Abstract
In the light of new cases of progressive multifocal leukoencephalopathy and induced autoimmunity in multiple sclerosis (MS) patients who received treatment with upcoming disease-modifying immunosuppressant drugs with a highly specific mode of action such as natalizumab, rituximab, or alemtuzumab, alternative oral treatment options for a subgroup of less severely affected MS patients are a major focus of drug development. These agents are currently investigated in phase III clinical trials and some of them are characterized by a favorable safety profile. With an emphasis on teriflunomide, the active metabolite of an immunosuppressant approved for the treatment of rheumatoid arthritis since 1998, a number of oral treatment options for patients with MS are discussed.
Keywords: teriflunomide, multiple sclerosis, immunosuppressant, oral drugs
Introduction
Since 1990, therapy for multiple sclerosis (MS) has dramatically improved. Immunomodulatory therapies of the first generation, including interferon-β (IFN-β) and glatiramer acetate, have become the standard of care in relapsing–remitting MS.1 The main advantage of those agents is their established positive safety profile, although efficacy is only partial. Unfortunately, the subcutaneous or intramuscular mode of application and local adverse effects at the sites of injection impair quality of life of and long-term acceptance by patients. Thus, new drugs for MS therapy have to prove to be superior to standard therapy in respect to efficacy, but also need to display a reasonable safety profile. In addition, intravenous and oral formulations might help to increase the rate of early treatment and overall compliance. Natalizumab, a monoclonal antibody directed against the alpha4-beta1-integrine, was the first drug of a second generation of immunoactive agents, approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) for highly active MS or MS refractory to first line treatment. It has been specifically designed to target a critical step of leukocyte extravasation from the blood into the central nervous system.2,3 Phase III clinical trials have clearly shown its advantages: high efficacy and a maximum of compliance by intravenous monthly infusion.4,5 Immediately after completion of a phase III trial that led to its approval by the FDA, safety issues, and most notably the risk of progressive multifocal leukoencephalopathy (PML), became apparent.6–8 Restriction of natalizumab to patients with highly active MS or patients, not responding to first line treatment, was not congruent with the inclusion criteria of these studies but based on risk–benefit considerations. Just recently, new cases of PML occurring in patients receiving natalizumab monotherapy have been published and the long-term safety data will decide how broadly this drug can be administered to patients.9,10 Interestingly, this safety issue is most likely not restricted to natalizumab, but is also relevant for other currently investigated monoclonal antibodies, including rituximab or alemtuzumab.11,12 Although the mode of action of these drugs is highly specific, their administration to patients apparently goes along with an increased risk of potential life threatening infectious (eg, risk of progressive multifocal leukoencephalopathy in natalizumab or rituximab) or autoimmune (eg, risk of autoimmune thrombocytopenia and thyroid disease in alemtuzumab)13 complications. As MS is a disease of low mortality in a young population and treatment primarily seems to be effective in the early inflammatory state of disease when patients suffer only from a low grade of impairment/disability, the risk–benefit consideration is crucial. Although low, the risk of a potential life-threatening complication in the MS population demands critical patient selection for the second generation of immunosuppressants and high standards of safety surveillance plans. Thus, in parallel with the development of new monoclonals targeting specific critical steps not only of autoimmunity but also of immunosurveillance and resistance to infections, concepts of more general modes of action resulting in a less severe immunosuppression have been tested in clinical trials. In the context of these studies, oral formulations are highly appreciated by patients, improving quality of life, and increasing adherence to therapy.14–16 Oral immunomodulatory or immunosuppressant drugs characterized by a maximum of compliance combined with a good safety–benefit ratio will likely become the third category of drugs available for MS treatment in the nearer future.
Teriflunomide and its mode of action
One promising oral agent in the treatment of MS is teriflunomide, the active metabolite of leflunomide, an approved therapy for rheumatoid arthritis since 1998.17–20 The ability to noncompetitively and reversibly inhibit the mitochondrial enzyme dihydro-orotate dehydrogenase (DHODH), relevant for the de novo synthesis of pyrimidine, is believed to exert the most important therapeutic effect.21–24 By inhibiting DHODH and diminishing DNA synthesis, teriflunomide has a cytostatic effect on proliferating B and T cells.25 In addition, teriflunomide inhibits protein tyrosine-kinase activity, reducing T-cell proliferation, activation, and production of cytokines.26–28 A more recent study showed that teriflunomide also interferes with the interaction between T cells and antigen-presenting cells (APC) crucial for T cell immune responses.29 Furthermore, there is some evidence that teriflunomide might block tumor necrosis factor-α (TNF-α) induced nuclear factor κB (NF κB) activation,30 inhibit cell adhesion molecules and matrix metalloproteinases.31,32 Also, in vitro data proved teriflunomide to diminish oxygen free-radical production and neutrophil chemotaxis, to augment levels of the immunosuppressive cytokine transforming growth factor-β1 (TGF-β1) and to inhibit cyclooxygenase-2 activity.32–35
Pharmacokinetics
Phase II clinical trials for leflunomide showed that teriflunomide is highly protein bound in plasma (99.3%) and has a low distribution volume. Its half-life is about two weeks in humans. It is cleared by hepatic metabolism and enterohepatic circulation can be prevented by cholestyramine decreasing the half-life of the drug to one or two days.36 Teriflunomide inhibits the cytochrome p450 2C9 isoenzyme and thereby enhances the anticoagulant effect of warfarin.36 Because its excretion is mainly hepatic, leflunomide is not contraindicated in renal insufficiency, although it should be used with caution in these circumstances.37
Effects of leflunomide and teriflunomide on experimental autoimmune encephalomyelitis
Studies in experimental autoimmune encephalomyelitis (EAE)–an animal model of MS–showed the immunomodulatory potential of leflunomide as well as of teriflunomide and proved both agents to be effective in ameliorating the disease course. In one study, the effect on disease activity was investigated in a T helper cell type 1 cell-borne monophasic disease model induced in Lewis rats by adoptive transfer of myelin basic protein (MBP)-specific T cell lines. In 12 Lewis rats treated with leflunomide for seven days, leflunomide suppressed clinical signs of EAE. Interestingly, significantly reduced motor disability was observed even in uridine-substituted animals suggesting additional mechanisms of action independent from the depletion of cellular pyrimidine. In vitro, MBP-specific T cell lines that had been antigen-activated in the presence of teriflunomide produced less IFN-γ and showed reduced chemotaxis.38 Just recently, a study in a Dark Agouti rat model of EAE showed teriflunomide to be effective in reducing behavioral, electrophysiological, and histopathological deficits.39 The Dark Agouti rat model of EAE is believed to more closely mimic the chronic clinical course in MS40 and is induced by a single subcutaneous injection of rat spinal cord homogenate. Teriflunomide delayed disease onset and decreased disease severity in this model in a dose-dependent manner. In addition, histopathological data demonstrated inhibition of up to 90% of inflammation, demyelination, and axonal loss. Furthermore, therapeutic dosing of teriflunomide prevented delayed conduction and a decrease in the amplitude of somatosensory evoked potentials.39
Results of phase II clinical trial
Study design
In 2006, the first randomized, double-blind, placebo-controlled phase II study to assess efficacy and safety of oral teriflunomide in MS-patients with relapses was published.41 One hundred and seventy nine patients with relapsing–remitting MS (n = 157) or secondary progressive MS with relapses (n = 22) and an Expanded Disability Status Scale (EDSS)42 score of <6 were randomized to receive either placebo (n = 61), teriflunomide 7 mg/day (n = 61) or teriflunomide 14 mg/day (n = 57). Patients aged 18 to 65 years with clinically confirmed MS43,44 were eligible for the trial. Patients were required to have two documented relapses within the previous three years and one during the preceding year. Patients on other immunosuppressant or immunomodulatory drugs within four months prior to the trial, except for corticosteroids, were excluded. Both male and female patients had to practice effective contraception during the trial and for 24 months after drug discontinuation or undergo a washout procedure. Magnetic resonance imaging (MRI) scans were performed every six weeks during the treatment phase of 36 weeks and activity was measured by pre- and postgadolinium-enhanced T1-weighted (T1 and T1-Gd) and by T2-weighted (T2) sequences. The primary efficacy endpoint was the number of combined unique (CU) active lesions (a combination score of the number of new and persisting Gd-T1 and T2 lesions) per MRI scan during the 36-week treatment phase. Secondary outcomes were MRI-based and included the number of T1-lesions, the number of T2 lesions, the number of patients with CU active, T1 and T2 active lesions and the percentage change from baseline to endpoint in burden of disease (measured in T2 lesion volume). Secondary clinical measures included the number of patients with MS relapses, the annualized relapse rate, and the number of relapsing patients requiring a course of steroids. In addition, the number of patients with an increase in disability was assessed, measured in an increase in EDSS >1 in patients with a baseline EDSS score of ≤5.5 or an increase in EDSS score of >0.5 in patients with a baseline EDSS score > 5.5. EDSS rating was performed every 12 weeks during treatment phase.
Efficacy of teriflunomide on MRI surrogate markers
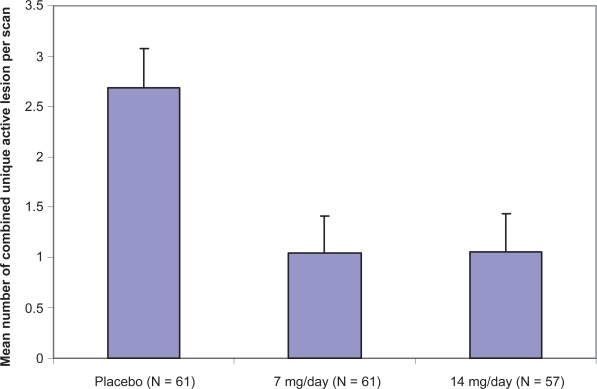
Treatment with either teriflunomide 7 or 14 mg/day resulted in the significant suppression of 61.1% or 61.3%, respectively (p < 0.03 or p < 0.01) of MRI activity measured in the mean number of CU active lesions per scan (Figure 1).
Figure 1.
Efficacy of teriflunomide on the primary outcome measure in the phase II study.
The decrease of cumulative mean number of CU lesions became significant by 12 weeks and was maintained for the full 36 weeks of treatment period. Regarding secondary MRI-endpoints, teriflunomide 7 or 14 mg/day also significantly reduced the median number of T1 and T2 lesions per scan over the treatment period. In addition, the number of patients with T1, CU active and T2 lesions was lower in both of the teriflunomide-treated groups. Finally, the burden of disease measured in the median change from baseline was significantly diminished in the teriflunomide 14 mg/day group (−4.1% versus 5.2%, p < 0.02).
Efficacy of teriflunomide on clinical measures
The proportion of patients showing an increase in disability measured on the EDSS score at endpoint versus baseline was significantly lower in the 14 mg/day teriflunomide group compared with placebo (7.4% versus 21.3%; p < 0.04). Annualized relapse rates were lower in both treatment groups compared to placebo without reaching statistical significance. Although not significant, a greater proportion of patients (77% versus 62%) was relapse-free in the 14 mg teriflunomide group and less patients in this group required steroids compared to placebo (14% versus 23%).
The safety profile of leflunomide
Leflunomide was first approved for treatment of rheumatoid arthritis in 1998. Based on the experience in this indication, its active metabolite teriflunomide seems to have a comparably well investigated safety profile. The most common adverse effects associated with leflunomide are gastrointestinal symptoms (diarrhea, dyspepsia, nausea/vomiting, abdominal pain, oral ulcers).18,19,45–48 Most of these symptoms decline after the first two weeks of treatment. Liver toxicity, most prominent in patients with pre-existent liver disease or concurrent use of other hepatotoxic drugs, seems to be one of the most serious safety issues. In the 2003 Cochrane review, the pooled absolute risk difference is calculated with 8% and the number needed to treat in order to have one person with elevated liver function tests was 12.5.19 In rare cases, severe hepatic injury with fatal outcome in some patients occurred in the post-marketing phase of leflunomide in rheumatoid arthritis.49 Because of an increased risk within the first six months of treatment, a monthly check of liver enzymes has been recommended and if stable, every six to eight weeks thereafter.36 Mild allergic reactions in the leflunomide group were more likely to occur when compared to placebo.19 Further adverse effects are reversible alopecia, rash, mild weight loss and headache.46,50 There is a low risk of leukopenia and pancytopenia.50–52 Although infection rates were not found to be significantly different between leflunomide and placebo in patients with rheumatoid arthritis within randomized trials,19,53 there is some evidence from the post marketing period for a slightly elevated risk of opportunistic exogenous and endogenous infections. Cases of pulmonary tuberculosis, Pneumocystis jieroveci pneumonia and other pulmonary infections have been reported.54 One case of PML in a patient with systemic lupus erythematosus on leflunomide was observed.55 However, this patient had been treated with various other immunosuppressant drugs before (prednisone, azathioprin, chloroquine, danazol, cyclosporine A, methotrexate), and was switched from methotrexate to leflunomide about five months before onset of PML symptoms. The incidence of drug-related hypertension ranges between 1.1% and 6.8%.46,47,50,56 Leflunomide was found to be teratogenic when administered to rats, rabbits, and mice.36,57–59 Therefore, leflunomide and its metabolite are considered to be teratogenic in human and are contraindicated in pregnancy. Based on animal data, teriflunomide levels <0.02 mg/L on two occasions >14 days apart before pregnancy are considered to have minimal risk. As mentioned above, drug clearance is accelerated by administration of chole-styramine.36 No malignancies in patients receiving leflunomide for rheumatoid arthritis have been observed so far.
The safety profile of teriflunomide
For the use of teriflunomide in MS in the 2006 published phase II clinical trial, serious adverse events (SAE) have been reported in 19 patients including elevated liver enzymes, hepatic dysfunction, neutropenia, rhabdomyolysis, and trigeminal neuralgia without any significant differences between the groups (teriflunomide 7 mg/day: five SAE, teriflunomide 14 mg/day: seven SAE, placebo: seven SAE). Nasopharyngitis, alopecia, nausea, increases in alanine aminotransferase levels, paresthesia, back pain, limb pain, diarrhea, and arthralgia were more commonly reported in patients on teriflunomide without any significant differences between the groups. Adverse events (AE) resulting in study exit were observed in 15 patients (four in placebo, three in teriflunomide 7 mg/day and eight in teriflunomide 14 mg/day). Six patients were withdrawn form the study because of abnormal alanine aminotransferase levels (three in placebo, one in teriflunomide 7 mg/day, and two in teriflunomide 14 mg/day), other reasons for withdrawal were alopecia, erythema multiforme, urticaria, condyloma accuminatum, dyspepsia, and hypertension. There were no deaths in any of the treatment groups.41
Ongoing studies of teriflunomide in MS
Currently two phase III studies investigate the effect of teriflunomide 7 and 14 mg/day versus placebo on clinical endpoints, the annualized relapse rate, and the accumulation of disability measured in EDSS. Final data collection dates for above mentioned trials on primary outcome measures are expected in October 2010 and in September 2011.60 There is currently one phase III trial studying the ability of teriflunomide to delay conversion of the clinically isolated syndrome to definite MS (time frame of two years), its primary completion date is expected to be in April 2011. Two phase II clinical studies investigate safety of teriflunomide when added to either glatiramer acetate or interferon β. In addition, safety-extension trials are ongoing for the 2006 published phase II study and are also planned for phase III clinical trials.60
Perspective: Teriflunomide’s potential role in the treatment of MS
Teriflunomide is currently one of five oral agents tested in phase III clinical studies for the treatment of relapsing–remitting forms of MS (Table 1).
Table 1.
Oral drugs in phase III for RR-MS61
| Phase III (indication, comparator) | Phase II (Primary end point) | Adverse effects | |
|---|---|---|---|
| Teriflunomide | (1) CIS, placebo (2) RR-MS, placebo (3) RR-MS, IFN-β | MRI41 (mean number of CU active lesions per scan): 7 or 14 mg/day: reduction by 61% | GI symptoms, hepatotoxicity, low risk of pancytopenia, low risk of endogenous infections, teratogenicity |
| Cladribine | (1) CIS, placebo (2) RR-MS, placebo (3) RR-MS, add-on to IFN-β | Not performed for oral formulation | Not published for oral formulation |
| Fingolimod | (1) RR-MS, placebo (2) RR-MS, placebo (3) RR-MS, IFN-β (4) PP-MS | Mri62 (median total number of gadolinium-enhanced lesions on MRI): 1.25 mg or 5 mg or placebo: 1 (P < 0.001) or 3 lesions (P = 0.006) or 5 lesions | Nasopharyngitis, dyspnea, headache, GI-symptoms, hepatotoxicity, case of the posterior reversible encephalopathy syndrome, cardiovascular side effects |
| Laquinimod | (1) RR-MS, placebo (2) RR-MS, IFN-β | MRI63 (cumulative number of active lesions over 24 weeks): reduction by 44% | Iritis and burning sensation; during follow-up acute tonsillitis, one case of breast cancer |
| BG12 | (1) RR-MS, placebo (2) RR-MS, glatiramer acetate | MRI64 (total number of new gadolinium-enhancing lesions on MRI week 12 to 24): reduction by 69% | Abdominal pain, flushing, hot flush, headache, fatigue |
Abbreviations: CIS, clinically isolated syndrome; CU, combined unique; IFN, interferon; GI, gastrointestinal; MRI, magnetic resonance imaging; MS, multiple sclerosis; RR, relapsing–remitting; PP, primary progressive.
While previous clinical trials consistently showed superior efficacy of each single compound compared with placebo regarding different MRI based primary outcome measures, most agents still have to show to be more effective on clinical endpoints in the currently recruiting trials. As documented for high-dose teriflunomide, some of the oral drugs already have proven to impact beneficially on relapse rate or sustained disability. In phase III trials, comparative outcomes between the tested agent and the first line therapy (glatiramer acetate or IFN-β) will decide about the future drug ranking and modalities of approval. Although different forms of application make a double blind approach more challenging, the rater blinded study directly comparing alemtuzumab to IFN-β1a published recently showed the advantage of this study concept.13 However, at the same time this trial showed alemtuzumab to be superior to standard therapy regarding efficacy, it also brought up safety issues, predominantly the issue of induced secondary autoimmunity.13 Additional cases of PML in MS under monotherapy of natalizumab have already increased the efforts to control risks, to identify certain patients at risk but also to look for other therapeutic options especially for less severely affected patients.9 In this respect, given the long-term favorable experience gathered with leflunomide in the treatment of rheumatoid arthritis, teriflunomide appears more promising than other drugs currently evaluated. New oral agents, eg, the sphingosin-1 phosphate receptor superagonist FTY720/fingolimod, clearly, like other powerful immunointerventional strategies, require close monitoring in a possible post-marketing setting when administered to a comparably young population with almost normal life expectancy. The risks and disadvantages of leflunomide have been studied for years in a population similar to MS patients. While hepatotoxicity seems to be manageable with close laboratory monitoring, washout procedures already have been defined in the face of the slightly increased risk of infection and to accelerate drug clearance in women who wish to become pregnant. Therefore, administration of teriflunomide to MS patients seems to be comparable safe.
Looking at currently running phase II clinical trials, the idea of combination therapy as an add-on to the established first line therapy is of growing interest. A key question in those trials is whether or not the two combined agents have an additive effect in a complex not yet fully understood autoimmune disease.65 The ideal combination would yield additive or even super-additive, synergistic effects regarding efficacy, without increasing toxicity. Phase II clinical trials for combination therapy including teriflunomide and other oral drugs might bring up further therapeutic options. Regarding the safety profile of leflunomide, there is some evidence of an increased frequency of hepatotoxicity or infectious complications when combined with other immunosuppressants, eg, methotrexate.66 Notably, one case of PML in a patient with systemic lupus erythematodes on leflunomide after treatment with various other immunosuppressants emphazises the need for critical assessment and vigorous monitoring.55
Looking at study completion dates, oral immunomodulatory or immunosuppressant drugs are most likely to enlarge the arsenal of approved drugs for MS within the next years. The results of these trials need to be carefully reviewed to develop a consensus about which drug should be administered to an individual patient. If promising results of phase II trials can be replicated in phase III, given its well investigated safety profile, teriflunomide could be one of the first oral drugs available.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wiendl H, Toyka KV, Rieckmann P, et al. Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J Neurol. 2008;255(10):1449–1463. doi: 10.1007/s00415-008-0061-1. [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356(25):2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 3.Rice GPA, Hartung H, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64(8):1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 5.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 6.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 7.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 8.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353(4):362–8. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 9.Hartung H. New cases of progressive multifocal leukoencephalopathy after treatment with natalizumab. Lancet Neurol. 2009;8(1):28–31. doi: 10.1016/S1474-4422(08)70281-3. [DOI] [PubMed] [Google Scholar]
- 10.TYSABRI Update: Patients Treated with TYSABRI as of the end of December 2008. March 13, 2009. Accessed March 17, 2009. Available from: http://library.corporate-ir.net/library/14/148/148682/items/328677/D96A171A-3815-4766-940D-BE6E2DAD8F0A_biib-Tysabri13Mar09.pdf
- 11.Kranick SM, Mowry EM, Rosenfeld MR. Progressive multifocal leukoencephalopathy after rituximab in a case of non-Hodgkin lymphoma. Neurology. 2007;69(7):704–706. doi: 10.1212/01.wnl.0000267325.06000.d9. [DOI] [PubMed] [Google Scholar]
- 12.Martin SI, Marty FM, Fiumara K, et al. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43(1):16–24. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 13.Coles AJ, Compston DAS, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 14.Kieseier BC, Wiendl H. Oral disease-modifying treatments for multiple sclerosis: the story so far. CNS Drugs. 2007;21(6):483–502. doi: 10.2165/00023210-200721060-00005. [DOI] [PubMed] [Google Scholar]
- 15.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 16.Tallantyre E, Evangelou N, Constantinescu CS. Spotlight on teriflunomide. Int MS J. 2008;15(2):62–68. [PubMed] [Google Scholar]
- 17.Li EK, Tam L, Tomlinson B. Leflunomide in the treatment of rheumatoid arthritis. Clin Ther. 2004;26(4):447–459. doi: 10.1016/s0149-2918(04)90048-3. [DOI] [PubMed] [Google Scholar]
- 8.Maddison P, Kiely P, Kirkham B, et al. Leflunomide in rheumatoid arthritis: recommendations through a process of consensus. Rheumatology (Oxford) 2005;44(3):280–286. doi: 10.1093/rheumatology/keh500. [DOI] [PubMed] [Google Scholar]
- 19.Osiri M, Shea B, Robinson V, et al. Leflunomide for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2003;(1):CD002047. doi: 10.1002/14651858.CD002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozman B. Clinical experience with leflunomide in rheumatoid arthritis. Leflunomide Investigators’ Group. J Rheumatol Suppl. 1998;53:27–32. [PubMed] [Google Scholar]
- 21.Bruneau JM, Yea CM, Spinella-Jaegle S, et al. Purification of human dihydro-orotate dehydrogenase and its inhibition by A77 1726, the active metabolite of leflunomide Biochem J 1998336(Pt 2):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox RI. Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol Suppl. 1998;53:20–26. [PubMed] [Google Scholar]
- 23.Furst DE. Leflunomide, mycophenolic acid and matrix metalloproteinase inhibitors. Rheumatology (Oxford) 1999;38(Suppl 2):14–18. [PubMed] [Google Scholar]
- 24.Greene S, Watanabe K, Braatz-Trulson J, Lou L. Inhibition of dihydro-orotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol. 1995;50(6):861–867. doi: 10.1016/0006-2952(95)00255-x. [DOI] [PubMed] [Google Scholar]
- 25.Cherwinski HM, McCarley D, Schatzman R, Devens B, Ransom JT. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J Pharmacol Exp Ther. 1995;272(1):460–468. [PubMed] [Google Scholar]
- 26.Fox RI, Herrmann ML, Frangou CG, et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 1999;93(3):198–208. doi: 10.1006/clim.1999.4777. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Williams JW, Bremer EG, Finnegan A, Chong AS. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem. 1995;270(21):12398–12403. doi: 10.1074/jbc.270.21.12398. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Blinder L, Shen J, et al. In vivo mechanism by which leflunomide controls lymphoproliferative and autoimmune disease in MRL/MpJ-lpr/lpr mice. J Immunol. 1997;159(1):167–174. [PubMed] [Google Scholar]
- 29.Zeyda M, Poglitsch M, Geyeregger R, et al. Disruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation. Arthritis Rheum. 2005;52(9):2730–2739. doi: 10.1002/art.21255. [DOI] [PubMed] [Google Scholar]
- 30.Manna SK, Aggarwal BB. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. J Immunol. 1999;162(4):2095–2102. [PubMed] [Google Scholar]
- 31.Déage V, Burger D, Dayer JM. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metal-loproteinases-1 over that of interleukin-1beta and metalloproteinases. Eur Cytokine Netw. 1998;9(4):663–668. [PubMed] [Google Scholar]
- 32.Kraan MC, Reece RJ, Barg EC, et al. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 2000;43(8):1820–1830. doi: 10.1002/1529-0131(200008)43:8<1820::AID-ANR18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Burger D, Begué-Pastor N, Benavent S, et al. The active metabolite of leflunomide, A77 1726, inhibits the production of prostaglandin E(2), matrix metalloproteinase 1 and interleukin 6 in human fibroblast-like synoviocytes. Rheumatology (Oxford) 2003;42(1):89–96. doi: 10.1093/rheumatology/keg038. [DOI] [PubMed] [Google Scholar]
- 34.Cao WW, Kao PN, Aoki Y, et al. A novel mechanism of action of the immunomodulatory drug, leflunomide: augmentation of the immunosuppressive cytokine, TGF-beta 1, and suppression of the immunostimulatory cytokine, IL-2. Transplant Proc. 1996;28(6):3079–3080. [PubMed] [Google Scholar]
- 35.Hamilton LC, Vojnovic I, Warner TD. A771726, the active metabolite of leflunomide, directly inhibits the activity of cyclo-oxygenase-2 in vitro and in vivo in a substrate-sensitive manner. Br J Pharmacol. 1999;127(7):1589–1596. doi: 10.1038/sj.bjp.0702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.sanofi-aventis. ARAVA® Tablets (leflunomide) 10 mg, 20 mg, 100 mg. Prescribing information. July 2007. Accessed March 18, 2009. Available from: http://products.sanofi-aventis.us/arava/arava.pdf
- 37.Beaman JM, Hackett LP, Luxton G, Illett KF. Effect of hemodialysis on leflunomide plasma concentrations. Ann Pharmacother. 2002;36(1):75–77. doi: 10.1345/aph.1A127. [DOI] [PubMed] [Google Scholar]
- 38.Korn T, Magnus T, Toyka K, Jung S. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide – mechanisms independent of pyrimidine depletion. J Leukoc Biol. 2004;76(5):950–960. doi: 10.1189/jlb.0504308. [DOI] [PubMed] [Google Scholar]
- 39.Merrill JE, Hanak S, Pu S, et al. Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. J Neurol. 2009;256(1):89–103. doi: 10.1007/s00415-009-0075-3. [DOI] [PubMed] [Google Scholar]
- 40.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor PW, Li D, Freedman MS, et al. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66(6):894–900. doi: 10.1212/01.wnl.0000203121.04509.31. [DOI] [PubMed] [Google Scholar]
- 42.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 43.Paty DW, Oger JJ, Kastrukoff LF, et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology. 1988;38(2):180–185. doi: 10.1212/wnl.38.2.180. [DOI] [PubMed] [Google Scholar]
- 44.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 45.Mladenovic V, Domljan Z, Rozman B, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum. 1995;38(11):1595–1603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- 46.Smolen JS, Kalden JR, Scott DL, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353(9149):259–266. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- 47.Strand V, Cohen S, Schiff M, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999;159(21):2542–2550. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- 48.van Riel PLCM, Smolen JS, Emery P, et al. Leflunomide: a manageable safety profile. J Rheumatol Suppl. 2004;71:21–24. [PubMed] [Google Scholar]
- 49.Aventis Pharmaceuticals. Important prescribing information. October 2003. Accessed March 18, 2009. Available from: http://www.fda.gov/medwatch/SAFETY/2003/arava_deardoc.pdf
- 50.Emery P, Breedveld FC, Lemmel EM, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000;39(6):655–665. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- 51.Auer J, Hinterreiter M, Allinger S, Kirchgatterer A, Knoflach P. Severe pancytopenia after leflunomide in rheumatoid arthritis. Acta Med Austriaca. 2000;27(4):131–132. doi: 10.1046/j.1563-2571.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- 52.Hill RL, Topliss DJ, Purcell PM. Pancytopenia associated with leflunomide and methotrexate. Ann Pharmacother. 2003;37(1):149. doi: 10.1345/aph.1C293. [DOI] [PubMed] [Google Scholar]
- 53.Kalden JR, Schattenkirchner M, Sörensen H, et al. The efficacy and safety of leflunomide in patients with active rheumatoid arthritis: a five-year follow-up study. Arthritis Rheum. 2003;48(6):1513–1520. doi: 10.1002/art.11015. [DOI] [PubMed] [Google Scholar]
- 54.Jenks KA, Stamp LK, O’Donnell JL, Savage RL, Chapman PT. Leflunomide-associated infections in rheumatoid arthritis. J Rheumatol. 2007;34(11):2201–2203. [PubMed] [Google Scholar]
- 55.Warnatz K, Peter HH, Schumacher M, et al. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann Rheum Dis. 2003;62(1):50–57. doi: 10.1136/ard.62.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozman B, Praprotnik S, Logar D, et al. Leflunomide and hypertension. Ann Rheum Dis. 2002;61(6):567–569. doi: 10.1136/ard.61.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukushima R, Kanamori S, Hirashiba M, et al. Inhibiting the teratogenicity of the immuno-suppressant leflunomide in mice by supplementation of exogenous uridine. Toxicol Sci. 2009;108(2):419–426. doi: 10.1093/toxsci/kfp022. [DOI] [PubMed] [Google Scholar]
- 58.Fukushima R, Kanamori S, Hirashiba M, et al. Critical periods for the teratogenicity of immune-suppressant leflunomide in mice. Congenit Anom (Kyoto) 2009;49(1):20–26. doi: 10.1111/j.1741-4520.2008.00217.x. [DOI] [PubMed] [Google Scholar]
- 59.Fukushima R, Kanamori S, Hirashiba M, et al. Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor leflunomide in mice. Reprod Toxicol. 24(3–4):310–316. doi: 10.1016/j.reprotox.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 60.US National Institutes of Health. Search for: teriflunomide multiple sclerosis - List Results - ClinicalTrials.gov. 2009. Accessed March 18, 2009. Available from: http://clinicaltrials.gov/ct2/results?term=teriflunomide+multiple+sclerosis
- 61.US National Institutes of Health. Search for relevant trials on: Home – ClinicalTrials.gov. 2009. Accessed March 19, 2009. Available from: http://clinicaltrials.gov/
- 62.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 63.Polman C, Barkhof F, Sandberg-Wollheim M, et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology. 2005;64(6):987–991. doi: 10.1212/01.WNL.0000154520.48391.69. [DOI] [PubMed] [Google Scholar]
- 64.Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372(9648):1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 65.Hofstetter HH, Stüve O, Hartung H. Is 1+1 0, 1, 2, or 11? Arithmetics of antiinflammatory agents in autoimmunity. Exp Neurol. 2009;217(1):4–6. doi: 10.1016/j.expneurol.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Curtis JR, Beukelman T, Onofrei A, et al. Elevated liver enzyme tests among rheumatoid arthritis and psoriatic arthritis patients treated with methotrexate and/or leflunomide Ann Rheum Dis 2009January15[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]