Abstract
The 60-kDa cysteine-rich outer membrane protein genes of Chlamydia psittaci, Chlamydia pneumoniae, and Chlamydia trachomatis have very different 5' ends, but two areas flanking this variable region show absolute sequence conservation. This observation permitted differentiation of the three species of Chlamydia by the polymerase chain reaction (PCR), forming the basis of a diagnostic test for chlamydial infections. The PCR product containing the variable region of the respective 60-kDa CrP genes was also subjected to restriction endonuclease digestion, enabling differentiation of individual type strains of C. psittaci. Differentiation was possible between lymphogranuloma venereum and trachoma isolates of C. trachomatis. The PCR-based diagnostic test was successful with all strains of chlamydiae studied. The PCR primers showed high specificity and did not produce any product with common bacterial pathogens that may share the same sites of infection.
Full text
PDF


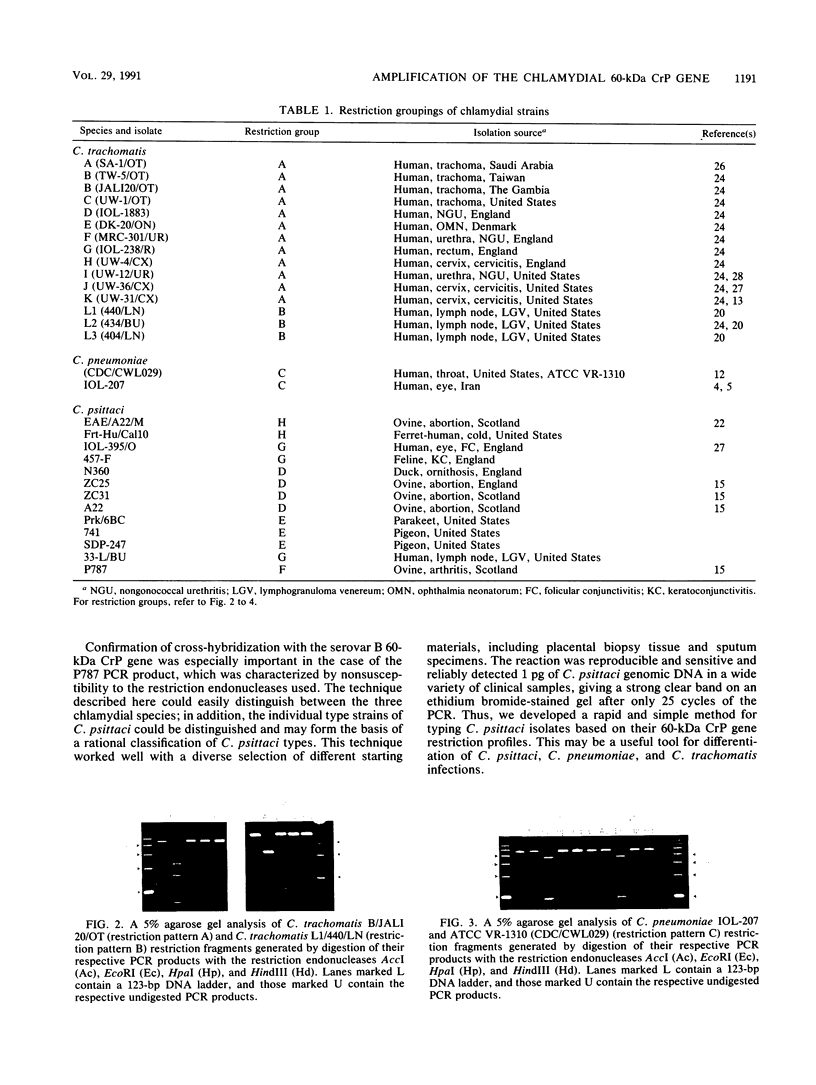


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Stephens R. S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989 Jan;171(1):285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer R. S., Treharne J. D., Jones B. R., Herring J. Chlamydial infection. Results of micro-immunofluorescence tests for the detection of type-specific antibody in certain chlamydial infections. Br J Vener Dis. 1972 Dec;48(6):452–459. doi: 10.1136/sti.48.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsey T., Darougar S., Treharne J. D. Prevalence in human beings of antibodies to Chlamydia IOL-207, an atypical strain of chlamydia. J Infect. 1986 Mar;12(2):145–152. doi: 10.1016/s0163-4453(86)93608-x. [DOI] [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Genetic diversity of avian and mammalian Chlamydia psittaci strains and relation to host origin. J Bacteriol. 1989 May;171(5):2850–2855. doi: 10.1128/jb.171.5.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C. A., Lim-Fong R., Prasad E., Kibsey P. C. Comparison of a DNA probe with culture for detecting Chlamydia trachomatis directly from genital specimens. Mol Cell Probes. 1990 Feb;4(1):25–31. doi: 10.1016/0890-8508(90)90036-y. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Miceli M., Sublett J. E. Synthesis of disulfide-bonded outer membrane proteins during the developmental cycle of Chlamydia psittaci and Chlamydia trachomatis. J Bacteriol. 1986 Feb;165(2):379–385. doi: 10.1128/jb.165.2.379-385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. M., Gaydos C. A., Quinn T. C. Detection and differentiation of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae by DNA amplification. J Infect Dis. 1990 Oct;162(4):984–987. doi: 10.1093/infdis/162.4.984. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T., Alexander E. R. TRIC type K, a new immunologic type of Chlamydia trachomatis. J Immunol. 1974 Aug;113(2):591–596. [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesire R. R., Maclean I. W., Shewen P. E., Winston S. E. Identification of genus-specific epitopes on the outer membrane complexes of Chlamydia trachomatis and Chlamydia psittaci immunotypes 1 and 2. Infect Immun. 1989 Sep;57(9):2914–2918. doi: 10.1128/iai.57.9.2914-2918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMP J. T., McEWEN A. D., WATT J. A. A., NISBET D. I. Enzootic abortion in ewes; transmission of the disease. Vet Rec. 1950 Apr 29;62(17):251–254. doi: 10.1136/vr.62.17.251. [DOI] [PubMed] [Google Scholar]
- Schachter J., Smith D. E., Dawson C. R., Anderson W. R., Deller J. J., Jr, Hoke A. W., Smartt W. H., Meyer K. F. Lymphogranuloma venereum. I. Comparison of the Frei test, complement fixation test, and isolation of the agent. J Infect Dis. 1969 Sep;120(3):372–375. doi: 10.1093/infdis/120.3.372. [DOI] [PubMed] [Google Scholar]
- Spears P., Storz J. Chlamydia psittaci: growth characteristics and enumeration of serotypes 1 and 2 in cultured cells. J Infect Dis. 1979 Dec;140(6):959–967. doi: 10.1093/infdis/140.6.959. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Gale J. L. Three new immunologic types of trachoma-inclusion conjunctivitis organisms. J Immunol. 1973 Mar;110(3):873–879. [PubMed] [Google Scholar]
- Wang S., Grayston J. T. Chlamydia trachomatis immunotype J. J Immunol. 1975 Dec;115(6):1711–1716. [PubMed] [Google Scholar]
- Watson M. W., Lambden P. R., Clarke I. N. The nucleotide sequence of the 60 kDa cysteine rich outer membrane protein of Chlamydia psittaci strain EAE/A22/M. Nucleic Acids Res. 1990 Sep 11;18(17):5300–5300. doi: 10.1093/nar/18.17.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. W., Lambden P. R., Ward M. E., Clarke I. N. Chlamydia trachomatis 60 kDa cysteine rich outer membrane protein: sequence homology between trachoma and LGV biovars. FEMS Microbiol Lett. 1989 Dec;53(3):293–297. doi: 10.1016/0378-1097(89)90233-4. [DOI] [PubMed] [Google Scholar]
- Watson M. W., al-Mahdawi S., Lamden P. R., Clarke I. N. The nucleotide sequence of the 60 kDa cysteine rich outer membrane protein of Chlamydia pneumoniae strain IOL-207. Nucleic Acids Res. 1990 Sep 11;18(17):5299–5299. doi: 10.1093/nar/18.17.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]