the presence of a prominent rough endoplasmic reticulum (RER) and mitrochondria (m); N represents nucleus. Scale bar is 500 nm.
To characterize further the FO conduits, pLNs from naïve mice were prepared for ultrastructural analysis. Electron microscopic (EM) examination of the SCS floor and the underlying B cell follicles revealed the presence of conduit structures originating from the floor of the SCS and extending deep into the B cell area (Figure 3D i, ii). The B cell area conduits are composed of bundles of collagen-like fibrils partly wrapped by stromal cells that closely resemble FRC defined for paracortical conduits (Sixt et al., 2005)(Figure 3D iii, iv). The openings (approximately 1 μm) of conduits to the SCS would provide access for passive drainage of small Ags into the follicles (Figure 3D ii). However, the tightly packed collagen fibers (less than 10 nm spacing) would exclude free diffusion of large molecules. Thus, based on ultrastructural analysis, the conduit structures extending into the B cell area are similar to those identified in the paracortical regions (Gretz et al., 2000; Sixt et al., 2005).
Acquisition of small Ag from conduits leads to B cell activation
Earlier studies proposed that Ags of a size comparable to that of TEL drain passively via fenestrations in the SCS floor into the underlying follicles where they are bound by cognate B cells (Pape et al., 2007). Our finding that conduit filling with s.c.-injected TEL precedes the detectable appearance in the interstitium and on B cells (Figure 2C) suggests that conduits might in addition facilitate more efficient direct delivery of Ag to B cells. To test this possibility, limiting amounts of TEL were injected into naïve mice that had been seeded with a mixture of fluorescently labelled naive MD4 and polyclonal B cells 20 hrs earlier. Under these conditions, only a fraction of MD4 B cells acquired Ag early after injection (Figure 4A and Movie S3), unlike what would be expected if the Ag was distributed through the follicle solely by diffusion. On closer inspection it was noticed that the majority of Ag+ B cells were closely associated with an Ag-filled conduit and that the distance of each MD4 B cell to the nearest conduit correlated with the amount of Ag it had acquired (Figure 4A–C). Moreover, the instantaneous velocity of cognate cells that bound TEL rapidly and transiently declined upon binding of TEL, while Ag-free MD4 and control B cells were unaffected (Figure S5 and Movie S1). TEL, like hen lysozyme (HEL) binds MD4 B cells with high affinity. To test whether the rapid kinetics of uptake of the high-affinity Ag TEL is representative of Ag uptake by naive B cells with commonly lower affinity for Ags, the experiment was repeated using duck lysozyme (DEL) which binds the MD4 BCR at 1000 fold lower affinity. Indeed, when a similar amount of DEL was injected s.c. and its entry into the draining pLN analyzed by MP-IVM, the kinetics of Ag accumulation on MD4 B cells were similar as with the high affinity TEL Ag (Figure S5 and Movie S4).
Figure 4.
Delivery of Ag to B cell by FO conduits leads to B cell activation. (A) A subregion of the same dataset as shown in Figure 2 is shown at different time-points. Short arrow indicates initial appearance of A633-TEL on a B cell in this region. Long arrow indicates a conduit in which A633-TEL is first detected. Arrowhead indicates MD4 B cells that remain free of or accumulate only minute amounts of A633-TEL. Time is shown in minutes and seconds. See also Movie S2. (B) A limiting amount of A633-TEL (red, containing 10 ng TEL) was injected into the footpad of a different mouse seeded with MD4 (blue) and WT B cells (green) and the draining LN monitored by MP-IVM (See also Movie S3) and the micrograph taken 170 sec after TEL injection. Magnified regions of interest highlight the close spatial relation of selected TEL+ MD4 B cells to TEL-containing conduits. Scale bar = 30 μm. (C) Quantification of TEL uptake by MD4 B cells (as measured by MFI) as a function of proximity to the nearest TEL+ conduit. Similar results were obtained in a separate experiment. (D) Electron micrographs of a B cell follicle (i and iii) and schematic drawing (ii) showing B cell processes in close contact with a conduit. The SLC that separate the SCS from the B cell follicle are indicated in orange. Scale bars are 1μm (i) and 500 nm (iii). (E) The acquisition of A633-TEL versus TEL-PE by MD4 B cells was assessed over a four hr period by FACS. Representative plots, gated on IgMa+ cells that acquired Ag in vivo (labelled MD4 cells were added ex vivo to capture free Ag), from four LNs analyzed in two experiments are shown. Average values ± SEM of Ag acquisition by MD4 B cells are shown in the graph. *p=0.004. **p=0.03. (F) Upregulation of CD86 on MD4 B cells by TEL or TEL-PE. The percentage of MD4 B cells expressing CD86 at 1, 2, 3, 4, 6 and 8 hrs after Ag injection. Average values ± SEM from at least two LNs from three experiments are shown. (G) Location of MD4 B cells in the inguinal LNs of mice that received no Ag, TEL or TEL-PE 8 hrs earlier. Results from two experiments were analyzed for the numbers of MD4 B cells localized to two zones, within the follicles, or in a ROI within 22 μm of the paracortical/follicular border or extrafollicular, and used to compile the graph.
One explanation for the increased efficiency of Ag uptake by MD4 cells most proximal to the conduits is that B cells interact directly with conduits as has been proposed for DC in the paracortical region (Sixt et al., 2005). Analyses of FO conduits by EM identified gaps in the stromal cell-wrapped conduit, as the cells appear to “grasp” the conduit without completely enveloping it (Figure 3D i–iii). Inspection of FO conduits by EM identified B cell pseudopods in direct contact with the collagen core (Figure 4D iii). Thus, Ag within the conduits is accessible to FO B cells via the gaps in the FSC envelope.
The finding that small Ag enters the follicles via conduits and is rapidly acquired by cognate B cells raised the question of whether Ag trafficking through conduits makes B cell activation more efficient. To compare the relative efficiency of uptake of large TEL-PE and small TEL Ag by cognate B cells, naïve mice were seeded with MD4 B cells and then injected s.c. in the hind flank with a mixture of fluorescently labelled Ag. Inguinal LNs were harvested at various times and analyzed by FACS. While some MD4 B cells acquired TEL within 30 min after injection, they were devoid of the large Ag TEL-PE. By 60 min, uptake of small TEL by MD4 B was maximal while only half as many MD4 B cells acquired TEL-PE. The acquisition of large TEL-PE by the MD4 B cells increased further at both 2 and 4 hrs nearing the maximal levels of small TEL acquired at 60 min. These results indicate that MD4 B cells acquired small TEL more rapidly than the larger Ag when they were co-injected (Figure 4E). Thus, small Ags draining via conduits are more readily accessible to cognate B cells than large Ags entering via other pathways.
To evaluate the quality of the interactions between cognate B cells and their Ag, i.e. whether they led to B cell activation, wild-type mice were seeded with MD4 B cells and 20 hrs later injected separately in the hind flank with either TEL-PE or TEL at equivalent TEL concentrations. Inguinal LNs were harvested at various times and MD4 B cells analyzed for upregulation of CD86 as an early marker of activation (Figure 4F). Within 4 hrs of Ag encounter, a higher frequency of MD4 B cells upregulated CD86 in response to small TEL compared to large TEL-PE (Figure 4F). By 6 and 8 hrs, roughly equivalent numbers of MD4 B cells that encountered either TEL or TEL-PE had upregulated CD86 (Figure 4F). Thus, B cell activation occurred more rapidly following exposure to small Ag (within 2 hrs) compared to MD4 B cells that encountered large TEL-PE.
B cell encounter with cognate Ag leads to their movement to the T-B border for interaction with cognate T cells (Garside et al., 1998; Okada et al., 2005); and this approach has been used as an indicator of B cell activation (Carrasco and Batista, 2007; Junt et al., 2007; Pape et al., 2007; Qi et al., 2006). To confirm B cell activation by small vs. large TEL Ags, the localization of MD4 cells at the T-B border was quantified (Figures 4G and S4). In control mice (no Ag), most MD4 B cells were observed within the follicle, with only 15% of MD4 B cells in a follicular border zone (approximately 22 μm inward from the edge of the follicle distal the SCS) or were extrafollicular. In contrast, in LNs from mice treated with small TEL 8 hrs earlier, 51% of MD4 B cells were located in the border/extrafollicular zone while TEL-PE-injected mice were similar to cells that had not been exposed to either TEL Ag (Figure 4G and S4). Notably, by 24 hrs a similar percentage of border/extrafollicular MD4 B cells were identified in LNs from TEL or TEL-PE treated mice (Figure S4). These observations indicate that the rapid delivery of TEL via FO conduits results in a more rapid activation of B cells compared to the delayed activation by large Ag which is excluded from the conduits.
Do FO conduits guide B cells to Ag via CXCL13 chemokine?
Trafficking of T cells within the paracortical region is not thought to be entirely random but guided by conduit-forming FRC bearing specific chemokines that promote T cell motility in a haptotactic or haptokinetic fashion (Bajenoff et al., 2006; Mempel et al., 2006; Okada and Cyster, 2007; Worbs et al., 2007). Similarly, CXCL13, produced by stromal cells including FDC (CD35+) and FSC (CD35−), likely regulates B cell migration in follicles (Allen and Cyster, 2008; Cyster et al., 2000; Nolte et al., 2003). To examine whether FO conduits harbor CXCL13 in addition to small lymph-borne Ags, naïve mice were injected in the footpad with labelled TEL, pLNs fixed in situ and cryosections analyzed by immunostaining. Staining with anti-CXCL13 identified a reticular pattern within the B cell region that co-localized with both FDC (CD35+) and conduits (identified by fibronectin and collagen I) (Figure 5A, B and C). As expected, the T cell area was negative for CXCL13.
Figure 5.
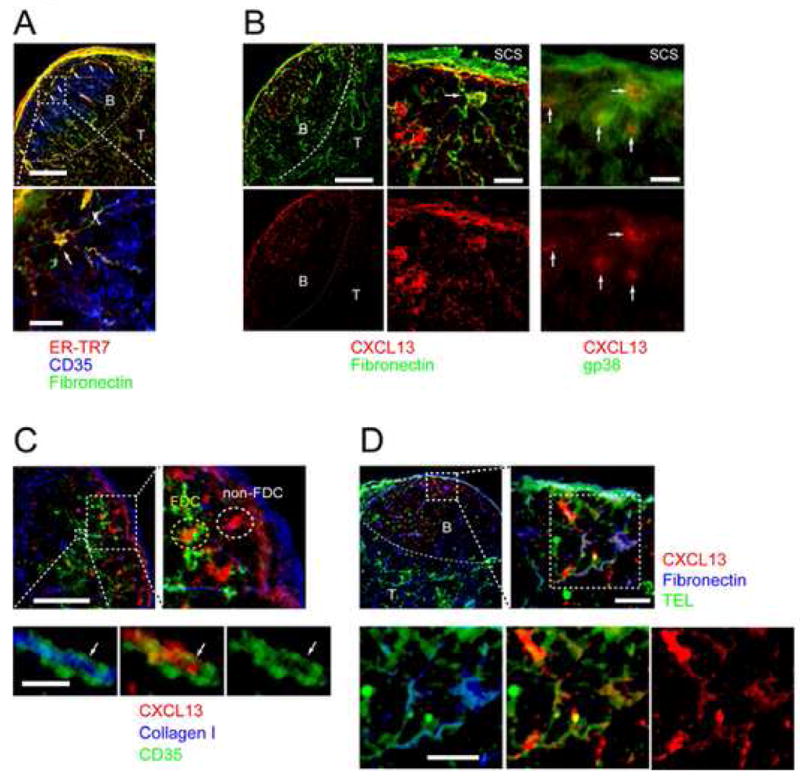
CXCL13 is associated with FO conduits. Immunofluorescence micrographs of B cell follicles stained with antibodies against (A) Fibronectin (green), ER-TR7 (red) and CD35 (blue). T-B boundaries are indicated by hatched lines. Scale bars are 100 μm (top) and 20 μm (bottom). (B) LN cryosections stained for CXCL13 (red), fibronectin (green, left and middle columns, scale bars are 100 and 20 μm) and gp38 (green, right column, scale bar is 20 μm). (C) Localization of CXCL13 (red) in the follicles: collagen I (blue), CD35 (green). Hatched boxes indicate magnified areas. Scale bars are 100 μm for overview and 20 μm and 5 μm for large and small magnified area respectively. (D) Location of Ag and CXCL13 in LN follicles. Ag (TEL, green), CXCL13 (red) and fibronectin (blue). Hatched boxes indicate magnified areas. Scale bars are 100 μm for overview and 20 μm for magnified areas.
In addition to the conduit associated reticular pattern, dense CXCL13 staining co-localized with FSC (gp38+) and FDC proximal and distal, respectively, to the SCS (Figure 5B and C). Moreover, higher magnification images suggest that the CXCL13 is wrapped by both FDC and conduit structures (Figure 5C). Thus, the staining pattern suggests that CXCL13 secreted by FSC enters the conduit and could provide an attractant to guide B cells to a likely site of Ag exposure. However, an alternative interpretation is that FSC-secreted chemokine binds to the outer surface of the FO conduit as proposed for conduits in the T cell area. Immunostaining of sections from LNs containing labelled TEL identify a similar pattern of staining for fibronectin, CXCL13 and TEL Ag (Figure 5D). Thus, conduits include CXCL13, possibly to provide an attractant to B cells to survey the conduits for the presence of Ag as proposed for DCs in the T cell area (Bajenoff et al., 2006).
It has been described that chemokines produced at the site of inflammation, such as CCL2, can drain into LNs, putatively via T cell area conduits, in order to influence cellular recruitment via HEV (Palframan et al., 2000). To examine further a possible role for conduits in the delivery of lymph-borne chemoattractants to or in the distribution of locally produced chemokines within the B cell area, mice were injected in the footpad s.c. with a small volume of recombinant CCL21 (SLC). LNs were immediately fixed in situ and harvested for analysis. CCL21 is normally found in the T cell area and as expected only a background level was observed within the B cell follicles in uninjected mice (Figure S7, left panels). By contrast, the follicular zone stained positive for CCL21 within pLNs of mice injected with the chemokine (Figure S7, right panels). Examination of sections at higher magnification revealed CCL21 co-localization with FO conduits (Figure S7). Thus the results are consistent with a model in which lymph-borne chemoattractants also enter the follicles (and paracortical region) via conduits.
Discussion
It is generally accepted that protein Ags retained on FDC provide an important depot for encounter by FO B cells (Kosco and Gray, 1992; Kelsoe, 2000; Fu et al., 1997; Cyster et al., 2000; Rajewsky, 1996). How Ag is delivered to FDC is less clear. Using a combination of MP-IVM, EM, confocal and flow cytometric analyses, we observe a novel pathway whereby small soluble Ag drains passively into the B cell zone through a FO conduit system that connects the SCS with FDC areas. Moreover, the FO conduit network, which is less dense but structurally similar to that of the T-cell zone, contains CXCL13. Thus, the conduit system provides a source of Ag as well as a possible pathway or network to guide B cells to Ag within the follicles in a similar manner as identified in the paracortex for T cells.
Qi et al. found that DC loaded with HEL ex vivo and adoptively transferred into mice present Ag to HEL-specific B cells within the paracortical region (Qi et al., 2006). They proposed that conduit-associated DC may take-up draining Ag and present it to B cells entering the T zone via high HEVs. Using a similar model Ag (HEL), Pape et al. tested the importance of DC in Ag transport in a mouse model in which CD11c+ cells were deleted prior to injection of Ag (Pape et al., 2007). They found that DC were not essential as the HEL drained rapidly into the follicles of the depleted mice and it was detected on specific B cells within 3.5 min. They proposed a model in which small protein Ags within the afferent lymph drain directly into the B cell follicles via gaps in the SCS floor and that cellular transport was not required. Our findings are consistent with their report as small Ag drained rapidly into follicles in an apparent cell-independent manner. However, an important difference is that TEL entered the follicles via discrete conduits rather than in a diffuse pattern, representing a second mechanism in which small Ags gain access to the B cell follicles. This discrepancy could result from the different techniques employed to visualize the Ag. In the Pape study, the draining LNs were flash frozen and cryosections imaged ex vivo without hydration. In our hands, Ag was only observed inside conduits when LNs were fixed in situ by perfusion via the lymph. It is possible that the flash freezing of the LNs obscured a discrete pattern within FO conduits. Moreover, the conduits, which are readily observed by MP-IVM, are not easily identified by confocal microscopy without staining with specific antibodies.
Immunostaining using antibodies specific for type I collagen identified co-localization of the labelled TEL. Furthermore, staining with Abs specific for basement membrane (fibronectin) or stromal cell surface markers (ER-TR7 and gp38) indicates that the Ag lies within, not outside, the conduit. Interestingly, the conduits appear to merge or overlap with the FDC network suggesting that small Ag are “delivered” directly and can be taken up if that they are tagged with complement ligand C3 (Fang et al., 1998; Fischer et al., 1998). Whether the primary role of conduits is channelling of chemokines and inflammatory mediators or delivery of B cell Ags is not clear. However, our results illustrate that they provide an efficient mechanism for delivery of small Ags from the SCS into the follicle where it is efficiently exposed to B cells.
The finding that TEL is taken-up by FDC more rapidly than larger Ags, such as PE and KLH, which are excluded from conduits, supports our observations that lymph-borne small Ags rapidly fill conduits and drain into the underlying follicles. In contrast, large Ags, such as PE, are bound by FO B cells and delivered to FDC in a complement-dependent manner, as reported (Phan et al., 2007). However, the uptake of TEL by naïve B cells was less efficient and the peak of uptake occured earlier than with PE. This suggests that either some TEL does diffuse into the follicles and is taken-up by B cells as an IC; or alternatively, TEL Ag enters into the interstitial space after being distributed in the follicle via conduits as suggested by our MP-IVM results. The observation of TEL entry into follicles via conduits is not explained by an injection of an excessive amount of Ag. In the current study, mice were injected with a mixture of TEL and TEL-PE with less than 1 μg of TEL. This represents considerably less Ag than used by Pape et al (Pape et al., 2007). Importantly, only the smaller TEL Ag was detected within the FO conduits.
Characterization of the FO conduits by ultrastructural analysis and immunostaining suggests that their structure is similar to that identified for those in the T zone. As reported by Gretz et al (Gretz et al., 2000), the FO conduits include a core of tightly packed collagen fibrils with spaces less than 10 nm that would explain the size exclusion of particles greater than 5.5 nm or approximately 70 kDa. As reported for T zone conduits, conduits in the follicles stained positive for fibronectin and perlecan suggesting that the collagen core was enveloped by a basement membrane (Sixt et al., 2005). Likewise, the FO conduits stained positive for ER-TR7 and gp38, which are markers for LN stromal cells.
In the current study, FO conduits were found to open into the SCS, which would provide access to small lymph-borne Ags. Although the conduits are “wrapped” by stromal cells, gaps were identified exposing the conduits to B cells. Indeed, MD4 B cells closest to conduits were observed to bind TEL Ag more rapidly than those more distal.
One difference between conduits in the follicles and in the T zone is that the former are less dense. One possible explanation for this difference is the presence of the FDC network, which is unique to the follicular region. Thus, the combination of FDC processes and FO conduits would provide a rich network for B cells to interact with Ag.
Rapid transport of small Ag into the follicles via conduits can accelerate its encounter by cognate B cells. Comparison of uptake and activation of MD4 B cells by TEL and TEL-PE revealed more rapid activation by the smaller Ag, based on CD86 expression and earlier migration of activated B cells to the T-B border. Moreover, tracking of Ag-bound cognate B cells within the first 15 min post-injection of small Ag revealed a dramatic slowing in their migration pattern relative to non-Ag binding control B cells. In the example of an infectious pathogen, this reduction in response time to microbially-derived Ags could provide a substantial benefit to the host (Ochsenbein et al., 1999). Thus, Ag is delivered to the B cell follicles via conduits within minutes after injection and in the presence of Ig and complement is taken up on FDC. These rapid kinetics could be critical in the case of a highly infectious pathogen. Since intact pathogens exceed the size limit of the conduits, the majority of the microbial Ag could be transported via B cells. However, small secreted bacterial products and/or viral “building blocks” could drain into the B cell zone via the conduits. Moreover, partial degradation of pathogens at the initial site of infection would release smaller products that gain access to the follicles via the FO conduits.
Recently, Bajenoff et al. reported that lymphocytes migrate through the T zone along FRC-ensheathed conduits presumably in response to chemokines bound to the cell surface (Bajenoff et al., 2006). Likewise, B cells were found to associate with FDC dendritic processes and it was proposed that they are guided by CXCL13. The observation of such a close contact supports a model in which Ag (and complement ligand) is exchanged between the two cell types. In the current study, FO conduits, FSC and FDC co-localized with CXCL13. Staining of cryosections with specific antibody suggests that both FSC and FDC are a source of CXCL13 as reported by Cyster et al (Allen and Cyster, 2008). Thus, one interpretation of our results is that FSC proximal to the SCS release CXCL13 from where it permeates the conduit network. Analogous to what has been described in the T cell area (Bajenoff et al., 2006), this would provide a mechanism by which B cells are guided in their migration through B follicles by the same structures that deliver Ag draining from peripheral tissues, maximizing their exposure to Ag. Whether CXCL13 enters the conduits or is bound to the outer surface of the FSC could not be determined definitively from our analysis. However, the finding that CCL21 injected s.c. in the footpad co-localizes with the FO conduits suggests that chemokines like small protein Ags enter follicles via this route.
In summary, we identified a novel network of conduits in peripheral LN that mediate rapid transport of small soluble protein Ags from the SCS into the B cell follicular region. The presence of conduits within the follicles provides an efficient mechanism to expose Ag to B cells and for rapid deposition on FDC.
Experimental Procedures
Mice
All mice were (CD45.1; CD45.2; MD4 (Goodnow et al., 1988); and Cr2−/− (Molina et al., 1996) were maintained on a C57BL/6 (B6) background and were housed at HMS and IDI animal facilities under SPF conditions. Studies were approved by the institutional animal care and use committees. Mice received food and water ad libitum.
Immunogens and immunization protocols
TEL and DEL were purified from fresh turkey eggs (Nicholas Turkey Breeding Farms, Sonoma, CA) as described (Prager and Wilson, 1971). B-PE and KLH were from Sigma Aldrich (St. Louis, MO). Ags were conjugated with Alexa Fluor 488 (A488), 568 (A568), or 633 (A633) carboxylic acid succinimidyl esters (Invitrogen, Carlsbad, CA) following manufacturer’s instructions. B-PE was covalently coupled to TEL using EDAC (Invitrogen, Carlsbad, CA) and Sulfo-NHS (Pierce Biotechnology, Rockford, IL) and purified by gel filtration chromatography. For in vivo IC formation, mice were passively immunized i.p. with 2 mg rabbit anti-lysozyme (Biodesign, Saco, ME); rabbit anti-KLH (GeneTex, San Antonio, TX); or rabbit anti-B PE (Rockland, Inc, Gilbertsville, PA) and 24 hrs later, injected s.c. in the hind flanks with a mixture of 10 μg A488-TEL and 10 μg B-PE. The draining inguinal LNs were isolated at various times for flow cytometry and confocal microscopy analyses.
Antibodies
The following antibodies were used for flow cytometry and immunohistochemistry: αCD3ε-Biotin, αCD19-PE, αCD21/35-FITC (7G6), αCD35-Cy5 (8C12), αCD35-Biotin, αCD45.1-PE and FITC, αCD45.2-FITC, αB220-PerCP, αIgMa-Biotin and Streptavidin-APC (BD Biosciences, San Jose, CA); αB220-Pacific Blue, αCD86-Biotin, Streptavidin-PE/Cy7 (eBiosciences, San Diego, CA); αB220-Cy5, goat anti-rabbit IgG-A488 (Invitrogen);. αcollagen I (Chemicon, Temecula, CA); αperlecan and αfibronectin (Neomarkers, Fremont, CA); αCXCL13-Biotin (R&D Systems Inc., Minneapolis, MN). Antibodies specific for ER-TR7, CD35 (8C12), gp38 (8.1.1), CD11c (N418), and B220 (6B2) were produced in house, and conjugated to A488 or A647 or detected with the appropriate Alexa-labelled secondary staining reagents.
Single cell suspensions and FACS analysis
Single-cell suspensions from LNs were blocked with anti-FcR (2.4G2) and stained with antibodies list above in PBS containing 1% FBS. Either FACSCalibur or a FACSAria (BD Biosciences, San Jose, CA) flow cytometers and FlowJo software (Tree Star, Inc., Ashland, OR) were used for analysis.
Histology
Cryosections of OCT (Tissue tek, Torrence, CA)-embedded LN were prepared as described (Verschoor et al., 2001), incubated with anti-FcR (2.4G2) before treatment with antibodies listed above in EBSS containing 1.5% BSA and 0.1% saponin for conduit components and CXCL13. Images were acquired using a DM6000 fluorescence microscope and an SP2 confocal microscope (Leica Microsystems, UK), and processed using Leica image viewer, Adobe Photoshop and ImageJ software. In some cases pseudocolor channels were swapped for visual representation.
B Cell Isolations, Labelling and Adoptive Transfers
Naïve (CD43−) B cells from WT and MD4 spleens were magnetically enriched (Miltenyi Biotec, Auburn, CA) to greater than 90% purity. For MP-IVM studies, splenocytes were labelled with CellTracker dyes (Invitrogen, Carlsbad, CA), CMFDA at 2.5 μM or CMAC at 20 μM, for 15 min followed by extensive washing prior to B cell isolation. Recipient mice received a 1:1 mixture of labelled WT and MD4 B cells (3×107 cells/mouse) or MD4 only (1.5×107) for Ag uptake and B cell activation assays i.v. at least 20 hrs prior to Ag injection.
Multiphoton intravital microscopy (MP-IVM)
MP-IVM was performed as described (Mempel et al., 2004). For detailed methods see Supplemental Methods.
Electron Microscopic Analysis
For detailed methods, see Supplemental Methods.
Supplementary Material
Acknowledgments
We wish to thank D. Vargas for exceptional animal care, R. Yeamans for technical assistance, Dr. N. Barteneva at the IDI Flow and Imaging Cytometry Core facility and Maria Ericsson at the Harvard Medical School EM Facility for technical assistance. R.R. is supported by a Marie Curie Fellowship (008873). MCC is supported by a grant from NIH (AI 039246). The authors have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney C, Herren S, Power CA, Gordon S, Martinez-Pomares L, Kosco-Vilbois MH. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190:851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xu C, Fu Y, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- Fischer MB, Goerg S, Shen L, Prodeus AP, Goodnow CC, Kelsoe G, Carroll MC. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Goodnow CG, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Hauser AE, Shlomchik MJ, Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nat Rev Immunol. 2007;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe G. The germinal center reaction. Immunology Today. 1995;16 doi: 10.1016/0167-5699(95)80146-4. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. Studies of the humoral immune response. Immunol Res. 2000;22:199–210. doi: 10.1385/IR:22:2-3:199. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kosco MH, Gray D. Signals involved in germinal center reactions. Immunol Rev. 1992;126:63–76. doi: 10.1111/j.1600-065x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- MacLennan I. Germinal Centers. Ann Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Martinez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S, Bonnefoy JY, Gordon S. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25:867–869. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Nolte MA, Belien JA, Schadee-Eestermans I, Jansen W, Unger WW, van Rooijen N, Kraal G, Mebius RE. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J Exp Med. 2003;198:505–512. doi: 10.1084/jem.20021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbien A, Pinschewer D, Odermatt B, Carroll M, Hengartner H, Zinkernagel R. Protective T cell-independent antiviral antibody responses are dependent on complement. J Exp Med. 1999;190:1165–1174. doi: 10.1084/jem.190.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi- dependent T cell motility in the lymph node. J Immunol. 2007;178:2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Catron DM, Itano AA, Jenkins MK. The Humoral Immune Response Is Initiated in Lymph Nodes by B Cells that Acquire Soluble Antigen Directly in the Follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- Prager EM, Wilson AC. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971;246:5978–5989. [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Szakal AK, Holmes KL, Tew JG. Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J Immunol. 1983;131:1714–1727. [PubMed] [Google Scholar]
- Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Verschoor A, Brockman MA, Knipe DM, Carroll MC. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J Immunol. 2001;167:2446–2451. doi: 10.4049/jimmunol.167.5.2446. [DOI] [PubMed] [Google Scholar]
- Victoratos P, Lagnel J, Tzima S, Alimzhanov MB, Rajewsky K, Pasparakis M, Kollias G. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006;24:65–77. doi: 10.1016/j.immuni.2005.11.013. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youd ME, Ferguson AR, Corley RB. Synergistic roles of IgM and complement in antigen trapping and follicular localization. Eur J Immunol. 2002;32:2328–2337. doi: 10.1002/1521-4141(200208)32:8<2328::AID-IMMU2328>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.