Abstract
We evaluated the efficacy and safety of a1 - blocker doxazosin for treatment of lower urinary tract symptoms (LUTS) compatible with benign prostatic hypertrophy (BPH). Fourteen randomized controlled trials enrolled 6261 men, average age 64 years, who had moderately severe LUTS and flow impairment. Compared with baseline measures and placebo effect, doxazosin resulted in a statistically significant improvement in both LUTS and flow. However, when compared with placebo, the average magnitude of symptom improvement (International Prostate Symptom Score [IPSS] improvement <3 points) typically did not achieve a level detectable by patients. Combined doxazosin and finasteride therapy improved LUTS and reduced the risk of overall clinical progression of BPH compared to each drug separately in men followed over 4 years. Reported mean changes from baseline in the IPSS were −7.4, −6.6, −5.6, and −4.9 points for combination therapy, doxazosin, finasteride, and placebo, respectively. Combination therapy reduced the need for invasive treatment for BPH and the risk of long-term urinary retention. The absolute reductions compared with placebo were less than 4% and primarily seen in men with prostate gland volume >40 mL or PSA levels >4 ng/mL. Efficacy was comparable with other a1–blockers. Withdrawals from treatment for any cause were comparable to placebo. Dizziness and fatigue occurred more frequently with doxazosin compared to placebo.
Keywords: benign prostatic hypertrophy (BPH), doxazosin, a1-adrenoceptor antagonists, lower urinary tract symptoms, systematic review
Introduction
Benign prostatic hypertrophy (BPH) is a common condition that can result in bothersome lower urinary tract symptoms (LUTS). These symptoms may be obstructive (weak urine flow, hesitancy, straining, incomplete emptying) or irritative (frequency, nocturia urgency) (Medina et al 1999). LUTS consistent with BPH are estimated to occur in over 30% of men 65 years or older (Chapple 2001). Treatment costs in the US, exclusive of outpatient pharmaceuticals, exceeds $1 billion dollars and accounts for nearly 8 million physician visits annually (Wei et al 2004). Therapeutic strategies to alleviate LUTS are based on symptom severity, prostate characteristics, and physician or patient preference. These include minimally invasive surgical techniques, lifestyle modification, herbal preparations and prescribed medications, and transurethral resection of the prostate (TURP) (AUA 2003).
Alpha1-adrenoreceptor antagonists (α1–blockers) are a primary medical approach to treating LUTS. α1–blockers commonly used to treat LUTS include doxazosin, terazosin, alfuzosin and tamsulosin. The goal of this review is to evaluate the efficacy and adverse events of nonuroselective α1-blocker doxazosin for LUTS associated with BPH and its effectiveness and safety compared with other medical therapies, including α1-blockers and 5-alpha reductase inhibitors. Our review emphasizes results from randomized controlled trials. We also summarize findings from a comprehensive review of treatment options for BPH prepared for the American Urological Association (AUA) (AUA 2003). Because LUTS is a chronic medical condition we assessed the effectiveness of doxazosin on preventing progression of disease long-term and tolerability as well as improving existing symptoms and flow measures.
Subjects and methods
Search strategy
This report is an update of a previously published systematic review (MacDonald et al 2004). MEDLINE was searched from 1966 to May 2006 combining an optimally sensitive Cochrane Collaboration search strategy with the MeSH headings ‘prostatic hyperplasia’, ‘LUTS’, and ‘doxazosin’, ‘alpha-blockers’, ‘Adrenergic Alpha-Antagonists’, ‘alpha 1-adrenoceptor’ or ‘alpha-1 blocker’ including all subheadings (Dickersin et al 1994). The Cochrane Library, the Cochrane Prostatic Diseases and Urologic Cancers Review Group specialized registry, and reference lists of identified trials and reviews were also searched. No language restrictions were applied to the search.
Selection criteria
Studies were included if men had symptomatic BPH, subjects were randomly assigned to doxazosin or a control (placebo, pharmacologic, or surgical treatment), and study duration was at least 4 weeks. We also reviewed and summarized findings from the AUA 2003 Clinical Treatment Guidelines for BPH report (AUA 2003).
Data extraction and study appraisal
Two reviewers (Roderick MacDonald and Timothy Wilt) independently determined whether studies met inclusion criteria. Study and demographic characteristics, enrollment criteria, outcomes, adverse effects, and number and reasons for dropout were then extracted. Authors and pharmaceutical manufacturers were contacted in attempts to obtain missing or additional information. As a measure of methodological study quality, the quality of concealment of treatment allocation for randomization was determined based on the scale developed by Schultz (1 = poorest quality, 2 = unclear, 3 = best quality) (Schultz et al 1995). We assessed blinding methods to the treatment, if intention-to-treat analysis was used, and the percent lost to follow up or withdrawn from study protocol.
Statistical methods
The primary outcome measure was defined a priori to be improvement in urological symptoms as measured by a validated symptom score eg, International Prostate Symptom Score or AUA (IPSS). Secondary outcome measures included peak urine flow, global assessment of symptom severity, complications associated with long-term progression of BPH (clinical progression measured by a 4-unit change in symptom score, need for invasive treatment such as prostatectomy, laser or microwave therapy, and urinary retention), adverse effects, and the incidence of withdrawals from treatment or participants lost to follow up.
Percentage improvements from baseline for treatment and control were calculated for the primary efficacy outcomes. If feasible, efficacy and adverse event data were pooled and analyzed in the Cochrane Collaboration Review Manager (RevMan 4.2, Cochrane Collaboration, Oxford, UK) software (CC 2001). Weighted mean differences (WMD), the difference between treatment and control pooled means at endpoint, along with 95% confidence intervals (CI) were calculated for continuous variables. Relative risk ratios (RR), comparing study intervention subjects with control subjects and their 95% CI were calculated for categorical adverse event and withdrawal data. A fixed-effects model was used if there was no evidence of heterogeneity between the studies, based on the chi-square test for heterogeneity (DerSimonian and Laird 1986). Previous work has established levels of symptom score change that are noticeable to patients based on their baseline symptom severity (Barry et al 1995). Therefore, we also assessed whether mean symptom improvements compared with placebo or control exceeded these levels as well as the percentage of subjects who achieved “clinically detectable differences.”
Description of studies
The search strategy identified 14 trials, 10 placebo-controlled, meeting inclusion criteria (Table 1). (Rollema et al 1991; Christensen et al 1993; Janknegt and Chapple 1993; Chapple et al 1994; Fawzy et al 1995; Gillenwater et al 1995; Kaplan et al 1995; Roehrborn and Siegel 1996; Akan et al 1998; Andersen et al 2000; Kirby 2003; Kirby et al 2003; McConnell et al 2003; de Reijke and Klarskov 2004; Lee et al 2005). Two of the placebo-controlled trials had finasteride, a 5-alpha (5-α) reductase inhibitor, monotherapy, and combined doxazosin/finasteride (combination therapy) arms (Kirby et al 2003; McConnell et al 2003). The report by Roehrborn (Roehrborn and Siegel 1996) was a pooled analysis of 3 double-blinded, placebo-controlled trials, 2 published (Fawzy et al 1995; Gillenwater et al 1995) and 1 unpublished. Not shown in Table 1 is a meta-analysis of 5 placebo-controlled studies (Janknegt and Chapple 1993), 3 published (Rollema et al 1991; Christensen et al 1993; Chapple et al 1994) and 2 unpublished included only in the safety analyses. Three studies compared doxazosin with α1–blockers alfuzosin (de Reijke and Klarskov 2004), terazosin (Kaplan et al 1995), and tamsulosin (Kirby 2003). Treatment allocation was unclear in all but 1 study (McConnell et al 2003). All studies were double-blinded with the exception of the 2 trials in which blinding methods were not stated (Akan et al 1998) or not used (Kaplan et al 1995). All studies were published in English.
Table 1.
Description of randomized trials of doxazosin for treatment of LUTS suggestive of benign prostatic hyperplasia
| Reference/Study design | Doxazosin, mg/day(n) | Control (n) | Description of subjects, inclusion criteria | Study duration* |
|---|---|---|---|---|
| Akan et al 1998 | ||||
| Fixed-dose, blinding | 4 mg(24) | Placebo(19) | Turkish men >48 years old (mean 62) with | 6 weeks |
| unclear | symptoms of “prostatism” identified by global physician assessment. | |||
| Andersen et al 2000 | ||||
| Dose-titration to 4 mg or 8 mg, double-blinded | (1). 1–8 mg (318);
(2). GITS* 4 or 8 mg (311) |
Placebo(155) | Scandinavian men between 50–80 years old (mean 65) with BPH: IPSS† score ≥12; peak urine flow [PUF] of 5–15 mL/s in a total voided volume [TVV] of ≥150 mLs. | 17 weeks |
| Chapple et al 1994 | ||||
| Dose-titration, double-blinded | 1–4 mg(67) | Placebo (68) | Normotensive/mildly hypertensive British men, mean age 67, white race 88%, with symptomatic urodynamically confirmed benign prostatic obstruction: PUF <15 mL/s with a TVV > 150 mLs. | 14 weeks |
| Christensen et al 1993 | ||||
| Fixed-dose, double-blinded | 4 mg (52) | Placebo (48) | Normotensive/mildly hypertensive Danish men, mean age 67, with symptomatic (moderatesevere symptoms) BPH who were candidates for transurethral resection of the prostate. | 10 weeks |
| Fawzy et al 1995 | ||||
| Dose-titration to 2 mg, 4 mg, or 8 mg, double-blinded | 1–8 mg (50) | Placebo (50) | Normotensive American men, age >45 years (mean 62), white race 96%, with symptomatic (BPH: AUA† score ≥10; PUF of 5–15 mL/s in a TVV of 125–500 mLs. | 17 weeks |
| Gillenwater et al 1995 | ||||
| Fixed-dose, double-blinded | 2 mg, 4 mg, 8 mg, 12 mg (199) | Placebo (49) | American men with mild to moderate essential hypertension, 45 years or older (mean 64) with symptomatic BPH (PUF of 5–15 mL/s in a TVV 150–500 mLs; post-void residual volume <200 mLs; daytime micturition ≥4 and nocturia ≥2. | 16 weeks |
| Kirby et al 2003 [PREDICT] | ||||
| Dose-titration to 4 or 8 mg, double-blinded | (1) 1–8 mg (275);
(2) Combined with finasteride 5 mg (286) |
(1) Placebo (270);
(2) Finasteride 5 mg (264) |
European men between with 50 and 80 years (mean 64) with moderate to severe BPH: IPSS score ≥12; PUF of 5–15 mL/s; and enlarged prostate determined by digital rectal exam (DRE). | 54 weeks |
| McConnell et al 2003 | ||||
| [MTOPS] Dose-titration to 4 mg or 8 mg, double-blinded | (1) 1–8 mg (756);
(2) Combined with finasteride 5 mg (786) |
(1) Placebo (737);
(2) Finasteride 5 mg (768) |
American men, 50 years or older (mean 63), white race 82%, black race 9%, Hispanic 7%, with moderate to severe BPH: AUA score ≥8; PUF of 4–15 mL/s; TVV ≥125 mLs. | 234 weeks mean follow-up |
| Roehrborn and Siegel 1996† | ||||
| Fixed-dose, double-blinded | 0.5 mg, 1 mg,
2 mg, 4mg(98) |
Placebo(98 total) | Normotensive men, age ≥45 years with symptomatic BPH: AUA score ≥10; PUF of 5–15 mL/s in a TVV of 125–500 mL; post-void residual volume £250 mLs. | 16 weeks |
| Rollema et al 1991 | ||||
| Fixed-dose, double-blinded | 1 mg, 2 mg, 4 mg(50) | Placebo(17) | Normotensive or mildly hypertensive Dutch men between 50–80 years (mean 65) with >2 symptoms of “prostatism:” unidentified symptom score >6 out of 15; PUF <10 mL/s. | 5 weeks |
| de Reijke and Klarskov 2004 | mean dose 6.1 mg(105) | Alfuzosin mean dose 8.8 mg(105) | Dutch and Scandinavian men aged 49–80 years (mean 63) with moderate to severe BPH: IPSS score ≥12; PUF of 5–15 mL/s in a TVV ≥150 mL; and enlarged prostate determined by DRE. | 16 weeks |
| Kaplan et al 1995 | ||||
| Fixed-dose | 4 mg (22) | Terazosin 5 mg; (21) | American normotensive men aged 50–80 years (mean 59.6) with symptomatic | 234 weeks |
| BPH: Boyarsky score ≥8; PUF of 5–15 mL/s in a TVV of 150 mLs. | mean follow-up | |||
| Kirby 2003 | ||||
| Crossover, dose-titration, double-blinded | GITS 4 mg or 8 mg (48) | Tamsulosin 0.4 or 0.8 mg(50) | British hypertensive men aged 50–80 years (mean 65), white race 98%, with symptomatic BPH: IPSS score ≥12; PUF of 5–15 mL/s in a TVV of 150 mLs; and enlarged prostate determined by DRE. | 20 weeks |
| Lee et al 2005 | ||||
| Fixed-dose, double-blinded | (1) GITS 4 mg (76)
(2) Combined with propiverine 20 mg (142) |
Korean men aged 50–80 years (mean 66) with overactive bladder for ≥6 months and urodynamically proven bladder outlet obstruction (Abrams-Griffith score ≥20). | 8 weeks | |
Note: *Includes run-in periods;
A pooled analysis of 3 trials; Fawzy (all subjects from the study with follow-up efficacy data), Gillenwater (4 mg, 8 mg and placebo), and an unpublished study. The unpublished study 3 characteristics are presented here.
Abbreviations: GITS, gastrointestinal therapeutic system.
Baseline characteristics
A total of 6261 men (doxazosin n = 2413, placebo n = 1460, active control n = 1208, doxazosin-finasteride combination therapy n = 1054, doxazosin-propiverine combination therapy n = 152) were randomized. The mean age was 64 years. The dose of doxazosin was generally 4 mg or 8 mg, either titrated to response or a fixed dose. Three studies assessed the efficacy of controlled release gastrointestinal therapeutic system (GITS) formulation of doxazosin, a placebo-controlled study with a standard formulation treatment arm, a crossover study versus tamsulosin, and a study comparing doxazosin-propiverine combination with doxazosin montherapy (Andersen et al 2000; Kirby 2003; Lee et al 2005). Study duration ranged from 5 weeks to 4.5 years with 3 trials lasting at least one year. Of the four placebo-controlled trials reporting racial characteristics, 86% of the participants were white, 7% black (Chapple et al 1994; Fawzy et al 1995; Andersen et al 2000; McConnell et al 2003). Men in the one active-controlled trial that reported racial characteristics were overwhelmingly white (Kirby 2003). Two studies specifically recruited men with mild to moderate essential HTN (Gillenwater et al 1995; Kirby 2003). One trial enrolled men with overactive bladder (OAB) with concomitant BPH (Lee et al 2005).
Severity of LUTS at baseline did not differ by treatment group based on symptom scores and peak urine flow rates. The mean baseline IPSS in 5 placebo-controlled trials was 17.1 points, indicative of moderate BPH. The mean baseline peak urine flow was 10.1 milliliters per second (mL/sec) in 6 placebo-controlled trials and the trial versus terazosin.
Results
Doxazosin vs placebo (n = 10 studies)
Urinary symptom scores
Statistically significant improvements in urinary symptom scores were reported in 6 trials (Fawzy et al 1995; Gillenwater et al 1995; Akan et al 1998; Andersen et al 2000; Kirby et al 2003; McConnell et al 2003), shown in Table 2. The IPSS was used to evaluate LUTS in 5037 men (Fawzy et al 1995; Akan et al 1998; Andersen et al 2000; Kirby et al 2003; McConnell et al 2003). Improvements in the IPSS were maintained in the 2 dose-titration studies 1 year or longer in duration.
Table 2.
Outcomes data from individual studies of doxazosin for treatment of LUTS suggestive of benign prostatic hyperplasia
| Reference/N (#) |
Symptom score (SS): points or Symptoms Improvement |
Change % (decrease) vs. baseline/p-value between placebo/control |
Peak Flow Rate: mL/s |
Change % (increase) vs baseline/p-value between placebo/control | ||
|---|---|---|---|---|---|---|
| baseline* | mean change | |||||
| Drug (mg/d)/Control | baseline* | mean change | ||||
| Placebo-controlled trials, long-term study duration (≥52 weeks) | ||||||
| Kirby N = 1095 | IPSS** | |||||
| Doxazosin (D) 4 mg or 8 mg | 17.1 ± 4.2 (SD) | −8.3 ± 0.4 (SE) | −49%/<0.05 vs. F, P | 10.4 ± 2.5 (SD) | 3.6 ± 0.3 | 35%/<0.05 vs F, P |
| Finasteride 5 mg (F) | 17.1 ± 4.4 (SD) | −6.6 ± 0.4 | −39% | 10.2 ± 2.5 (SD) | 1.8 ± 0.3 | 18% |
| Combination D+F | 17.3 ± 4.7 (SD) | −8.5 ± 0.4 | −49%/<0.05 vs. F, P | 10.4 ± 2.7 (SD) | 3.8 ± 0.3 | 37%/<0.05 vs. F, P |
| Placebo (P) | 17.2 ± 4.5 (SD) | −5.7 ± 0.4 | −33% | 10.8 ± 2.5 (SD) | 1.4 ± 0.3 | 13% |
| MTOPS N = 3047 | AUA** | |||||
| Doxazosin 4 mg or 8 mg | 17.0 ± 5.8 (SD) | −6.6 | −39%/<0.001 vs. F, P | 10.3 ± 2.5 (SD) | 4.0 | 39%/<0.001 vs F, P |
| Finasteride 5 mg | 17.6 ± 5.9 (SD) | −5.6 | −32% | 10.5 ± 2.5 (SD) | 3.2 | 30% |
| Combination D+F | 16.8 ± 5.8 (SD) | −7.4 | −44%/<0.001 vs. F, P, | 10.6 ± 2.5 (SD) | 5.1 | 48%/<0.001 vs. F, P |
| 0.035 vs. D | 0.002 vs. D | |||||
| Placebo (P) | 16.8 ± 5.9 (SD) | −4.9 | −29% | 10.5 ± 2.6 (SD) | nr† | |
| Placebo-controlled trials, mid-term study duration (>12 weeks <52 weeks) | ||||||
| Andersen N = 795 | IPSS | |||||
| Doxazosin 4 mg or 8 mg | 17.8 ± 4.5 | −8.4 ± 0.3 | −47%/<0.001 | 10.0 ± 2.8 | 2.2 ± 0.2 | 22%/<0.001 |
| Dox.–GITS†† 4 mg or 8 mg | 17.7 ± 4.3 | −8.0 ± 0.3 | −45%/<0.001 | 10.3 ± 2.6 | 2.6 ± 0.2 | 25%/<0.001 |
| Placebo | 18.0 ± 4.3 | −6.0 ± 0.4 | −33% | 9.9 ± 2.6 | 0.8 ± 0.3 | 8% |
| Chapple N = 135 | Modified Boyarsky | |||||
| Doxazosin 4 mg | “Slight differences” between doxazosin and placebo in both obstructive and irritative symptom scores. | 9.1 ± 0.5 | (SE) | 2.6 ± 0.7 | 29%/0.09 | |
| Placebo | 9.1 ± 0.5 | 1.1 ± 0.6 | 12% | |||
| Fawzy N = 100 | AUA | |||||
| Doxazosin 2 mg, 4mg or 8 mg | 14.2 ± 3.6(SD) | −5.7 | −39%/<0.001 | 9.7 ± 2.5 | 2.9 | 30%/<0.01 |
| Placebo | 15.6 ± 3.3 | −2.5 | −17% | 9.9 ± 2.4 | 0.7 | 7% |
| Gillenwater N = 248 | Modified Boyarsky–Severity score (total) | |||||
| Doxazosin 2 mg | 28.2 ± 3.6(SD) | −2.8 | −10%/ns‡ | nr | 1.5 | –/ns |
| Doxazosin 4 mg | 30.0 ± 4.6 | −5.0 | −17%/<0.01 | nr | 2.3 | –/<0.05 |
| Doxazosin 8 mg | 30.0 ± 4.0 | −4.2 | −14%/<0.05 | nr | 3.3 | –/<0.01 |
| Doxazosin 12 mg | 29.0 ± 4.6 | −3.6 | −12%/ns | nr | 3.6 | –/<0.01 |
| Placebo | 28.0 ± 5.0 | −2.5 | −9% | nr | 0.1 | – |
| Modified Boyarsky-Bothersomeness score (total) | ||||||
| Doxazosin 2 mg | 37.1 ± 5.8(SD) | −3.4 | −9%/ns | |||
| Doxazosin 4 mg | 38.1 ± 5.8 | −5.3 | −14%/<0.05 | |||
| Doxazosin 8 mg | 37.4 ± 6.6 | −4.7 | −13%/ns | |||
| Doxazosin 12 mg | 37.4 ± 5.6 | −4.9 | −13%/ns | |||
| Placebo | 36.3 ± 6.5 | −3.0 | −8% | |||
| Roehrborn N = 339 (A pooled analysis that included data Fawzy (dose titration), Gillenwater and unpublished data from Pfizer (both fixed-dose studies) | ||||||
| Based on the AUA and Boyarsky (Severity) symptom scores = 100 points | ||||||
| Doxazosin 4 or 8 mg | 47.1 ± 0.9 (SE) | −16.4 | −35%/0.0001 | 10.0 ± 0.2 (SE) | 2.2 ± 0.3 | 22%/0.0017 |
| Placebo | 48.2 ± 1.1 | −9.8 | −20% | 10.0 ± 0.2 | 0.9 ± 0.3 | 9% |
| Placebo-controlled trials, short-term study duration (≤12 weeks) | ||||||
| Akan N = 43 | IPSS | |||||
| Doxazosin 4 mg | 18 | −10 | −56%/<0.05 | 10 | 3.2 | 32%/<0.05 |
| Placebo | 18 | −7 | −39% | 10 | 1.8 | 18% |
| Christensen N = 100 | Patient subjective overall assessment | |||||
| Doxazosin 4 mg | 79% reporting improvement | 0.001 | 7.6 ± 3.7 (SD) | 1.5 | 20%/0.11 | |
| Placebo | 44% reporting improvement | 7.5 ± 3.5 | −0.3 | −4% | ||
| Rollema N = 67 | Symptom score not identified | |||||
| Doxazosin 1 mg | 7.8 | −1.2 | −15%/nr | 6.0 | 1.8 | 30%/nr |
| Doxazosin 2 mg | 7.8 | −1.6 | −21% | 6.5 | 3.1 | 48% |
| Doxazosin 4 mg | 7.8 | −1.3 | −17% | 8.2 | 1.1 | 13% |
| Placebo | 8.1 | −1.5 | −19% | 8.6 | −0.8 | −9% |
| Active-controlled trials: versus α1-receptor antagonists (n = 3) | ||||||
| de Reijke N = 210 | IPSS | |||||
| Doxazosin mean 6.1 mg | 19.1 ± 5.2 (SD) | −9.2 ± 0.6 (SE) | −48%/<0.05 | 10.0 ± 3.3 | 2.5 ± 0.4 | 25%/ns |
| Alfuzosin mean 8.8 mg | 18.0 ± 4.8 | −7.4 ± 0.6 | −41% | 10.6 ± 3.1 | 2.8 ± 0.4 | 26% |
| Kaplan N = 43 | Boyarsky –Total | |||||
| Doxazosin 4 mg (AM) | 11.6 | −4.9 | −42%/ns for all groups | 9.0 | 2.8 | 31%/ns for all groups |
| Doxazosin 4 mg (PM) | 12.0 | −5.0 | −42% | 9.2 | 3.1 | 34% |
| Terazosin 5 mg (AM) | 12.1 | −4.6 | −38% | 9.2 | 3.0 | 33% |
| Terazosin 5 mg (PM) | 11.5 | −5.4 | −47% | 8.9 | 3.1 | 35% |
| Kirby N = 52 | IPSS | |||||
| Dox.–GITS 4 or 8 mg | 16.4 ± 6.4 (SD) | −8.0 ± 0.5 | −50%/0.019 | 10.4 ± 3.14 | 2.6 ± 0.4 | 25%/0.089 |
| Tamsulosin 0.4 or 0.8 mg | 16.1 ± 6.8 | −6.4 ± 0.5 | −40% | 10.3 ± 4.35 | 1.7 ± 0.4 | 17% |
| Lee N = 228 | IPSS | |||||
| Dox.-GITS 4 mg | 20.6 ± 7.2 (SD) | −7.3 | −35%/ns | 10.5 ± 4.2 | 1.7 | 16%/ns |
| Combination D+ Propiverine 20 mg | 22.0 ± 7.3 (SD) | −7.4 | −34% | 10.4 ± 4.3 | 1.0 | 10% |
Note: *±, Standard deviation (SD) or Standard error (SE);
International Prostate Symptom Score is equivalent to the American Urological Association Symptom Score (AUA-SS) in the United States;
nr, not reported;
GITS, gastrointestinal therapeutic system,
ns, not statistically significant.
The Medical Therapy of Prostatic Symptoms (MTOPS) trial was the largest and longest study conducted (McConnell et al 2003). The goal of MTOPS was to determine if combination medical therapy with an α1–blocker and a 5-α reductase inhibitor was superior to placebo or either drug alone at improving both baseline symptoms and preventing disease progress as determined by a worsening IPSS score of at least 4 points and/or need for surgical intervention. The mean change from baseline over 4 years was −6.6 points (39% improvement) for doxazosin compared with −4.9 points (29%) placebo (McConnell et al 2003). When compared with placebo, the mean change in IPSS scores for patients randomized to receive doxazosin (WMD = −1.7 points, p < 0.001) with this level of LUTS did not achieve a level previously determined to be noticeable by patients (ie, at least 3 point improvement). The yearlong Prospective European Doxazosin and Combination Therapy (PREDICT) trial reported a mean change from baseline of −8.3 points (49% improvement) for doxazosin versus −5.7 points (33%) for placebo (Kirby et al 2003). Similar to the MTOPS findings, the average change due to doxazosin compared with placebo in the PREDICT trial did not reach a clinically noticeable level (WMD = −2.6 points, p < 0.05).
Mean change in urinary symptom scale scores varied in studies that were “mid-length duration” (ie, >12 weeks <1 year). Modified unvalidated Boyarsky symptom scores were used in 2 studies involving 383 men (Chapple et al 1994; Gillenwater et al 1995). The fixed-dose study by Gillenwater found only the 4 mg dose statistically superior to placebo in improving both severity and bother scores (Gillenwater et al 1995). Roehrborn and Siegel transformed different symptom indices (AUA and Boyarsky) to produce a homogeneous pool of symptom and bother data in their pooled analysis (Roehrborn and Siegel 1996). Doxazosin resulted in significantly greater improvements in symptom severity and bother versus placebo (Roehrborn and Siegel 1996). Doxazosin GITS was as effective as standard doxazosin in improving symptoms compared with placebo (symptom score reductions from baseline for Doxazosin GITS, Doxazosin and Placebo = 8.0, 8.4, and 6.0 points respectively) (Andersen et al 2000). None of the improvements reached a clinically detectable difference compared with placebo.
Peak urinary flow
Doxazosin significantly improved peak urinary flow (PUF) in 6 studies compared with placebo (Fawzy et al 1995; Gillenwater et al 1995; Akan et al 1998; Andersen et al 2000; Kirby et al 2003; McConnell et al 2003) (Table 2). The percentage increases in peak flow for the mid-term trials were, on average, between 20%–30%. Long-term maintenance of these improvements was shown in MTOPS and PREDICT with MTOPS demonstrating 39% increase in peak flow after 4 years (McConnell et al 2003). Overall, mean change for PUF from baseline for doxazosin ranged from 1.5–3.6 milliliters per second (mL/sec). Mean change for placebo ranged from −0.3 mL/sec to 1.8, with improvements from −18% to 18%. The WMD from baseline for three studies, including the Roehrborn analysis that incorporated data from the Fawzy and Gillenwater trials, was 1.6 mL/sec (95% CI, 1.2–2.1) versus placebo (Roehrborn and Siegel 1996; Andersen et al 2000; Kirby et al 2003). The clinical importance of this is not known.
Doxazosin vs finasteride (n = 2 studies)
MTOPS and PREDICT also compared the effect of doxazosin with finasteride 5 mg monotherapy. Doxazosin was significantly more effective than finasteride in improving IPSS scores and PUF versus finasteride at 1 year (Kirby et al 2003a; McConnell et al 2003) At year 4, there was no difference in peak flow between doxazosin and finasteride (McConnell et al 2003).
Combination therapy (n = 2 studies)
Combination finasteride plus doxazosin therapy provided similar improvement in symptom scores and peak flow rates compared with doxazosin alone at 1 year (Kirby et al 2003; McConnell et al 2003). However, over a 4-year period, improvements in urinary symptoms and PUF were significantly greater with combination therapy versus doxazosin or finasteride alone (McConnell et al 2003). Mean change for combination therapy was −7.4 IPSS points compared with −6.6 (WMD = −0.8 points), −5.6, and −4.9 points for doxazosin, finasteride, and placebo, respectively. The median change from baseline for peak flow rate for combination therapy was 3.7 mL/sec (mean 5.1), 2.5 mL/sec (mean 4.0) for doxazosin, 2.2 mL/sec (mean 3.2) for finasteride, and 1.4 mL/sec for placebo.
The primary outcome measure of the MTOPS trial was the effect of doxazosin, finasteride, and combination therapy on the overall clinical progression of BPH. Clinical progression was defined as any occurrence of the following items: 1) increase of at least 4 AUA points from baseline; 2) acute urinary retention; 3) urinary incontinence; 4) renal insufficiency; or 5) recurrent urinary tract infections. Combination therapy significantly reduced the risk of overall clinical progression of BPH compared with the montherapies and placebo (p < 0.001 for all groups). There were 42 incidences of clinical progression for the combination therapy group, 73 for doxazosin, 78 for finasteride, and 122 for placebo at a mean follow-up of 4 years. Significant reductions were also observed for doxazosin and finasteride monotherapy versus placebo. Reduction in risk versus placebo was 66%, 39%, and 34% for combination therapy, doxazosin, and finasteride, respectively. The vast majority of “Clinical progression events” were due to increase of at least 4 AUA points from baseline. Among the men taking placebo, there were 122 cumulative clinical progression event (97/122 were due to at least a 4 point increase in AUA symptom score-item 1) compared with 42 events (36 item 1) for combination therapy (5.3%, absolute risk reduction (ARR) versus placebo 11.3%), 73 events (55 item 1) for doxazosin (9.7%, ARR 6.9%), and 78 events (65 item 1) for finasteride (10.2%, ARR 6.4%). Four cases of urinary retention (<1% of subjects, ARR 1.9%) were reported for combination therapy group compared with 6 for finasteride (<1%, ARR 1.7%), 9 for doxazosin (1.2%, ARR 1.3%), and 18 for placebo (2.4%). Subgroup analysis suggested that effectiveness of combination therapy was associated with prostate volume as measured by PSA levels or transrectal ultrasound. For example, in the 20% and 30% of men with baseline PSA 4 ng/ml or greater or ultrasound prostate volume greater than 40 ml the number needed to treat at 4.5 years was 4.9 and 4.7 respectively, compared with 8.4 in the entire cohort (Kaplan et al 2006)
Combination therapy reduced the need for minimally invasive therapy (eg, transurethral prostatectomy, laser surgery, or microwave thermotherapy) and risk of urinary retention compared with doxazosin. However, the absolute improvement versus placebo at 4 years was 3.5% for the combination therapy group compared with 3.2% and 1.6% for finasteride and doxazosin monotherapy, respectively (McConnell et al 2003).
Doxazosin vs other α1–blockers (n = 3 studies)
Treatment with doxazosin resulted in a greater decrease in IPSS score from baseline compared with alfuzosin, −9.2 points versus −7.4 points (p = 0.036) (de Reijke and Klarskov 2004). Doxazosin was not more effective in improving PUF over the 14-week study period. Doxazosin-GITS produced a greater improvement versus tamsulosin in the IPSS (−8.0 points vs −6.4 points, p = 0.019) but was not significantly more effective in improving PUF at the end of the 20-week study period (Kirby 2003). Doxazosin 4 mg and terazosin 5 mg were similar in efficacy in a study investigating the effect of dosing schedule and safety of the two α1–blockers over a mean follow-up of 42 weeks (Kaplan et al 1995).
Doxazosin vs Doxazosin plus Propiverine (n = 1 study)
Combined antimuscarinic propiverine and doxazosin GITS therapy was effective and relatively safe in treating men with OAB and BPH (Lee et al 2005). Both combination and doxazosin GITS monotherapy improved total IPSS, peak flow rate, urinary frequency and average micturition volume. Improvements in storage symptoms and urgency IPSS subscales were significantly greater in subjects treated with combination therapy.
Withdrawals and adverse events
Withdrawal from treatment and adverse event data for the placebo-controlled trials is summarized in Table 3. Men receiving doxazosin were less likely to stop treatment (15%) than men on placebo (20%) (Christensen et al 1993; Chapple et al 1994; Fawzy et al 1995; Gillenwater et al 1995; Andersen et al 2000; Kirby et al 2003; McConnell et al 2003). Withdrawals related to adverse events were higher in men in the doxazosin group (RR = 1.9; 95% CI, 0.9–4.0) and were often related to cardiovascular events such as dizziness although instances of hypotension were rare.
Table 3.
Withdrawals from treatment and adverse events: Number of men reporting
| Adverse events | n/N | % | n/N | % | Number of Studies Reporting | Relative Risk Ratio [95% CI] |
|---|---|---|---|---|---|---|
| Versus Placebo | Doxazosin | Placebo | ||||
| Withdrawals: all causes | 198/1282 | 15.4 | 125/640 | 19.5 | 6 | 0.93 [0.64 to 1.35] |
| Withdrawals: due to AEs | 108/1282 | 8.4 | 39/640 | 6.1 | 6 | 1.88 [0.88 to 4.01] |
| Any AE | 265/536 | 49.4 | 98/268 | 36.6 | 3* | 1.35 [1.12 to 1.62] |
| Dizziness | 163/1450 | 11.2 | 49/693 | 7.1 | 5* | 1.92 [1.40 to 2.61] |
| Headache | 99/1474 | 6.7 | 70/714 | 9.8 | 6* | 0.81 [0.39 to 1.72] |
| Asthenia | 93/1450 | 6.4 | 17/693 | 2.5 | 5* | 3.33 [1.97 to 5.61] |
| Postural hypotension | 32/1400 | 2.3 | 6/643 | <1 | 4 | 2.72 [1.21 to 6.15] |
| Somnolence | 21/471 | 4.5 | 8/412 | 1.9 | 2 | 2.31 [1.02 to 5.21] |
| Impotence | 16/275 | 5.8 | 9/269 | 3.3 | 1 | 1.71 [0.77 to 3.79] |
| Versus finasteride** | Doxazosin | Finasteride | ||||
| Withdrawals: all causes | 282/1031 | 27.4 | 265/1032 | 25.7 | 2 | 1.05 [0.87 to 1.26] |
| Withdrawals: due to AEs | 32/275 | 11.6 | 34/264 | 12.9 | 1 | 0.90 [0.57 to 1.42] |
| Dizziness | 43/275 | 15.6 | 21/264 | 8.0 | 1 | 1.97 [1.20 to 3.22] |
| Asthenia | 29/275 | 10.5 | 11/264 | 4.2 | 1 | 2.53 [1.29 to 4.96] |
| Postural hypotension | 16/275 | 5.8 | 2/264 | <1 | 1 | 7.68 [1.78 to 33.08] |
| Impotence | 16/275 | 5.8 | 13/264 | 4.9 | 1 | 1.18 [0.58 to 2.4] |
| Combination therapy versus placebo** | Combination therapy | Placebo | ||||
| Withdrawals: all causes | 89/286 | 31.1 | 76/270 | 28.1 | 1 | 1.11 [0.85 to 1.43] |
| Withdrawals: due to AEs | 35/286 | 12.2 | 30/270 | 11.1 | 1 | 1.10 [0.70 to 1.74] |
| Dizziness | 39/286 | 13.6 | 20/269 | 7.4 | 1 | 1.83 [1.10 to 3.06] |
| Asthenia | 29/286 | 9.1 | 11/269 | 4.1 | 1 | 2.48 [1.26 to 4.86] |
| Postural hypotension | 8/286 | 2.8 | 4/269 | 1.5 | 1 | 1.88 [0.57 to 6.17] |
| Impotence | 30/286 | 10.5 | 9/269 | 3.3 | 1 | 3.14 [1.52 to 6.48] |
| Versus alfuzosin** | Doxazosin | Alfuzosin | ||||
| Discontinuations: all causes | 12/105 | 14.3 | 18/105 | 17.1 | 1 | 0.67 [0.34 to 1.31] |
| Discontinuations: due to AEs | 12/105 | 14.3 | 7/105 | 6.7 | 1 | 1.71 [0.70 to 4.18] |
| Dizziness | 14/99 | 14.1 | 11/93 | 11.8 | 1 | 1.20 [0.57 to 2.50] |
| Asthenia | 5/99 | 5.1 | 5/93 | 5.4 | 1 | 0.94 [0.28 to 3.14] |
| Postural hypotension | 2/99 | 2.0 | 3/93 | 3.2 | 1 | 0.63 [0.11 to 3.66] |
| Impotence | 0/99 | 0.0 | 1/93 | 1.1 | 1 | 0.31 [0.01 to 7.60] |
| Versus tamsulosin** (crossover study) | Doxazosin-GITS | Tamsulosin | ||||
| Any AE | 38/48 | 79.2 | 39/50 | 78.0 | 1 | 1.01 [0.83 to 1.25] |
| Treatment-related AE | 18/48 | 37.5 | 20/50 | 40.0 | 1 | 0.94 [0.57 to 1.54] |
| Dizziness | 8/48 | 16.7 | 8/50 | 16.0 | 1 | 1.04 [0.43 to 2.55] |
| Asthenia | 6/48 | 12.5 | 12/50 | 24.0 | 1 | 0.52 [0.21 to 1.28] |
| Headache | 6/48 | 12.5 | 8/50 | 16.0 | 1 | 0.78 [0.29 to 2.08] |
| Hypotension | 4/48 | 8.3 | 2/50 | 0.4 | 1 | 2.08 [0.40 to 10.85] |
| Somnolence | 4/48 | 8.3 | 2/50 | 0.4 | 1 | 2.08 [0.40 to 10.85] |
| Retrograde ejaculation | 0/48 | 0.0 | 2/50 | 0.4 | 1 | |
| Versus terazosin† | Doxazosin | Terazosin | ||||
| Discontinuations: all causes | 3/22 | 13.6 | 5/21 | 23.8 | 1 | 0.57 [0.16 to 2.10] |
| Discontinuations: due to adverse events | 3/22 | 13.6 | 5/21 | 23.8 | 1 | 0.57 [0.16 to 2.10] |
| Dizziness | 1/22 | 4.5 | 3/21 | 14.3 | 1 | 0.32 [0.04 to 2.82] |
| Headache | 1/22 | 4.5 | 1/21 | 4.8 | 1 | 0.95 [0.06 to 14.30] |
| Versus combination doxazosin and propiverine therapy | Doxazosin | Combination therapy | ||||
| Discontinuations: all causes† | 9/76 | 11.8 | 21/152 | 13.8 | 1 | 0.86 [0.41 to 1.78] |
| Discontinuations: due to adverse events | 1/69 | 1.4 | 7/142 | 4.9 | 1 | 0.29 [0.04 to 2.34] |
| Dry mouth | 4/69 | 5.8 | 26/142 | 18.3 | 1 | 0.32 [0.12 to 0.87] |
| Dizziness | unclear | 8/142 | 5.6 | 1 | ||
| Difficult voiding | 1/69 | 1.4 | 4/142 | 2.8 | 1 | 0.51 [0.06 to 4.52] |
| Constipation | 0/69 | 0.0 | 3/142 | 2.1 | 1 | 0.29 [0.02 to 5.57] |
| Blurred vision | 1/69 | 1.4 | 2/142 | 1.4 | 1 | 1.03 [0.09 to 11.15] |
| Significant post-void residual Volume | 0/69 | 0.0 | 2/142 | 1.4 | 1 | 0.41 [0.02 to 8.40] |
Note: *Includes meta-analysis by Janknegt, pooling studies by Chapple, Christensen, Rollema and 2 unpublished trials;
Includes only men treated with Doxazosin versus the respective controls from individual studies;
Includes 17 subjects withdrawing consent and not receiving allocated intervention.
Abbreviations: AE, adverse event; CI, confidence interval.
Most placebo-controlled trials reported specific adverse events data. The incidence of dizziness, asthenia, and postural hypotension were significantly greater compared with men in the placebo and finasteride groups. It is unclear from most of the trials whether the postural hypotension was symptomatic or asymptomatic. Rates of dizziness, asthenia, and postural hypotension were 11%, 6%, and 2% compared with 7%, 3%, and <1% for placebo. Reports of hypotension and somnolence, although small and infrequent, were more likely to occur in men treated with doxazosin (Janknegt and Chapple 1993; Chapple et al 1994; Fawzy et al 1995; Kirby et al 2003; McConnell et al 2003). Doxazosin-GITS had fewer adverse events compared with standard doxazosin. Dizziness and asthenia was reported in 5.7% and 3.2% of men on doxazosin-GITS compared with 8.4% and 5% of men receiving regular release formulations of doxazosin (Andersen et al 2000).
Withdrawal rates primarily related to adverse events were comparable between combination therapy with doxazosin and finasteride alone (Table 3) (Kirby et al 2003). However, the type of adverse event differed between treatment groups. Erectile dysfunction reported in PREDICT was significantly greater for combination therapy compared with doxazosin or finasteride separately (10.5% vs 5.8% and 4.9%, respectively, p < 0.01). In MTOPS, 27% on doxazosin alone discontinued, compared with 24% on finasteride and 18% on combination therapy (McConnell et al 2003). Dyspnea, abnormal ejaculation, and peripheral edema were reported more frequently in men treated with combination therapy. Generally, adverse events reported for combination therapy were similar to the events common with each monotherapy. Rates of dizziness, asthenia, postural hypotension, and somnolence were significantly greater with doxazosin and combination therapy. Erectile dysfunction and decreased libido were reported more frequently with use of finasteride and combination therapy.
In the crossover trial evaluating doxazosin-GITS to tamsulosin, no subject receiving doxazosin-GITS withdrew from the trial because of a treatment-related adverse event compared with 2 subjects receiving tamsulosin. The incidence of adverse events was generally similar between doxazosin-GITS and tamsulosin. There were no significant differences in the frequency of adverse events of doxazosin compared with alfuzosin and terazosin (Kaplan et al 1995; de Reijke and Klarskov 2004). In the doxazosin montherapy versus propiverine plus doxazosin trial, two monotherapy and 11 combination subjects withdrew from treatment prior to study completion (Lee et al 2005). Withdrawals due to adverse events occurred in one (1.4%) and seven (4.9%) of mono and combined therapy subjects, respectively. Dry mouth, a common antimuscarinic adverse event, was reported in 18% of combination subjects compared with 6% receiving doxazosin monotherapy (RR reduction = 0.32; 95% CI, 0.12–0.87).
Additional information from other studies of doxazosin
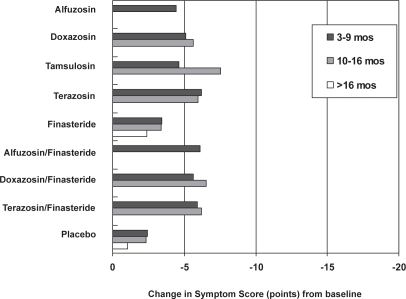
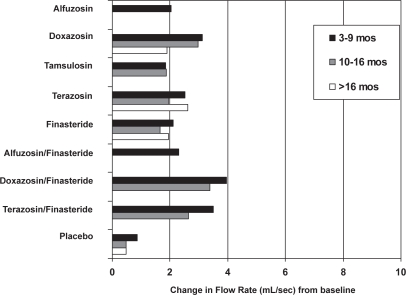
The AUA Guideline Report on the Management of Benign Prostatic Hyperplasia included results from nonrandomized studies (AUA 2003). They noted improvements in urinary symptoms and flow measures comparable with our findings with an average across all α1–blockers of approximately 2 to 2.5 points versus placebo (Figures 1 and 2). The median dose of doxazosin in analyzed trials was between 6 mg and 7 mg per day. Doxazosin resulted in an approximately 1–2 point improvement in the BPH Impact Index with slightly less improvement in longer term studies. While there were no direct comparison studies, the magnitude of symptom improvement reported for doxazosin was less then surgical or minimally invasive procedures. Analysis of selected clinically relevant adverse effects of pharmacologic therapies indicates that overall adverse effects and withdrawals are similar among α1–blockers, vary in the type of occurrence and only slightly in frequency, especially for symptomatic adverse events.
Figure 1.
AUA/IPSS Symptom Index score improvements from baseline for medical therapies by duration of follow-up. Missing bars indicate that data were not available. Copyright © 2003. Reproduced with permission from Roehrborn CG, McConnell JD, Barry MJ, et al. 2003. AUA guideline on the management of benign prostatic hyperplasia [online]. Accessed on 28 October 2004. AUA Education and Research, Inc. URL: http://auanet.org/guidelines/bph.cfm.
Figure 2.
Peak urine flow-rate improvements for medical therapies from baseline by duration of follow-up. Missing bars indicate that data were not available. Copyright © 2003. Reproduced with permission from Roehrborn CG, McConnell JD, Barry MJ, et al. 2003. AUA guideline on the management of benign prostatic hyperplasia [online]. Accessed on 28 October 2004. AUA Education and Research, Inc. URL: http://auanet.org/guidelines/bph.cfm.
Of note are results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attach Trial (ALLHAT). This randomized trial evaluated four classes of pharmacologic therapies (including doxazosin) for hypertension in patients 55 years of age or older. Many enrollees were men and undoubtedly some had LUTS of comparable severity with patients enrolled in BPH treatment trials. While the effectiveness on LUTS and urinary symptoms was not reported, the doxazosin arm of the trial was discontinued prematurely due to a higher risk of stroke, congestive heart failure and combined cardiovascular events compared with the diuretic chlorthalidone. While ALLHAT does not demonstrate that doxazosin is harmful, it suggests that use of doxazosin (and likely other alpha blockers) to treat both hypertension and LUTS is not warranted.
Discussion
The evidence from randomized controlled trials demonstrate that doxazosin, typically 4 mg/day or 8 mg/day, reduces LUTS and improves urinary flow rates compared with placebo and finasteride. Efficacy was comparable with α1–blockers terazosin, alfuzosin, and tamsulosin.
The GITS formulation of doxazosin was as effective and had slightly fewer adverse effects than the standard formulation of doxazosin. However, the average magnitude of improvement compared with placebo did not reach of level previously determined to be detectable and is therefore of questionable clinical significance.
Long-term, doxazosin did reduce the number of men with clinical progression of BPH compared with placebo (10% vs 17%). Combination doxazosin-finasteride therapy reduced the percentage of men having clinical progression compared with doxazosin (10% vs 5%) and the percent having at least a 4-point increase in AUA symptom score (7% vs 5%). Combination therapy also reduced the need for invasive treatment for BPO though the absolute difference compared with doxazosin was only 2%. The benefit appeared to be greatest and perhaps limited to men with at least moderately severe urinary symptoms and enlarged prostate glands (the latter as measured by a PSA >4 and/or a ultrasound volume >40 ml. Dizziness, fatigue, and postural hypotension were more frequent with doxazosin than with placebo. The combination of doxazosin plus finasteride resulted in a higher rate of adverse events then for either drug alone.
A limitation of many of the trials reported is the short-term study duration, with only 2 placebo-controlled studies lasting longer than 26 weeks (Kirby et al 2003; McConnell et al 2003). Only the MTOPS trial evaluated the long-term effect of doxazosin on urinary symptom progression and development of urinary retention, renal insufficiency, recurrent urinary tract infection, and/or need for surgery or a minimally invasive technique (McConnell et al 2003). The PREDICT study examined the occurrence of acute urinary retention and transurethral resection of the prostate in a post-hoc analysis (Kirby et al 2003). Similar to MTOPS, PREDICT found no benefit of combination therapy compared with doxazosin alone at 1 year. Thus the currently available evidence suggests that doxazosin provides an average improvement in urinary symptom scale scores of approximately 2 points versus placebo, which is maintained over 4 years. It reduces the percentage of men having at least a 4-point increase in urinary symptom scale scores by 7% versus placebo and 2% versus finasteride. The combination of doxazosin plus finasteride results in an additional 0.8 point reduction in the AUA symptom scale score, 2% reduction in men having at least a 4 point increase in AUA symptom score, and 2% reduction in use of invasive therapy due to BPO after 4 years of therapy.
These benefits need to be balanced against the increased medication cost and adverse events associated with combination therapy. Based on the MTOPS results and the monthly drug cost in the US for doxazosin (8 mg) of $24 and finasteride of $80, the cost for preventing one episode of at least a 4-point progression in AUA symptom scale scores over 4 years would be $15 728 for doxazosin (Number needed to treat [NNT] at 4 years = 13.7), $57 600 for finasteride (NNT at 4 years = 15), and $42 718 for combination therapy (NNT at 4 years = 8.4). Among men with a serum prostate-specific antigen level 4.0 ng per milliliter or greater, medication costs would equal to $23 878 for combination therapy (NNT at 4 years = 4.7) and $27 616 for finasteride (NNT at 4 years = 7.2) (McConnell et al 2003).
Systematic reviews are limited by the quality of evidence based on the available information. Adequate concealment of treatment of randomization was reported in only one study, a validated measure of study quality. Inconsistencies (eg, lack of standard deviations or errors) in data reporting precluded a pooled analysis of all studies. We were also unable to obtain additional data that would have enhanced pooling of study results despite multiple attempts to contact authors and the manufacturer of doxazosin (Pfizer Pharmaceuticals, New York, NY, USA). Few trials included in this systematic review provided racial characteristics and those that did were overwhelmingly white. Although race has not been shown to compromise efficacy (Fulton et al 1995), no BPH study has addressed the efficacy of doxazosin in black men. No studies have directly compared the long-term effectiveness, costs, durability, tolerability and satisfaction of alpha blockers with other surgical or minimally invasive options.
Conclusion
Doxazosin is generally well tolerated and improves LUTS and flow compared with baseline measures in men with symptomatic BPH. Efficacy was superior to placebo and finasteride and comparable with other α1–blockers. Compared with placebo, the average symptom improvement may not reach clinically noticeable levels though some men may achieve detectable benefits and/or have symptom progression prevented. Combination therapy with a 5-α reductase inhibitor was superior to doxazosin alone in reducing the risk for clinical progression and the need for invasive therapy due to BPH although the absolute risk reductions were 5% for clinical progression and 2% for invasive therapy. Benefit from combination therapy was greatest in men with a prostate volume greater than 40 mL or a prostate-specific antigen level greater than 4.0 ng/mL.
Acknowledgments
The authors would like to acknowledge Indulis Rutks for his work on literature search and article retrieval. This project was supported by the Department of Veterans Affairs Health Services Research and Development Service and the Minneapolis VA Center for Chronic Disease Outcomes Research. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
References
- Akan H, Basar M, Dalva I, et al. The early effects of doxazosin on benign prostatic hyperplasia. Arch Ital Urol Androl. 1998;70:41–4. [PubMed] [Google Scholar]
- Andersen M, Dahlstrand C, Hoye K. Double-blind trial of the efficacy and tolerability of doxazosin in the gastrointestinal therapeutic system, doxazosin standard, and placebo in patients with benign prostatic hyperplasia. Eur Urol. 2000;38:400–9. doi: 10.1159/000020315. [DOI] [PubMed] [Google Scholar]
- [AUA] AUA Practice Guidelines Committee AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- Barry MJ, Fowler FJ, Jr, Bin L, et al. A nationwide survey of practicing urologists: current management of benign prostatic hyperplasia and clinically localized prostate cancer. J Urol. 1997;158:488–91. doi: 10.1016/s0022-5347(01)64510-5. [DOI] [PubMed] [Google Scholar]
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–4. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- [CC] The Cochrane Collaboration . Review Manager (RevMan) [Computer program] Version 41 for Windows. Oxford; England: 2001. [Google Scholar]
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol. 1994;74:50–6. doi: 10.1111/j.1464-410x.1994.tb16546.x. [DOI] [PubMed] [Google Scholar]
- Chapple CR. Lower urinary tract symptoms suggestive of benign prostatic obstruction –Triumph: design and implementation. Eur Urol. 2001;39(Suppl 3):31–6. doi: 10.1159/000052565. [DOI] [PubMed] [Google Scholar]
- Christensen MM, Bendix Holme J, Rasmussen PC, et al. Doxazosin treatment in patients with prostatic obstruction. A double-blind placebo-controlled study. Scand J Urol Nephrol. 1993;27:39–44. doi: 10.3109/00365599309180412. [DOI] [PubMed] [Google Scholar]
- Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85–9. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- de Reijke TM, Klarskov P. Comparative efficacy of two α1-adrenoreceptor antagonists, doxazosin and alfuzosin, in patients with lower urinary tract symptoms from benign prostatic enlargement. BJU Int. 2004;93:757–62. doi: 10.1111/j.1464-410X.2003.04720.x. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;312:944–7. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzy A, Braun K, Lewis GP, et al. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J Urol. 1995;154:105–9. [PubMed] [Google Scholar]
- Fulton B, Wagstaff AJ, Sorkin EM. Doxazosin. An update of its clinical pharmacology and therapeutic applications in hypertension and benign prostatic hyperplasia. Drugs. 1995;49:295–320. doi: 10.2165/00003495-199549020-00011. [DOI] [PubMed] [Google Scholar]
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol. 1995;154:110–15. [PubMed] [Google Scholar]
- Janknegt RA, Chapple CR. Efficacy and safety of the alpha-1 blocker doxazosin in the treatment of benign prostatic hyperplasia. Analysis of 5 studies. Doxazosin Study Groups. Eur Urol. 1993;24:319–26. doi: 10.1159/000474321. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, McConnell JD, Roehrborn CG, et al. Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 ml or greater. J Urol. 2006;175:217–20. doi: 10.1016/S0022-5347(05)00041-8. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Soldo KA, Olsson CA. Terazosin and doxazosin in normotensive men with symptomatic prostatism: a pilot study to determine the effect of dosing regimen on efficacy and safety. Eur Urol. 1995;28:223–8. doi: 10.1159/000475055. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: The prospective European doxazosin and combination therapy (PREDICT) trial. Urology. 2003;61:119–26. doi: 10.1016/s0090-4295(02)02114-3. [DOI] [PubMed] [Google Scholar]
- Kirby RS. A randomized, double-blind crossover study of tamsulosin and controlled-release doxazosin in patients with benign prostatic hyperplasia. BJU Int. 2003;9:41–44. doi: 10.1046/j.1464-410x.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- Lee Lee KS, Choo MS, Kim DY, et al. Combination treatment with propiverine hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective, randomized, controlled multicenter study. J Urol. 2005;174:1334–8. doi: 10.1097/01.ju.0000173630.94559.fd. [DOI] [PubMed] [Google Scholar]
- MacDonald R, Wilt TJ, Howe RW. Doxazosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects. BJU Int. 2004;94:1263–70. doi: 10.1111/j.1464-410X.2004.05154.x. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Roehrborn C, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Eng J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- Medina JJ, Parra RO, Moore RG. Benign prostatic hyperplasia (the aging prostate) Med Clin North Am. 1999;83:1213–29. doi: 10.1016/s0025-7125(05)70159-0. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, McConnell JD, Barry MJ, et al. 2003AUA guideline on the management of benign prostatic hyperplasia [online]Accessed on 28 October 2004. AUA Education and Research, Inc. URL: http://auanet.org/guidelines/bph.cfm
- Roehrborn C, Siegel RL. Safety and efficacy of doxazosin in benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. Urology. 1996;48:406–15. doi: 10.1016/S0090-4295(96)00208-7. [DOI] [PubMed] [Google Scholar]
- Rollema HJ, Rosier P, Janknegt RA, et al. Efficacy of alpha-blocker (Doxazosin) in BPH appraised by pressure-flow (CLIM) analysis. Neurourol Urodynam. 1991;10:295–99. [Google Scholar]
- Schultz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Wei JT, Calhoun EA, Jacobsen SJ. Benign prostatic hyperplasia. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. US Department of Health and Human Services, Public Health Service, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease. Washington, DC: US Government Publishing Office; 2004. pp. 43–70. NIH Pubication No. 04-5512. [Google Scholar]