Abstract
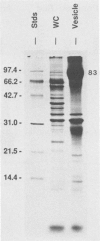
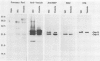
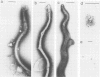
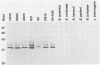
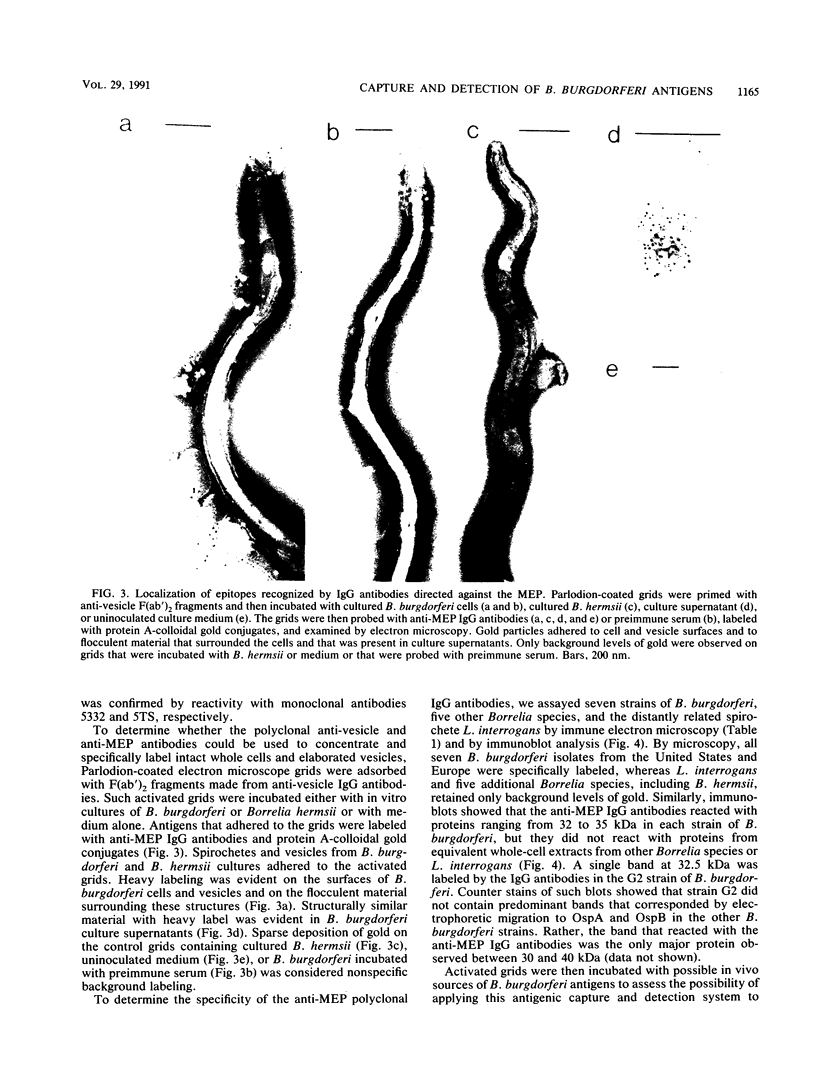
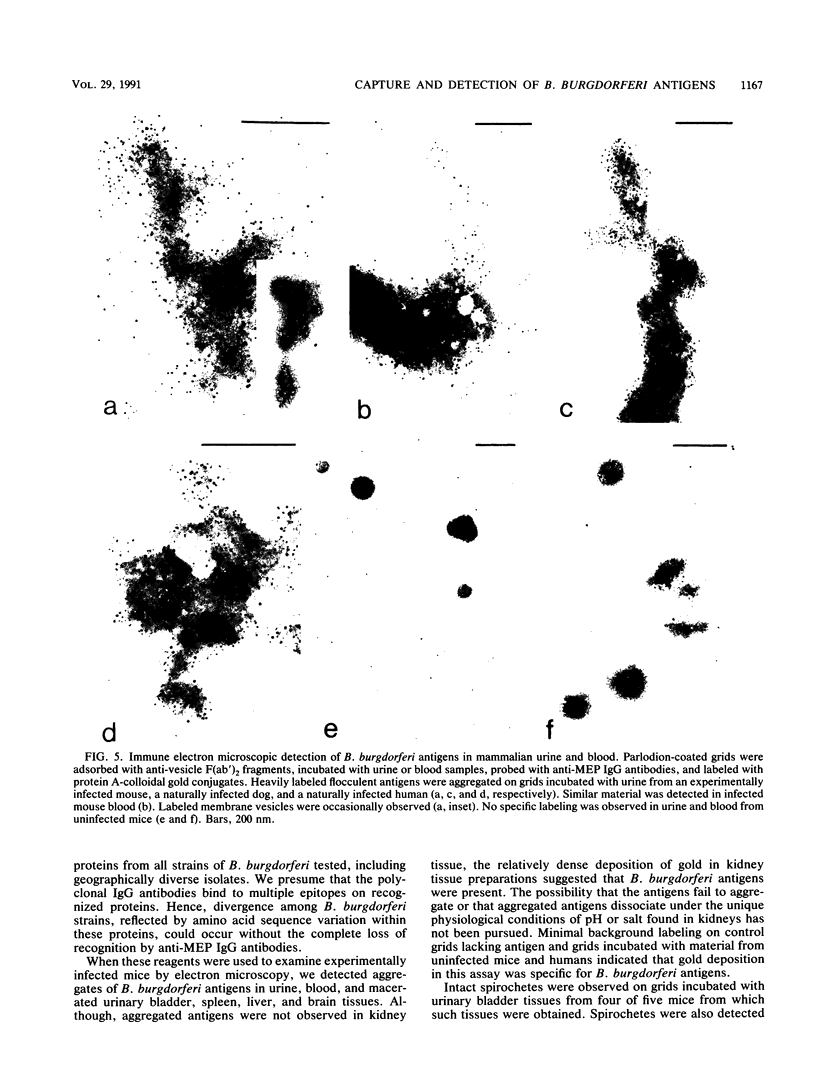
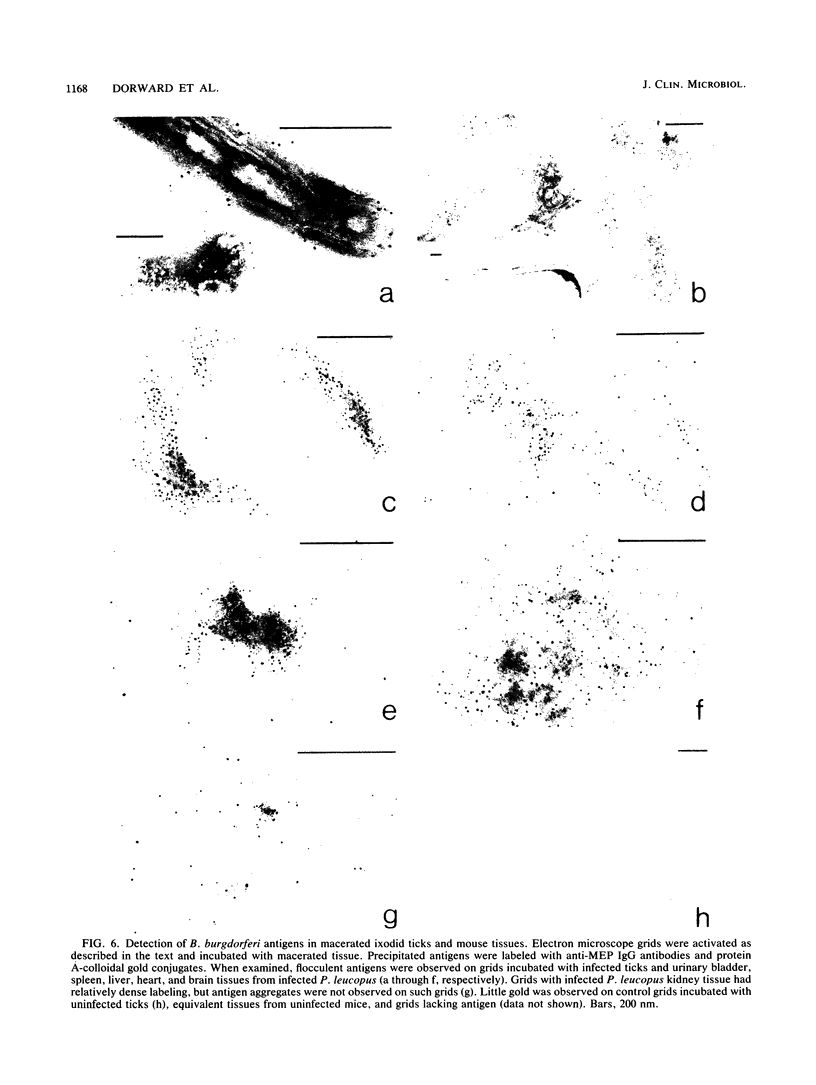
Current biological and serological techniques for demonstrating infections by Borrelia burgdorferi can be inconclusive. In order to monitor Lyme borreliosis, we developed a rapid and sensitive assay for B. burgdorferi antigens in infected hosts. Polyclonal rabbit antisera were raised against membrane vesicles and an 83-kDa vesicle-associated protein band that was purified from in vitro B. burgdorferi cultures. Immunoglobulin G (IgG) antibodies were recovered from these sera and tested for a species-specific reaction with several geographically diverse Borrelia isolates by immunoblot analysis. Parlodion-coated electron microscope grids were activated with anti-vesicle F(ab')2 fragments and then incubated with confirmed or experimental sources of spirochetal antigens. Such sources included cultured spirochetes; spirochete culture supernatants; samples of urine, blood, or serum from mice, dogs, and humans; triturates of Ixodes ticks; and bladder, spleen, liver, kidney, heart, or brain tissues from infected or control mice. Captured antigens were assayed by immune electron microscopy by using anti-83-kDa IgG antibodies and protein A-colloidal gold conjugates. The results indicated that B. burgdorferi appears to shed surface antigens which are readily detectable in urine, blood, and several organs from infected hosts. Such antigens were detectable in mouse urine at dilutions exceeding 10(-6). Intact spirochetes were frequently observed on grids incubated with blood, spleen, or bladder preparations, and B. burgdorferi was reisolated from the urinary bladders of all experimentally infected mice. These results indicated that B. burgdorferi antigens arise in a variety of host materials. Such antigens can be captured and identified with specific polyclonal antibodies, providing a sensitive assay for monitoring and studying Lyme borreliosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bosler E. M., Cohen D. P., Schulze T. L., Olsen C., Bernard W., Lissman B. Host responses to Borrelia burgdorferi in dogs and horses. Ann N Y Acad Sci. 1988;539:221–234. doi: 10.1111/j.1749-6632.1988.tb31856.x. [DOI] [PubMed] [Google Scholar]
- Callister S. M., Agger W. A., Schell R. F., Brand K. M. Efficacy of the urinary bladder for isolation of Borrelia burgdorferi from naturally infected, wild Peromyscus leucopus. J Clin Microbiol. 1989 Apr;27(4):773–774. doi: 10.1128/jcm.27.4.773-774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duray P. H., Steere A. C. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. doi: 10.1111/j.1749-6632.1988.tb31839.x. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Dorward D. W., Corwin M. D. Structural features of Borrelia burgdorferi--the Lyme disease spirochete: silver staining for nucleic acids. Scanning Microsc Suppl. 1989;3:109–115. [PubMed] [Google Scholar]
- Goodman J. L., Jurkovich P., Kramber J. M., Johnson R. C. Molecular detection of persistent Borrelia burgdorferi in the urine of patients with active Lyme disease. Infect Immun. 1991 Jan;59(1):269–278. doi: 10.1128/iai.59.1.269-278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde F. W., Johnson R. C., White T. J., Shelburne C. E. Detection of antigens in urine of mice and humans infected with Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol. 1989 Jan;27(1):58–61. doi: 10.1128/jcm.27.1.58-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Purification of outer membrane proteins of the gram-negative bacterium Neisseria gonorrhoeae. Anal Biochem. 1988 Sep;173(2):307–316. doi: 10.1016/0003-2697(88)90194-7. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Hovind-Hougen K., Svenungsson B., Stiernstedt G. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J Clin Microbiol. 1990 Mar;28(3):473–479. doi: 10.1128/jcm.28.3.473-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamoyi E., Nisonoff A. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. J Immunol Methods. 1983 Jan 28;56(2):235–243. doi: 10.1016/0022-1759(83)90415-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre R. B., Perng G. C., Johnson R. C. The 83-kilodalton antigen of Borrelia burgdorferi which stimulates immunoglobulin M (IgM) and IgG responses in infected hosts is expressed by a chromosomal gene. J Clin Microbiol. 1990 Jul;28(7):1673–1675. doi: 10.1128/jcm.28.7.1673-1675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A. Serologic diagnosis of Lyme disease. Ann N Y Acad Sci. 1988;539:154–161. doi: 10.1111/j.1749-6632.1988.tb31848.x. [DOI] [PubMed] [Google Scholar]
- Rawlings J. A., Fournier P. V., Teltow G. J. Isolation of Borrelia spirochetes from patients in Texas. J Clin Microbiol. 1987 Jul;25(7):1148–1150. doi: 10.1128/jcm.25.7.1148-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. N., Jr, McGee Z. A., Kaplan J., Hammond M. E., Larson J. K., Buchanan T. M., Schoolnik G. K. Ultrastructural localization of specific gonococcal macromolecules with antibody-gold sphere immunological probes. Infect Immun. 1984 Nov;46(2):361–366. doi: 10.1128/iai.46.2.361-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Schrumpf M. E., Karstens R. H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol. 1988 May;26(5):893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernstedt G., Gustafsson R., Karlsson M., Svenungsson B., Sköldenberg B. Clinical manifestations and diagnosis of neuroborreliosis. Ann N Y Acad Sci. 1988;539:46–55. doi: 10.1111/j.1749-6632.1988.tb31837.x. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]