Abstract
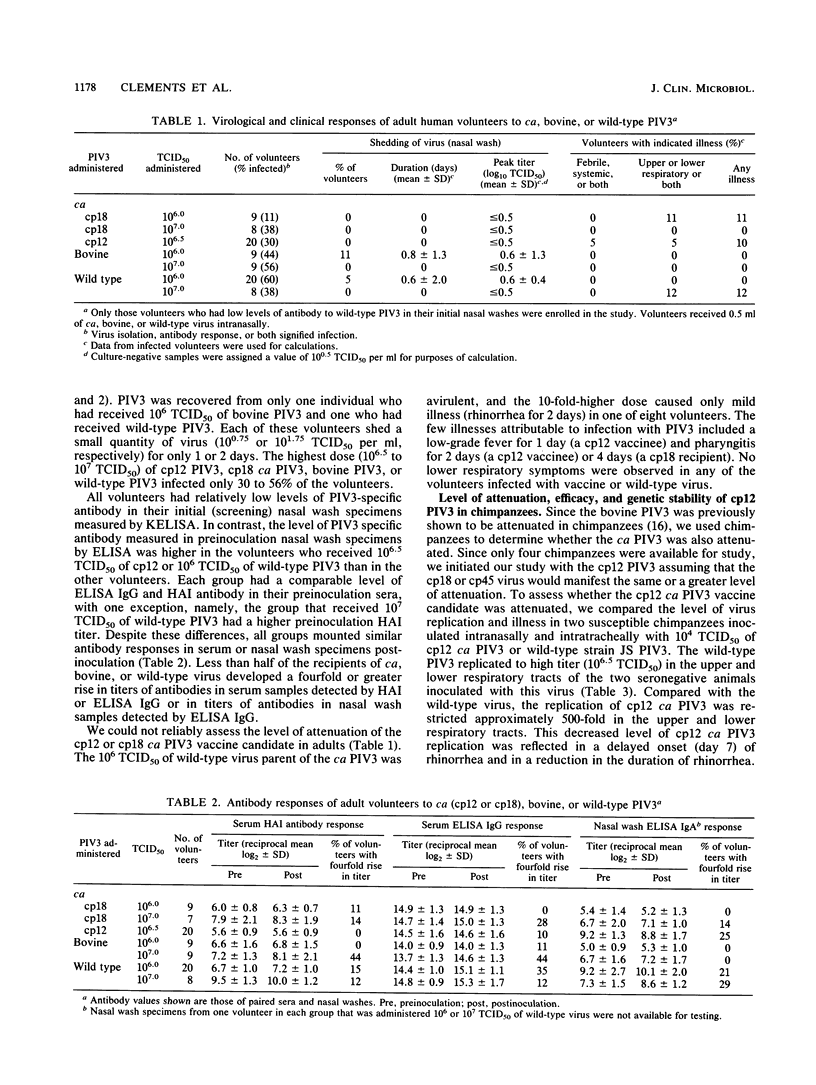
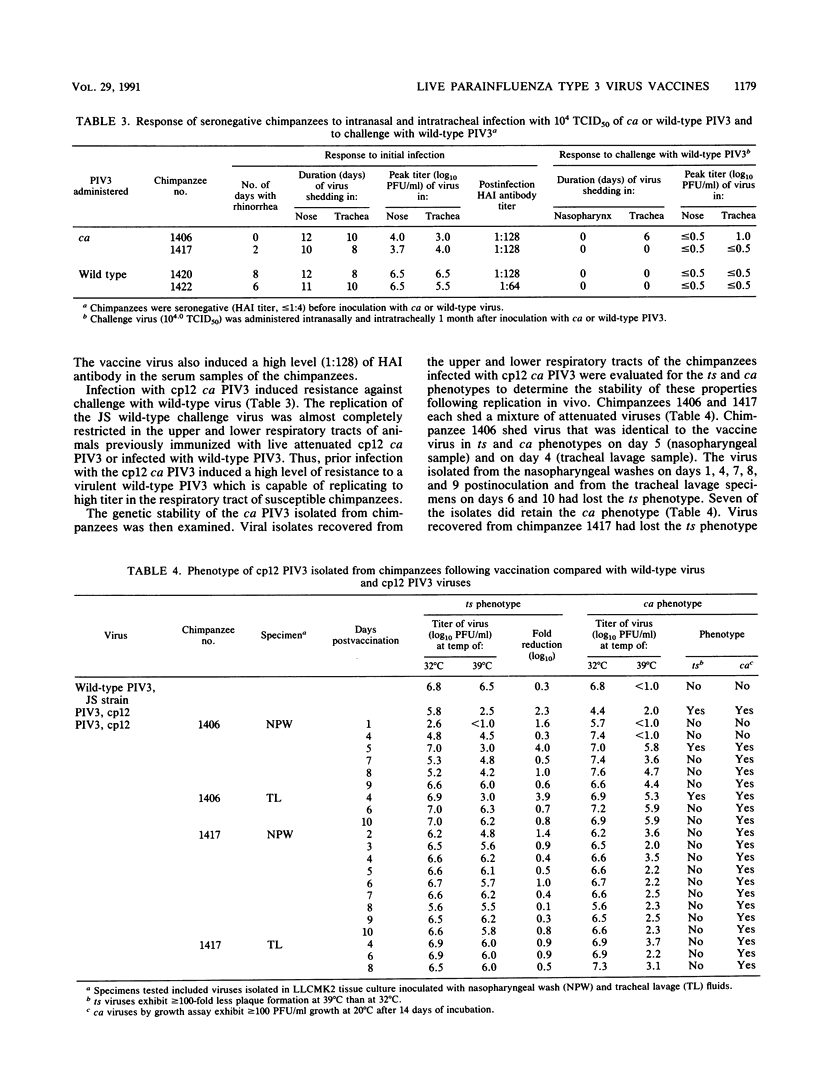
In an attempt to evaluate the level of attenuation of live parainfluenza type 3 virus (PIV3) vaccine candidates, we compared the responses of partially immune adult volunteers inoculated intranasally with 10(6) to 10(7) 50% tissue culture infective dose (TCID50) of bovine PIV3 (n = 18) or cold-adapted (ca) PIV3 (n = 37) with those of 28 adults administered 10(6) to 10(7) TCID50 of wild-type PIV3. The candidate vaccine viruses and the wild-type virus were avirulent and poorly infectious for these adults even though all of them had a low level of nasal antibodies to PIV3. To determine whether the ca PIV3 was attenuated, we then administered 10(4) TCID50 of ca PIV3 (cold-passage 12) or wild-type PIV3 intranasally and intratracheally to two fully susceptible chimpanzees, respectively, and challenged the four primates with wild-type virus 1 month later. Compared with wild-type virus, which caused upper respiratory tract illness, the ca PIV3 was highly attenuated and manifested a 500-fold reduction in virus replication in both the upper and lower respiratory tracts of the two immunized animals. Despite restriction of virus replication, infection with ca PIV3 conferred a high level of protective immunity against challenge with wild-type virus. The ca PIV3 which had been passaged 12 times at 20 degrees C did not retain its ts phenotype. These findings indicate that ca PIV3 may be a promising vaccine candidate for human beings if a passage level can be identified that is genetically stable, satisfactorily attenuated, and immunogenic.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belshe R. B., Hissom F. K. Cold adaptation of parainfluenza virus type 3: induction of three phenotypic markers. J Med Virol. 1982;10(4):235–242. doi: 10.1002/jmv.1890100403. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Arrobio J. O., Jeffries B. C., Wood S. C., Chanock R. M., Parrott R. H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. 3. Composite analysis of eleven consecutive yearly epidemics. Am J Epidemiol. 1973 Nov;98(5):355–364. doi: 10.1093/oxfordjournals.aje.a121565. [DOI] [PubMed] [Google Scholar]
- Brunell P. A., Brickman A., Steinberg S. Evaluation of a live attenuated mumps vaccine (Jeryl Lynn). With observations on the optimal time for testing serologic response. Am J Dis Child. 1969 Sep;118(3):435–440. doi: 10.1001/archpedi.1969.02100040437004. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H. ACUTE RESPIRATORY DISEASE IN INFANCY AND CHILDHOOD: PRESENT UNDERSTANDING AND PROSPECTS FOR PREVENTION. Pediatrics. 1965 Jul;36:21–39. [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H., JOHNSON K. M., KAPIKIAN A. Z., BELL J. A. MYXOVIRUSES: PARAINFLUENZA. Am Rev Respir Dis. 1963 Sep;88:SUPPL–166. doi: 10.1164/arrd.1963.88.3P2.152. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Murphy B. R. Use of temperature-sensitive and cold-adapted mutant viruses in immunoprophylaxis of acute respiratory tract disease. Rev Infect Dis. 1980 May-Jun;2(3):421–432. doi: 10.1093/clinids/2.3.421. [DOI] [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Murphy B. R. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet. 1984 Mar 31;1(8379):705–708. doi: 10.1016/s0140-6736(84)92222-0. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Tierney E. L., Murphy B. R. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J Clin Microbiol. 1986 Jan;23(1):73–76. doi: 10.1128/jcm.23.1.73-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Tierney E. L., Murphy B. R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986 Jul;24(1):157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., O'Donnell S., Levine M. M., Chanock R. M., Murphy B. R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983 Jun;40(3):1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookshanks-Newman F. K., Belshe R. B. Protection of weanling hamsters from experimental infection with wild-type parainfluenza virus type 3 (para 3) by cold-adapted mutants of para 3. J Med Virol. 1986 Feb;18(2):131–137. doi: 10.1002/jmv.1890180205. [DOI] [PubMed] [Google Scholar]
- Crookshanks F. K., Belshe R. B. Evaluation of cold-adapted and temperature-sensitive mutants of parainfluenza virus type 3 in weanling hamsters. J Med Virol. 1984;13(3):243–249. doi: 10.1002/jmv.1890130306. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Loda F. A., Clyde W. A., Jr, Senior R. J., Sheaffer C. I., Conley W. G., Denny F. W. Epidemiologic patterns of acute lower respiratory disease of children in a pediatric group practice. J Pediatr. 1971 Mar;78(3):397–406. doi: 10.1016/s0022-3476(71)80218-4. [DOI] [PubMed] [Google Scholar]
- Hall C. B., Walsh E. E., Long C. E., Schnabel K. C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991 Apr;163(4):693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Feldman S., Thompson J. M., Mahoney J. D., Wright P. F. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986 Jul;154(1):121–127. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- KAPIKIAN A. Z., CHANOCK R. M., REICHELDERFER T. E., WARD T. G., HUEBNER R. J., BELL J. A. Inoculation of human volunteers with parainfluenza virus type 3. JAMA. 1961 Nov 11;178:537–541. doi: 10.1001/jama.1961.03040450001001. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Jeffries B. C., Pyles G., Reid J. L., Chanock R. M., Parrott R. H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973 Sep;98(3):216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chanock R. M., Clements M. L., Anthony W. C., Sear A. J., Cisneros L. A., Rennels M. B., Miller E. H., Black R. E., Levine M. M. Evaluation of A/Alaska/6/77 (H3N2) cold-adapted recombinant viruses derived from A/Ann Arbor/6/60 cold-adapted donor virus in adult seronegative volunteers. Infect Immun. 1981 May;32(2):693–697. doi: 10.1128/iai.32.2.693-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Clements M. L., Tierney E. L., Black R. E., Stienberg J., Chanock R. M. Dose response of influenza A/Washington/897/80 (H3N2) avian-human reassortant virus in adult volunteers. J Infect Dis. 1985 Jul;152(1):225–229. doi: 10.1093/infdis/152.1.225. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Prince G. A., Collins P. L., Van Wyke Coelingh K., Olmsted R. A., Spriggs M. K., Parrott R. H., Kim H. W., Brandt C. D., Chanock R. M. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 1988 Aug;11(1):1–15. doi: 10.1016/0168-1702(88)90063-9. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARROTT R. H., VARGOSKO A., LUCKEY A., KIM H. W., CUMMING C., CHANOCK R. Clinical features of infection with hemadsorption viruses. N Engl J Med. 1959 Apr 9;260(15):731–738. doi: 10.1056/NEJM195904092601501. [DOI] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Ray R., Brown V. E., Compans R. W. Glycoproteins of human parainfluenza virus type 3: characterization and evaluation as a subunit vaccine. J Infect Dis. 1985 Dec;152(6):1219–1230. doi: 10.1093/infdis/152.6.1219. [DOI] [PubMed] [Google Scholar]
- Scott T. F., Bonanno D. E. Reactions to live-measles-virus vaccine in children previously inoculated with killed-virus vaccine. N Engl J Med. 1967 Aug 3;277(5):248–250. doi: 10.1056/NEJM196708032770506. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Snyder M. H., Banks S., Murphy B. R. Determination of antibody response to influenza virus surface glycoproteins by kinetic enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Oct;26(10):2034–2040. doi: 10.1128/jcm.26.10.2034-2040.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYRRELL D. A., BYNOE M. L., PETERSEN K. B., SUTTON R. N., PEREIRA M. S. Inoculation of human volunteers with parainfluenza viruses types 1 and 3 (HA 2 and HA 1). Br Med J. 1959 Nov 7;2(5157):909–911. doi: 10.1136/bmj.2.5157.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolpin M. D., Clements M. L., Levine M. M., Black R. E., Saah A. J., Anthony W. C., Cisneros L., Chanock R. M., Murphy B. R. Evaluation of a phenotypic revertant of the A/Alaska/77-ts-1A2 reassortant virus in hamsters and in seronegative adult volunteers: further evidence that the temperature-sensitive phenotype is responsible for attenuation of ts-1A2 reassortant viruses. Infect Immun. 1982 May;36(2):645–650. doi: 10.1128/iai.36.2.645-650.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolpin M. D., Massicot J. G., Mullinix M. G., Kim H. W., Parrott R. H., Chanock R. M., Murphy B. R. Genetic factors associated with loss of the temperature-sensitive phenotype of the influenza A/Alaska/77-ts-1A2 recombinant during growth in vivo. Virology. 1981 Jul 30;112(2):505–517. doi: 10.1016/0042-6822(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Tremonti L. P., Lin J. S., Jackson G. G. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J Immunol. 1968 Sep;101(3):572–577. [PubMed] [Google Scholar]
- Yanagihara R., McIntosh K. Secretory immunological response in infants and children to parainfluenza virus types 1 and 2. Infect Immun. 1980 Oct;30(1):23–28. doi: 10.1128/iai.30.1.23-28.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., London W. T., Murphy B. R. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988 Apr;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]


