Abstract
Neovascular age-related macular degeneration (AMD) is a visually devastating condition resulting from choroidal neovascularization and secondary photoreceptor loss. Ranibizumab and bevacizumab are medications that target vascular endothelial growth factor (VEGF). While other therapies have demonstrated some ability to reduce the risk of losing vision from neovascular AMD, most patients continue to lose some degree of central visual acuity. There is growing evidence that intravitreal administration of ranibizumab and bevacizumab is effective in significantly improving the visual acuity in patients with neovascular age-related macular degeneration.
Keywords: age-related macular degeneration, choroidal neovascularization, ranibizumab, bevacizumab
Introduction
Age-related macular degeneration (AMD) is a common condition affecting the central vision of older adults. It has been estimated to occur in approximately 25% (Klein et al 1992) of individuals over the age of 65. AMD usually begins with degenerative changes to Bruch’s membrane and the retinal pigment epithelium (RPE) in the central retina (macula), resulting in mild to moderate atrophy and dysfunction of the overlying neurosensory retina often referred to as dry AMD.
Approximately 25% (AED 2001) of AMD patients will develop severe vision loss from either geographic atrophy or choroidal neovascularization (CNV), commonly known as wet or neovascular AMD. CNV represents angiogenesis from the choroid into the sub-RPE or sub-retinal space and commonly results in profound central visual loss as photoreceptors are damaged then destroyed by exudation leading to intraretinal/subretinal/subRPE fluid, hemorrhage, and eventual fibrotic scarring (Figures 1–4). CNV have been classified into either classic or occult types based on vascular leakage characteristics on fluorescein angiography. These angiographically-defined types may be related to whether the neovascularization is anatomically located in the subretinal or sub-RPE space, respectively (SST 2006).
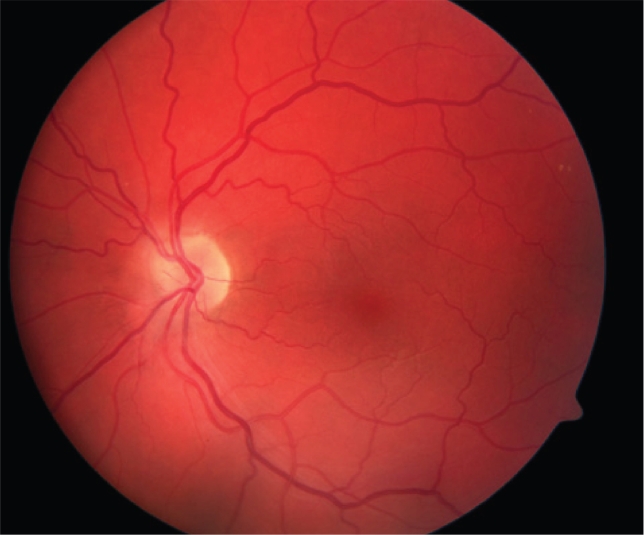
Figure 1.
Color fundus photograph of a normal left eye.
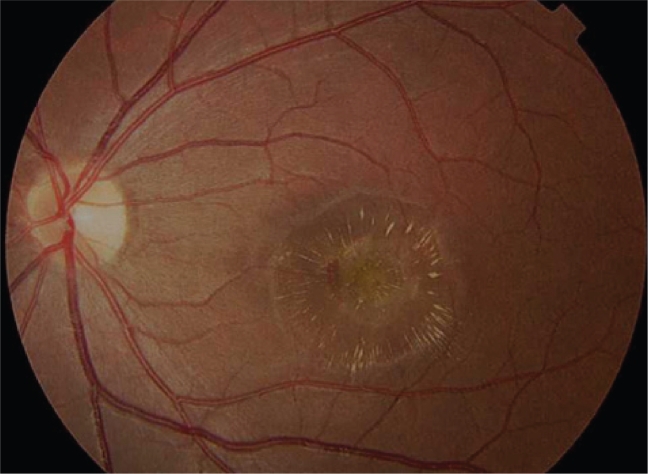
Figure 4.
Disciform scarring of right eye. This end-stage of choroidal neovascularization is represented subretinal fibrosis and photoreceptor loss.
Choroidal neovascularization development involves inflammation, angiogenesis, and, eventually, fibrosis. Vascular endothelial growth factor (VEGF) has been recognized as one important contributor (Kvanta et al 1996) in the complex and incompletely elucidated mechanisms of CNV development.
Current treatment of AMD
Antioxidant vitamin supplementation (AED 2001) has been found to benefit patients with high risk dry AMD, reducing the risk of visual loss by approximately 30% over 6 years. Treatment is aimed at arresting further atrophic changes of the RPE and the prevention of CNV.
Once individuals have developed neovascular AMD, therapies are employed which target obliteration or regression of neovascularization. Currently available and approved treatments for neovascular AMD include: focal thermal laser; photodynamic therapy with verteporfin (Visudyne, QLT Photo-therapeutics, Inc., Vancouver, British Columbia); and targeted anti-VEGF treatment with pegaptanib (Macugen, Eyetech Pharmaceuticals, New York, NY) and the recently US Food and Drug Administration-approved ranibizumab (RhuFab V2, Lucentis, Genentech, Inc., South San Francisco, CA).
Focal thermal laser may be used to photocoagulate CNV, but treatment is reserved for lesions not involving the center of the macula (fovea) as such treatment results in an immediate loss of central vision (MPSG 1991). Treatment does not prevent recurrent CNV formation. For CNV involving the fovea, a number of treatments have been shown to reduce the risk of central visual loss. Several treatment options are briefly described below.
Photodynamic therapy (PDT) uses the photoactive intravenous medication, verteporfin, which localizes preferentially to neovascularization. Once activated by nonthermal laser, verteporfin results in free radical production and subsequent damage to the neovascular tissue targeted. PDT has been shown to reduce visual loss in those individuals with small or predominantly classic subfoveal CNV (TAP 2001, 2003). However, most patients require multiple retreatments and collateral damage to adjacent healthy tissues may occur with resultant reduction in visual function (Reinke et al 1999).
Pegaptanib is an RNA oligonucleotide with affinity and specificity for the VEGF 165 amino acid isoform, the isoform thought to play the dominant role in pathologic neovascularization (Ishida et al 2003; McColm et al 2004). Given as an intravitreal injection every 6 weeks, it has been shown to significantly reduce visual loss in patients with CNV regardless of type or size (Gragoudas et al 2004).
In addition to the above approved therapies, other treatments are currently undergoing further evaluation. Corticosteroids possess anti-inflammatory and anti-angiogenic properties (Danis et al 1996; Ciulla et al 2001) and reduce vascular permeability (Penfold et al 2000). Early case series of treatment combining intravitreal steroids with PDT have demonstrated promise (Spaide et al 2003) and larger, prospective, randomized studies are currently underway to determine whether such combination treatment may result in better visual outcomes than with PDT alone. However, intra- or peri-ocular corticosteroid use may be limited by induced glaucoma and cataracts in a significant number of patients (Smithen et al 2004).
Anecortave acetate is a cortisene molecule, specifically designed to retain the anti-angiogenic properties of corticosteroids while eliminating the aforementioned side-effects and is administered as a posterior juxtascleral depot injection every 6 months (Figure 5) (AAC 2003). A recent study comparing anecortave acetate with PDT for the treatment of CNV found that it did not meet the specified noninferiority criteria (Slakter et al 2006).
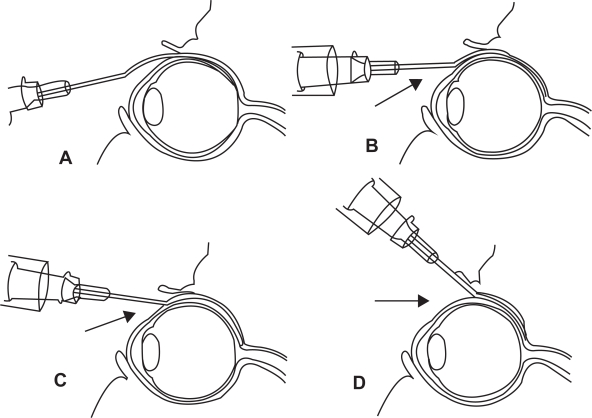
Figure 5.
Technique of posterior juxtascleral depot injection involves the introduction of cannula into the subconjunctival/subtenon’s space and subsequent advancement behind and adjacent to globe.
Surgical removal of CNV in AMD has been studied in a randomized, controlled trial and was found not to be better than observation alone with regards to maintenance of vision (SST 2004a, 2004b).
Ranibizumab
Treatment rationale and principles
VEGF is an important mediator of angiogenesis, playing a major role in endothelial cell proliferation and migration, and greatly increases vascular permeability. It exists as multiple isoforms with VEGF-165 the most pathogenic. VEGF acts as the ligand for 2 endothelial cell membrane-bound receptors, VEGFR-1 and VEGFR-2, with the latter being the mediator for angiogenesis (Robinson and Stringer 2001).
Levels of VEGF are found to be elevated in those individuals with proliferative diabetic retinopathy (Adamis et al 1994), ischemic central retinal vein occlusion (Aiello et al 1994), and CNV (Kvanta et al 1996). Further, experimentally increased expression of VEGF has been shown to be sufficient to induce neovascularization in primates (Tolentino et al 1996).
A potential strategy in the treatment of neovascular AMD would therefore be to inhibit the action of VEGF to inhibit or cause regression of vision-threatening CNV while also reducing intraretinal and subretinal fluid accumulation by reducing vascular permeability associated with VEGF activity.
Introduction of monoclonal antibodies to VEGF have been shown to inhibit development of ocular neovascularization in primates (Adamis et al 1996) and anti-angiogenic agents have been shown to increase vessel closure in combination with PDT in cultured retinal capillary endothelial cells (Renno et al 2000).
At present, only three anti-VEGF agents have been approved for use in humans. Pegaptanib and ranibizumab for neovascular AMD (see above) and bevacizumab (Avastin, Genentech, Inc., South San Francisco, CA), a recombinant, monoclonal antibody to VEGF, approved for treatment of metastatic colorectal cancer (Hurwitz et al 2004).
Ranibizumab (RhuFab V2, Lucentis, Genentech, Inc., South San Francisco, CA) is a 48 kDA, recombinant, humanized, monoclonal antibody fragment (Fab portion) derivative of bevacizumab with specificity to all isoforms of VEGF and is designed to inhibit angiogenesis and reduce vascular permeability in neovascular AMD. Given as an intravitreal injection (Figure 6), it has been designed with the theoretical advantage over whole IgG antibodies of being able to better cross the internal limiting membrane and thus gain better access to CNV in the subretinal space (Mordenti et al 1999; Gaudreault et al 2005).
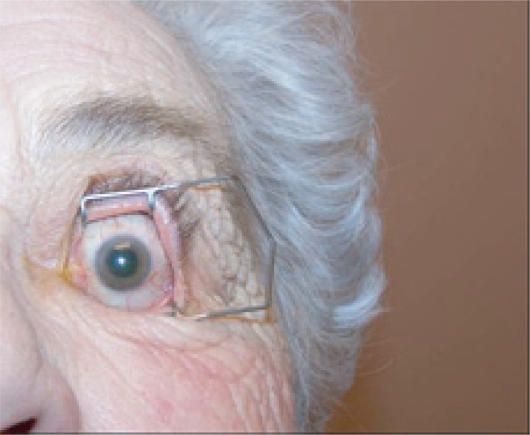
Figure 6a.
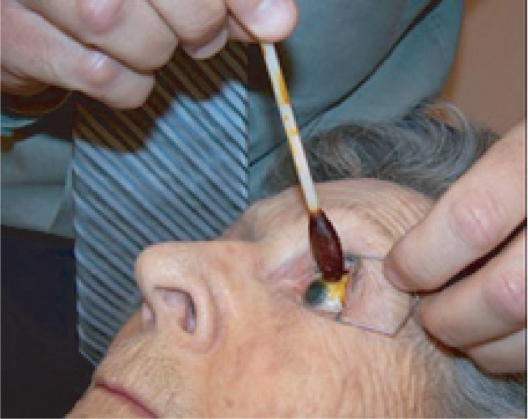
Preparation of the eye for an intravitreal injection involves exposure of eye with speculum following administration of topical anesthetic drops. Additional anesthesia is then given by a topical (anesthetic gel or drop-soaked pledget) or subconjunctival route.
Animal studies
When given as a bilateral 500μg or 2000μg intravitreal injection to monkeys (Guadreault et al 20005), ranibizumab distributes quickly to the retinal tissues with peak concentrations being found at 6 hours and 1 day, respectively, and a terminal half-life of 2.6 days. Concentrations of ranibizumab in the neurosensory retina were approximately two times greater than in the RPE/Bruch’s membrane complex, with both being greater than 1000-fold the concentration of VEGF normally detected in the retina. Maximum serum concentrations were detected 6 hours following administration of either dose and serum half-life was approximately 3.5 days. Maximum serum concentrations were found to be low, with 150 ng/mL and 616 ng/mL detected in the lower and higher dose groups, respectively. No antibody production to ranibizumab was detected up to 11 days post-injection.
Intravitreal ranibizumab given every two weeks has been studied in the laser-induced model of CNV in monkeys. Significant prevention of CNV formation in susceptible animals and reduction of CNV activity expressed as angiographic leakage in those animals already harboring neovascularization was found (Krzystolik et al 2002). All study eyes developed transient intraocular inflammation which resolved over 1 week and became less pronounced with subsequent injections.
In a similar study of laser-induced CNV in cynomolgus monkeys, intravitreal ranibizumab plus PDT (each given every 2 weeks) was found to result in fewer subjects with angiographic leakage compared to PDT alone, although this result was not statistically significant given the small sample size (Husain et al 2005). Of 12 eyes receiving ranibizumab plus PDT, none had angiographic leakage 42 days following treatment, whereas 2 of 12 eyes receiving only PDT demonstrated persistent leakage. Histopathological specimens in the combination group demonstrated no patent vessels in the area of the CNV. Specimens in the PDT-only group showed rare open vessels. No other architectural differences were noted between the 2 groups.
Human studies
In humans, ranibizumab was well tolerated in single intravitreal doses to 500μg in a study involving 27 subjects with doses ranging from 150–1000μg. Significant intraocular inflammation was found in the 2 subjects given a ranibizumab dose of 1000μg (Rosenfeld, Schwartz, et al 2005). Some degree of ocular inflammation was seen in 44% of subjects with all but 3 of 27 (including the 2 subjects in the 1000μg dose group) experiencing only mild, transient inflammation. 78% of patients experienced some mild, self-limited adverse events (eg, subconjunctival hemorrhage, conjunctival edema, or pain) related to the injection itself but not the medication.
There are currently 2 large phase III trials, MARINA and ANCHOR, evaluating the efficacy in treating neovascular AMD with ranibizumab over a 24 month period. Both of these pivotal studies have been published recently. Their results are summarized below (Brown et al 2006; Rosenfeld et al 2006).
The MARINA (Minimally classic/occult trial of Anti-VEGF antibody RhuFab V2 In the treatment of Neovascular AMD) Trial prospectively randomized 716 patients 1:1:1 to receive monthly intravitreal ranibizumab (300μg or 500μg) or sham injections for 24 months. At inclusion, subjects had fluorescein angiographic evidence of subfoveal minimally classic or occult with no classic CNV secondary to AMD and no prior history of PDT treatment.
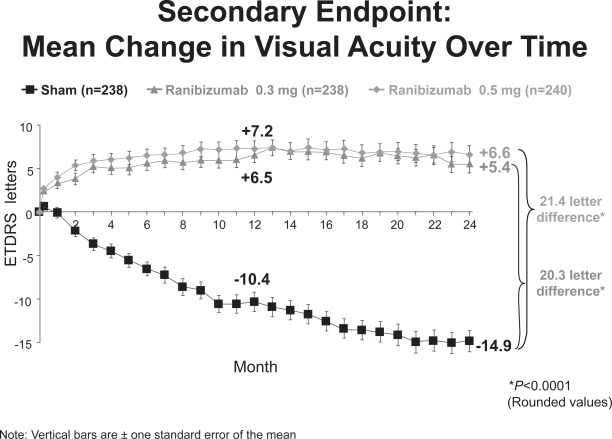
At 24 months, 91% of subjects in the ranibizumab groups maintained or improved vision (defined as a loss of fewer than 15 ETDRS letters in visual acuity) compared with 53% of subjects in the control/sham group (p < 0.0001) (Heier 2006). 26% of subjects receiving 300μg of ranibizumab and 33% of those receiving 500μg gained at least 15 letters of vision compared with 4% in the control group (p < 0.0001). On average, subjects in the ranibizumab group gained 5.4 and 6.6 letters of visual acuity in the 300μg and 500μg groups, respectively, while those in the control group lost 14.9 letters (Figure 7).
Figure 7.
MARINA 2 year visual acuity results (Genentech, South San Francisco, CA).
Ocular side effects were uncommon in the ranibizumab group: endophthalmitis (a presumed or proven infection of the vitreous) and intraocular inflammation were reported in 1.3 and 1.7% of ranibizumab subjects, respectively. No difference in systemic hypertension was found between treatment and control groups, although a slightly higher rate of thromboembolic events was found in the 500μg and 300μg groups compared with controls (4.6 vs 4.6 vs 3.8%).
The ANCHOR (Anti-VEGF antibody for the treatment of Predominantly Classic Choroidal Neovascularization in AMD) Trial prospectively randomized 423 patients 1:1:1 to receive monthly intravitreal ranibizumab (300μg or 500μg) or PDT every 3 months as indicated for 24 months. Patients in the ranibizumab group were also eligible to receive PDT every 3 months if they showed evidence of angiographic leakage from CNV. At inclusion, subjects had fluorescein angiographic evidence of subfoveal predominantly classic CNV secondary to AMD and no prior history of PDT treatment.
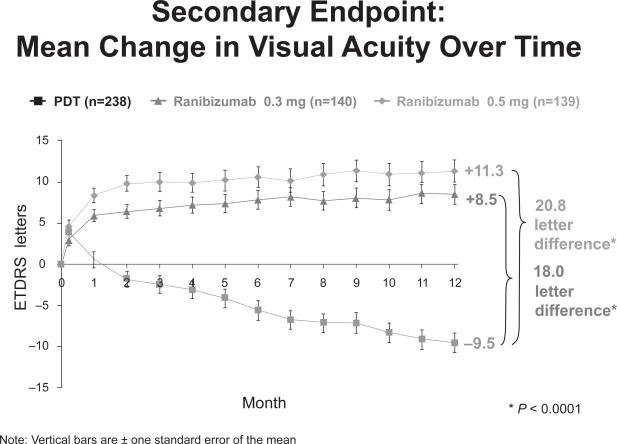
At 12 months, approximately 95% of subjects in the ranibizumab group maintained or improved vision compared with 64% of those treated with PDT alone. On average, subjects in the 300μg and 500μg ranibizumab groups gained 8.5 and 11 letters of visual acuity, respectively, compared with a loss of 9.5 letters in the PDT group (Figure 8).
Figure 8.
ANCHOR 1 year visual acuity results (Genentech, South San Francisco, CA).
Adverse events were uncommon, with less than 1% of subjects in each group experiencing endophthalmitis, intraocular inflammation, or cerebrovascular events. The frequency of myocardial infarction was slightly higher in the 500μg ranibizumab group than in the other two groups (2.1 vs 0.7%).
The PIER trial (Phase IIIb, Multicenter, Randomized, Double-Masked, Sham Injection-Controlled Study of the Efficacy and Safety of Ranibizumab) has enrolled 184 neovascular AMD patients with or without classic CNV to receive intravitreal ranibizumab once per month for the first three months followed thereafter by doses once every three months for a total of 24 months, with the goal of determining whether less frequent subsequent dosing will maintain treatment efficacy.
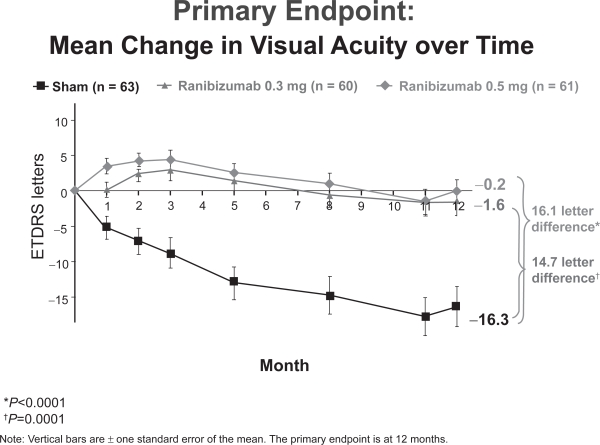
At 12 months, 83 and 90% of subjects receiving 300μg and 500μg of ranibizumab maintained or improved vision compared with 49% of those in the control group (p < 0.0001). Unlike MARINA and ANCHOR, subjects treated with this lower frequency subsequent dosing of ranibizumab lost approximately 1 letter of visual acuity on average. Control patients, however, lost 16.3 letters at 12 months (Figure 9). No cases of endophthalmitis, uveitis, or thromboembolic events were encountered in this small group of subjects. Rates of hypertension were similar between all groups.
Figure 9.
PIER 1 year visual acuity results (Genentech, South San Francisco, CA).
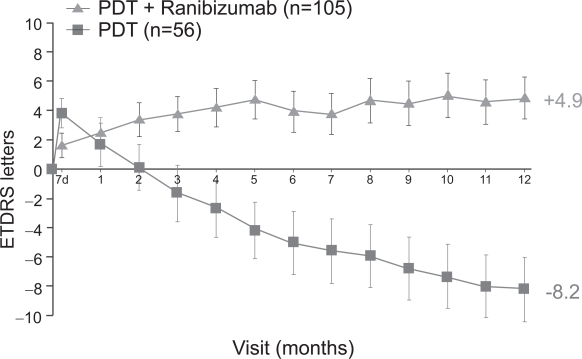
A smaller phase I/II study, FOCUS (RhuFab V2 Ocular Treatment Combining the Use of Visudyne to Evaluate Safety), studied the efficacy of monthly intravitreal ranibizumab (500μg) in combination with PDT compared with PDT only in 162 patients with predominantly classic CNV (Heier et al 2006). At 12 months, approximately 90% of subjects in the ranibizumab plus PDT group maintained or improved vision compared with 68% in the PDT-only group. 24% in the combination group and 5% in the PDT-only group gained at least 15 letters of vision. On average, those in the combination group gained 5 letters of vision, compared with a loss of 8 letters of vision in the PDT-only group (Figure 10). Interestingly, subjects in the combination treatment group did not have improved outcomes when compared with similar subjects treated with ranibizumab alone in the ANCHOR study. Whether combination treatment will reduce the total number of intravitreal and/or PDT treatments required overall has not yet been studied.
Figure 10.
FOCUS 1 year visual acuity results (Genentech, South San Francisco, CA).
Another small, open-label phase I/II study, PrONTO (Prospective OCT Imaging of Patients with Neovascular AMD with Intra-Ocular Lucentis), has studied a variable dosing regimen for intravitreal ranibizumab in neovascular AMD of any lesion type. A 500μg intravitreal dose was given monthly for 3 months to 40 subjects. Follow-up was conducted monthly thereafter and ranibizumab was only re-injected if there was evidence of persistence or recurrence of CNV. At 12 months, subjects required an average of only 5.5 injections and only 5% of patients required 12 injections (ie. needing monthly injections for the entire follow-up period) (Rosenfeld 2006). 37.5% of all subjects required only 3 or 4 injections. Similar to the MARINA and ANCHOR studies, subjects gained an average of 9.3 letters. Despite a similar average number of injections to the PIER study, visual acuity results appear more favourable in PrONTO. Although these two protocols have not been directly compared, additional ranibizumab doses at the first indication of CNV persistence/recurrence may be responsible for the improved outcomes.
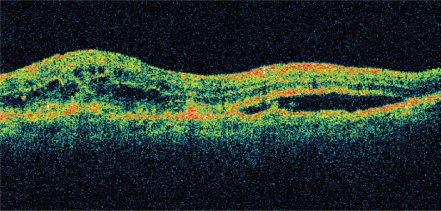
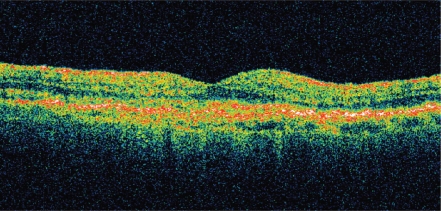
Although visual acuity was the most important outcome measured in each of the above studies, significant anatomic resolution of intraretinal, subretinal, and sub-RPE fluid has been seen with intravitreal ranibizumab. As demonstrated by optical coherence tomography (OCT), resolution of fluid may occur soon after the initial dose and is crucial for visual improvement (Figure 11).
Figure11a.
OCT of neovascular AMD pre-ranibizumab treatment, visual acuity 20/100. Note intra- and sub-retinal fluid and macular thickening.
Abbreviations: AMD, age-related macular degeneration; OCT, optical computed tomography.
It is important to note that intravitreal ranibizumab is the first treatment for neovascular AMD to demonstrate an improvement in visual acuity and that this effect has been repeatedly shown in multiple randomized, controlled clinical trials.
Bevacizumab
Owing to the preliminary results above and the published data regarding pegaptanib, enthusiasm has been generated for targeted anti-VEGF therapy. As bevacizumab (Avastin) is an approved, commercially available, and structurally and functionally similar compound to ranibizumab (albeit whole antibody compared to an antibody fragment), several early studies on its use in neovascular AMD have been published.
In the first report of SANA (Systemic Avastin for Neo-vascular AMD), an open-label, uncontrolled study, 9 patients with subfoveal CNV secondary to AMD were treated with 2 to 3 doses of intravenous bevacizumab (5mg/kg) every 2 weeks and re-evaluated at 12 weeks (Michels et al 2005). Patients enrolled had either persistent CNV despite PDT or CNV unsuitable for PDT. The authors hypothesized that systemic bevacizumab could be effective by gaining access to the subretinal and sub-RPE space by leaking from the CNV and binding to extracellular VEGF. Subjects experienced a mean 12 letter increase in visual acuity, a mean 177μm reduction in central retinal thickness, and reduction or absence of angiographic leakage was found in all. No serious systemic or ocular side effects were encountered although there was a mild, transient elevation in systolic blood pressure. Ongoing patient enrollment and longer term follow-up continue.
As intravenous bevacizumab has been associated with serious systemic side-effects (to be further discussed below), intravitreal bevacizumab has been offered as a treatment for CNV. The reduced dose (and therefore cost) of medication required may also be considered an advantage over systemic administration.
The first report of intravitreal bevacizumab involved a patient with persistent CNV despite PDT and pegaptanib treatments. 1.0mg of intravitreal bevacizumab was given and resulted in stabilization of visual acuity and resolution in subretinal fluid and angiographic leakage at 4 weeks after treatment (Rosenfeld, Moshfeghi, et al 2005). Despite previous studies showing only limited inner retinal penetration of whole antibody through the inner retina in monkeys Mordenti et al 1999), the authors hypothesized that intravitreal administration of bevacizumab might be effective because: the aged or diseased human retina may allow penetration differently than the nonhuman primate model studied; limited retinal penetration of full-length antibody or inhibition of vitreous VEGF may be sufficient to result in a clinically therapeutic effect; or that bevacizumab may penetrate the retina differently than the whole antibody used in the primate model.
Consistent with the above observation, a recent study has shown that intravitreal bevacizumab may pass to all layers of the rabbit neurosensory retina, though absent in the RPE and choroid, within 24 hours of administration (Shahar et al 2006). Bevacizumab was present at 7 days following administration but absent at 4 weeks. Further, the 2.5 mg/0.1 mL bevacizumab dose did not produce any differences in full-field electroretinography (ERG) or flash visual evoked potentials at 4 weeks compared with control eyes in the same animal receiving intravitreal injections of saline. Although a direct correlation between rabbit and human retina cannot be made, the retinal toxicity of intravitreal bevacizumab in the milligram range is unlikely given the smaller vitreous volume of the rabbit eye compared with human (<2 ml vs 5 mL) and the relatively large dose used in this study (and hence a much higher effective concentration of drug).
In a similar study of rabbit eyes, intravitreal bevacizumab doses of 0.5 mg to 5.0 mg produced no reduction in ERG amplitudes at 2 weeks following administration compared with control eyes injected with saline (Manzano et al 2006). There was no histologic evidence of retinal toxicity although one of three eyes receiving the 5mg dose had some inflammatory cells in the vitreous. Studies examining the toxic effects of serial injections have yet to be conducted.
Electrophysiologic responses to intravitreal bevacizumab in humans with neovascular AMD have also been reported (Maturi et al 2006). 5 patients underwent full-field ERG testing to evaluate for generalized toxic effects to the retina and 4 patients underwent multifocal ERG testing to evaluate changes in macular function following intravitreal injection of 1.25 mg of bevacizumab. No significant change in either ERG modality was noted at 1 month. However, improvements in macular function may have been blunted by the inclusion of patients with recurrent/persistent CNV following PDT, pegaptanib, or focal laser treatments only. All subjects had stable or improved visual acuity and 75% had reduced central macular thickness at 1 month.
Larger retrospective case series have also been reported in patients with neovascular AMD. 81 eyes of 79 patients received monthly intravitreal bevacizumab (1.25 mg/0.05 cc) injections, with 37% and 49% experiencing complete resolution of retinal edema, subretinal fluid, and pigment epithelial detachment at 4 and 8 weeks, respectively (Avery et al 2006). Median visual acuity improved from 20/200 to 20/80 at both 4 and 8 weeks. Of note, 78% of patients had previously failed treatment with PDT and/or pegaptanib. No ocular or systemic side effects were reported in this short-term study.
Another short-term, retrospective study of 266 eyes of 266 patients receiving monthly intravitreal bevacizumab for neovascular AMD found an improvement in mean visual acuity from 20/184 to 20/109 at 3 months (Spaide et al 2006). Mean central macular thickness decreased from 340μm to 247μm over the same period. 69.7% of patients had been previously treated with either PDT, pegaptanib, anecortave acetate, or intravitreal triamcinolone. No serious ocular side effects were reported. 2 patients each developed a myocardial infarction and transient ischemic attack, with 2 of the 4 having a previous history or significant risk factors for these conditions.
Given its abilities to inhibit angiogenesis and to reduce vascular permeability, applications in other ocular conditions have been suggested for intravitreal bevacizumab. Its use in proliferative diabetic retinopathy (Avery 2006; Spaide and Fisher 2006), macular edema secondary to central retinal vein occlusion (Rosenfeld, Fung, et al 2005; Iturralde et al 2006), pseudophakic cystoid macular edema (Mason et al 2006), and neovascular glaucoma (Davidorf et al 2006) has been reported in small case series, with regression of neovascularization or reduction in macular edema noted in all cases.
Potential complications
Complications from intraocular treatment may generally be classified into those related to the mode of drug delivery (ie, the intravitreal injection) or those related to the drug itself. Over a 1 year study period involving 890 eyes, each receiving 9 intravitreal injections of pegaptanib: 1.3% of patients developed endophthalmitis, 0.6% developed a traumatic injury to the crystalline lens, and 0.7% developed a retinal detachment (Gragoudas et al 2004). Although the per injection rate of each of these serious adverse events is low, their importance should not be minimized considering the cumulative risk over a long treatment period and the increased frequency of ranibizumab dosing (ie, 4 weeks vs 6 weeks for pegaptanib). Data from the MARINA and ANCHOR ranibizumab studies confirm similar rates of endophthalmitis and raise concern. Studies examining potential injection-related complications over a longer follow-up period have yet to be performed but will be important in assessing the overall safety of serial intraocular injections given over a lengthy treatment period.
Systemic intravenous administration of bevacizumab has been shown to increase the risk of hypertension (p < 0.01) in patients being treated for metastatic colorectal cancer (Hurwitz et al 2004). Doses used were the same as those used to treat CNV in the SANA study (5 mg/kg). Though rates of leukopenia, diarrhea, proteinuria, and thrombotic events were slightly higher in the bevacizumab group compared with controls, the difference did not reach statistical significance.
In a study of intravenous bevacizumab for the treatment of metastatic renal cell cancer, there was a statistically significant increased rate of epistaxis, hypertension, hematuria, and proteinuria in patients treated with bevacizumab compared with controls (Yang et al 2003). However, these adverse events were only encountered in the “high dose” (10 mg/kg) dose and not in the “low dose” (3 mg/kg) bevacizumab treatment group.
Though it is reassuring that mortality, hypertensive disorders, hemorrhagic, and thromboembolic event rates were not different between patients receiving intravitreal pegaptanib compared with placebo over 54 weeks (Gragoudas et al 2004), this follow-up period is relatively short and many patients may require continued treatment. Further, systemic side effects of ranibizumab may be different given its ability to inhibit all isoforms of VEGF.
Despite nonstatistically significant results, intravitreal ranibizumab data indicate a trend towards higher rates of cerebrovascular events and myocardial infarction compared with controls in the MARINA and ANCHOR trials, although statistical significance and final data are still to be provided.
Although systemic ranibizumab concentrations are extremely low following intravitreal injections (Gaudreault et al 2005) (especially when compared with intravenous administration), the effect of systemic VEGF inhibition has yet to be determined. Maximum serum levels of ranibizumab following a single intravitreal injection in the monkey have been shown to be 150 ng/mL. However, serum levels of VEGF-165 in normal humans have been found to be only 159.7 pg/mL (with a 2 standard deviation range of 15–500 pg/mL) (Larsson et al 2002). The effect of a 1000-fold excess of systemic VEGF inhibition is not known although implications on wound healing and normal homeostatic processes must be considered. The short-term safety of VEGF inhibitors appears to be validated by the studies discussed above. However, long-term effects are yet to be determined.
Future directions
Whether a combination of treatments will be more effective than a single modality in preserving vision in patients with neovascular AMD remains to be proven. However, it would seem intuitive that treatments of differing mechanisms of action targeting different stages and structures of CNV pathogenesis should result in more favorable outcomes. Given the varying mechanisms, intensity, and cost of different therapies, it is conceivable that future combinations will employ some modalities in the acute exudative phase of CNV (eg, VEGF inhibitors, PDT) and others in the chronic, convalescent phase (eg, anecortave acetate, corticosteroids). Determining the best sequence and combination of modalities to employ remains a challenge for the future.
Selection of combination treatment will only become more complex as further treatments are developed. Promising therapies in development include: other VEGF inhibitors; VEGF traps; small interfering RNA (siRNA) to block translation/production of VEGF or its receptor; pigment epithelium-derived factors (PEDF) to counterbalance the angiogenic effects of VEGF; and tyrosine kinase inhibitors to block signal transduction following VEGFR binding.
Summary
Many different therapies for neovascular AMD have become available in the last 10 years. In addition to focal thermal laser, ophthalmologists have had photodynamic therapy and intravitreal pegaptanib to treat CNV. Both corticosteroid and anecortave acetate may prove to be useful adjuncts to the above, but require more evaluation. With recent FDA-approval of ranibizumab and bevacizumab showing great promise, it appears that there are now even greater treatment options for this visually devastating condition. It is particularly noteworthy that these compounds appear, for the first time, to provide a treatment for neovascular AMD producing significant improvements in visual acuity. However, enthusiasm must be tempered by concerns about long term safety, both ocular and systemic.
Optimal management of neovascular AMD remains elusive and further studies will be required to determine whether emerging therapies will prove to be effective and whether a combination of treatments offers a better overall visual result than any one therapy alone.
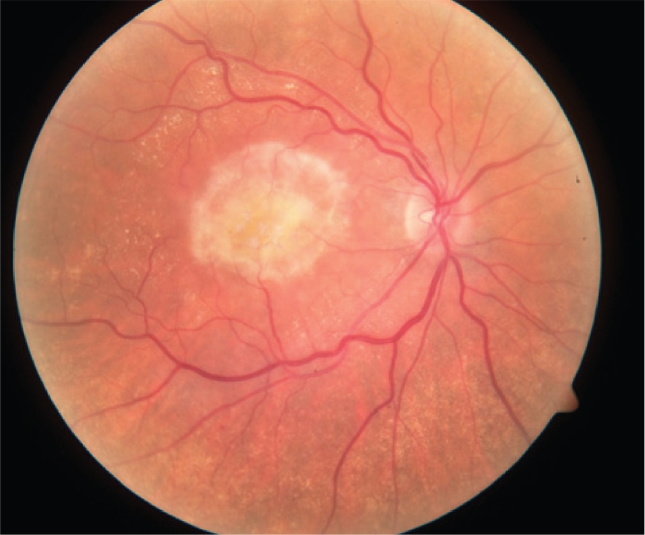
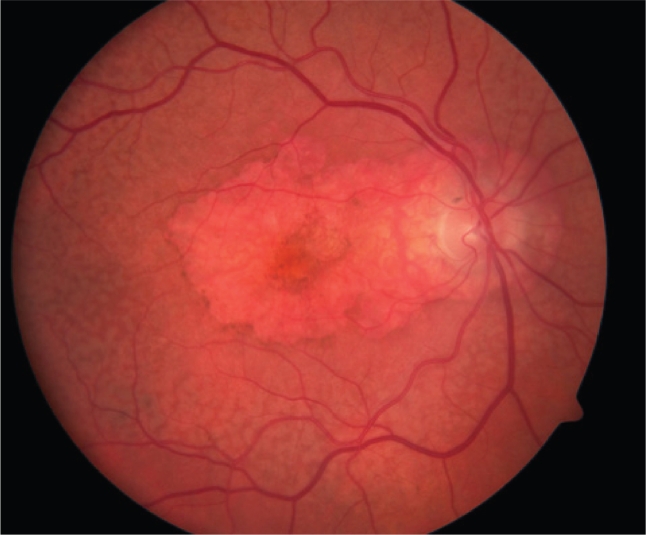
Figure 2.
Color fundus photograph of a right eye with central geographic atrophy. Note the central RPE pigment attenuation.
Abbreviations: RPE, retinal pigment epithelium.
Figure 3a.
Color fundus photograph of left subfoveal choroidal neovascularization demonstrating subretinal fluid, intraretinal lipid exudate, and subretinal hemorrhage.
Figure 3b.
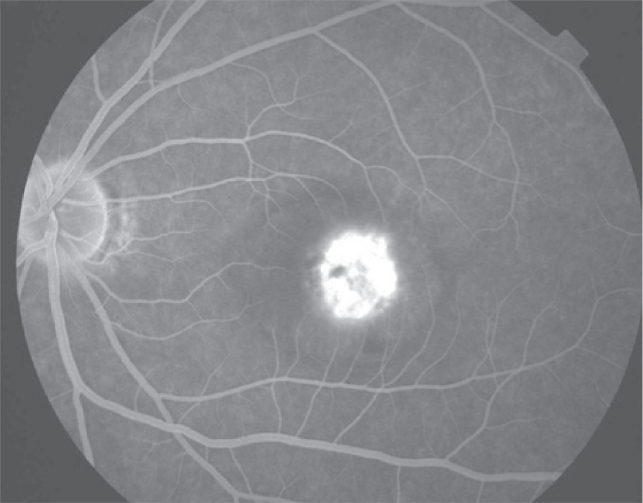
Early arteriovenous phase fluorescein angiogram demonstrating early central hyperfluorescence of the choroidal neovascularization. Note blockage of fluorescence by hemorrhage and relative lack of fluorescence in surrounding subretinal fluid.
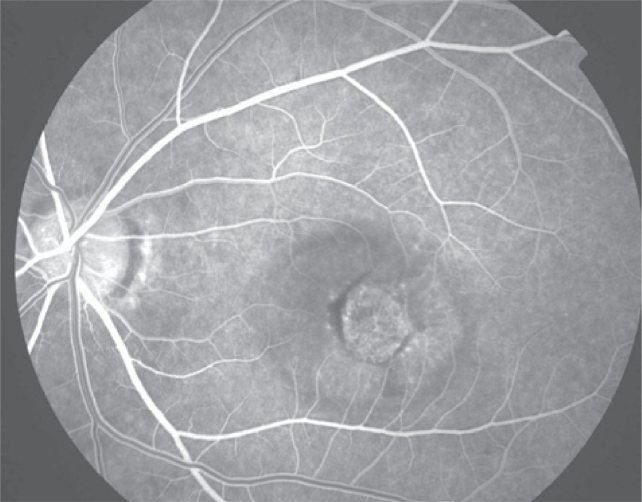
Figure 3c.
Late phase fluorescein angiogram with late leakage of the choroidal neovascularization (predominantly classic type).
Figure 6b.
Povidone-iodine is applied to ocular surface for sterile preparation.
Figure 6c.
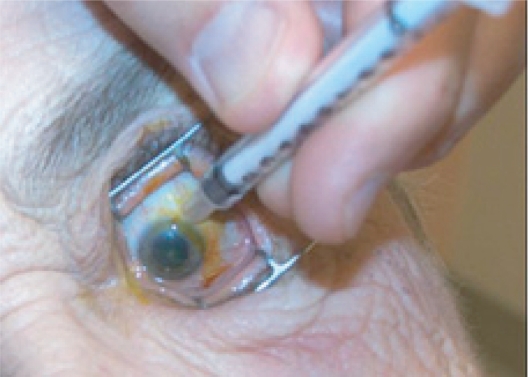
The injection site 3–4 mm posterior to limbus is marked using the end of a TB syringe. This ensures that the injection will be given through the pars plana. An injection given more anterior may result in hemorrhage due to trauma to the highly vascularized pars plicata. An injection given more posterior may result in retinal detachment.
Figure 6d.
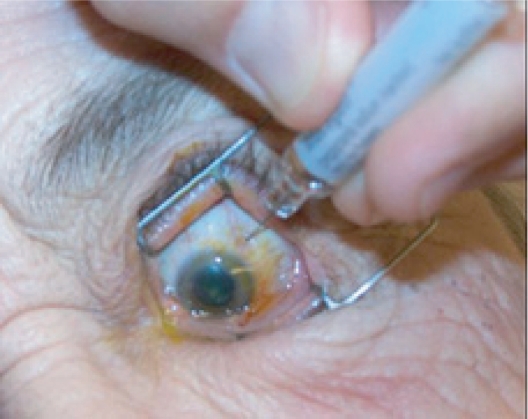
Intravitreal injection given with a 27- or 30-gauge needle through the pars plana.
Figure11b.
OCT of neovascular AMD 1-week post-ranibizumab treatment, visual acuity 20/40. Note significant resolution of intra- and sub-retinal fluid, reduction in macular thickness, and return of foveal contour.
Abbreviations: AMD, age-related macular degeneration; OCT, optical computed tomography.
References
- [AAC] The anecortave acetate clinical study group Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration. Ophthalmol. 2003;110:2372–85. doi: 10.1016/j.ophtha.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–50. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Shima DT, Tolention MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- [AED] Age-related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;110:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello L, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmol. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (avastin) treatment. Retina. 2006;26:352–4. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;335:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Criswell MH, Danis DP, et al. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol. 2001;119:399–404. doi: 10.1001/archopht.119.3.399. [DOI] [PubMed] [Google Scholar]
- Danis DP, Bingaman DP, Yang Y, et al. Inhibition of preretinal and optic nerve head neovascularization in pigs by intravitreal triamcinolone acetonide. Ophthalmol. 1996;103:2099–104. doi: 10.1016/s0161-6420(96)30383-7. [DOI] [PubMed] [Google Scholar]
- Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (avastin) injection. Retina. 2006;26:354–6. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of ranibizumab (rhuFab V2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Heier JS. Randomized, controlled phase III study of ranibizumab (Lucentis) for minimally classic or occult neovascular age-related macular degeneration: Two-year efficacy results of the MARINA study. Association for Research in Vision and Ophthalmology 2006 Annual Meeting; April 30–May 4, 2006; Fort Lauderdale, Florida. 2006. [Google Scholar]
- Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration. Arch Opthalmol. 2006;124:1532–42. doi: 10.1001/archopht.124.11.1532. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Husain D, Kim I, Gauthier D, et al. Safety and efficacy of intravitreal injection of ranibizumab in combination with verteporfin PDT on experimental choroidal neovascularization in the monkey. Arch Ophthalmol. 2005;123:509–16. doi: 10.1001/archopht.123.4.509. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–9. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (avastin) treatment of macular edema in central retinal vein occlusion: A short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Linton KL, et al. Prevalence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmol. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- Krzystolik MG, Afshari MA, Adamis EP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–46. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, et al. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–34. [PubMed] [Google Scholar]
- Larsson A, Skoldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis. 2002;5:107–10. doi: 10.1023/a:1021588227705. [DOI] [PubMed] [Google Scholar]
- Manzano RPA, Peyman GA, Khan P, et al. Testing of intravitreal toxicity of bevacizumab (avastin) Retina. 2006;26:257–61. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- Mason JO, Albert MA, Vail R. Intravitreal bevacizumab (avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006;26:356–7. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (avastin) treatment. Retina. 2006;26:270–4. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]
- McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004;10:512–20. [PMC free article] [PubMed] [Google Scholar]
- Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmol. 2005;112:1035–47. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Cutherbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labelled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–44. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- [MPSG] Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Five year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–14. [PubMed] [Google Scholar]
- Penfold PL, Wen L, Madigan MC, et al. Triamcinolone acetonide modulates permeability and intercellular adhesion molecule-1 (ICAM-1) expression of the ECV304 cell line: implications for macular degeneration. Clin Exp Immunol. 2000;121:458–65. doi: 10.1046/j.1365-2249.2000.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke MH, Canakis C, Husain D, et al. Verteporfin photodynamic therapy retreatment of normal retina and choroid in the cynomolgus monkey. Ophthalmol. 1999;106:1915–23. doi: 10.1016/S0161-6420(99)90401-3. [DOI] [PubMed] [Google Scholar]
- Renno RZ, Delori FC, Holzer RA, et al. Photodynamic therapy using lutex induces apoptosis in vitro, and its effect is potentiated by angiostatin in retinal capillary endothelial cells. Invest Ophthalmol Vis Sci. 2000;41:3963–71. [PubMed] [Google Scholar]
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–65. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. Ophthalmol. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–9. [PubMed] [Google Scholar]
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- Rosenfeld PJ, Schwartz SD, Blumenkranz MS, et al. Maximum tolerated dose of humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmol. 2005;112:1048–53. doi: 10.1016/j.ophtha.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ. Visual acuity outcomes following a variable-dosing regimen for ranibizumab (Lucentis) in neovascular AMD: The PrONTO study. Association for Research in Vision and Ophthalmology 2006 Annual Meeting; April 30–May 4, 2006; Fort Lauderdale, Florida. 2006. [Google Scholar]
- Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (avastin) Retina. 2006;26:262–9. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Slakter JS, Bochow T, D’Amico DJ, et al. Anecortave acetate (15 milligrams) versus photodynamic therapy for treatment of subfoveal neovascularization in age-related macular degeneration. Ophthalmol. 2006;113:3–13. doi: 10.1016/j.ophtha.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smithen LM, Ober MD, Maranen L, et al. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740–3. doi: 10.1016/j.ajo.2004.06.067. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fisher YL. Intravitreal bevacizumab (avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmol. 2003;110:1517–25. doi: 10.1016/S0161-6420(03)00544-X. [DOI] [PubMed] [Google Scholar]
- [SST] Submacular Surgery Trials (SST) Research Group Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: Ophthalmic Findings; SST report no. 11. Ophthalmol. 2004a;111:1967–80. doi: 10.1016/j.ophtha.2004.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [SST] Submacular Surgery Trials (SST) Research Group Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: Ophthalmic findings; SST report no. 13. Ophthalmol. 2004b;111:1993–2006. doi: 10.1016/j.ophtha.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [SST] Submacular Surgery Trials (SST) Research Group Comparison of 2D reconstructions of surgically excised subfoveal choroidal neovascularization with fluorescein angiographic features; SST report no. 15. Ophthalmol. 2006;113:267–79. doi: 10.1016/j.ophtha.2005.10.031. [DOI] [PubMed] [Google Scholar]
- [TAP] Treatment of age-related macular degeneration with photodynamic therapy (TAP) study group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two year results of 2 randomized clinical trials; TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- [TAP] Treatment of age-related macular degeneration with photodynamic therapy and verteporfin in photodynamic therapy study groups Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration; TAP and VIP report no. 1. Am J Ophthalmol. 2003;136:407–18. doi: 10.1016/s0002-9394(03)00223-x. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114:964–70. doi: 10.1001/archopht.1996.01100140172010. [DOI] [PubMed] [Google Scholar]
- Yang JC, Haworth L, Sherry RM, et al. A randomised trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]