Abstract
Purpose
Inflammation and increased metabolic activity associated with oxidative stress in irradiated normal tissues may contribute to both complications following radiotherapy and increased glucose uptake as detected by post-therapy FDG PET imaging. We sought to determine if increased glucose uptake in normal tissues after chemoradiotherapy is associated with increased toxicity.
Methods
Consecutive patients with locoregionally advanced head and neck cancers treated with IMRT and free of recurrence at one year were studied. FDG-PET imaging was obtained at 3 and 12 months post-treatment. SUV levels were determined at various head and neck regions. Functional outcome was measured using a quality of life questionnaire and weight loss and type of diet tolerated one year after therapy. A one-tailed Pearson correlation test was used to examine associations between SUV levels and functional outcome measures.
Results
SUV levels in the supraglottic and glottic larynx from FDG PET imaging obtained 12 months post-treatment were inversely associated with quality of life measures and were correlated with a more restricted diet one year after therapy. SUV levels at 3 months after therapy did not correlate with functional outcome. Increases in SUV levels in normal tissues between 3 and 12 months were commonly found in the absence of recurrence.
Conclusion
Altered metabolism in irradiated tissues persists one year after therapy. FDG PET scans may be used to assess normal tissue damage following chemoradiotherapy. These data support investigating hypermetabolic conditions associated with inflammation and\or oxidative stress as causal agents for radiation induced normal tissue damage.
Keywords: Head and Neck Neoplasms, Radiotherapy, Normal tissue toxicity, Positron Emission Tomography
INTRODUCTION
Definitive chemoradiotherapy for head and neck cancer frequently results in long-term tissue injury and dysfunction (1–10). Increasing the intensity of treatment increases locoregional control rates, but also results in greater toxicity. Speech and swallowing are functions affected by chemoradiotherapy. A dose-response relationship has long been established between laryngeal irradiation and speech outcome (11, 12). Higher radiation doses, particularly above 60 Gy, result in inferior speech related quality of life (QOL) (1). Volume of tissue irradiated also impacts on voice function after therapy (12). Likewise, increased radiation doses to the supraglottic larynx and nearby lateral pharyngeal walls are correlated with a more restricted diet, greater weight loss, and lower health-related QOL measures following chemoradiotherapy (1).
The mechanism, or mechanisms, responsible for chronic, late term toxicity after chemoradiotherapy are poorly understood. Various models have been proposed including chronic inflammation, and on-going tissue injury. Hauer-Jensen (13) and others (14) have proposed that radiation exposure results in a complex wound, with an excess of pro-inflammatory cytokines and perpetuation of tissue injury. Indeed, excess cytokines, including TGF-beta, are seen after irradiation and appear to correlate with some radiation induced toxicities, including radiation pneumonitis after thoracic irradiation (15). The ongoing inflammatory response is thought to lead to the fibrosis characteristic of late radiation reactions.
Inflammation creates an increase in metabolism associated with chronic oxidative stress (16). Glucose metabolism serves as a major source for reducing equivalents for the detoxification of hydroperoxides through the formation of pyruvate, which directly scavenges hydroperoxides, and NADPH, which acts as a cofactor for glutathione and thioredoxin dependent peroxidases (17–22). Therefore, the increased glucose consumption in tissues under going inflammatory processes [and corresponding increased 18F fluorodeoxyglucose (FDG) uptake seen on positron emission tomography (PET)] may represent a response to the severity of the oxidative stress associated with inflammation. In some cases, FDG uptake in inflammatory processes can be difficult to distinguish from FDG uptake in cancer. In addition, metabolic changes within irradiated tissues undergoing chronic inflammatory processes may also alter glucose uptake and retention after radiotherapy. Recently, Guerrero et al.(23) examined correlations between radiation dose delivered to lung and FDG uptake on post-treatment PET scans. They found a linear relationship between dose delivered and FDG uptake, providing evidence that glucose metabolism is altered in irradiated tissues. The increased use of FDG PET for post-treatment cancer surveillance affords the opportunity to examine glucose consumption not only in cancer tissues but also in adjacent normal tissues. Since an increasing number of patients are examined with FDG PET scans after chemoradiotherapy to assess tumor response, information regarding normal tissue FDG uptake in irradiated tissues is available.
The hypothesis of this work is that long-term tissue damage following chemoradiotherapy may result from processes such as chronic inflammation or oxidative metabolic derangements within irradiated normal tissues. This hypothesis would predict patients with high FDG uptake in normal tissues after radiotherapy may experience worse normal tissue toxicity. To test this hypothesis, metabolic activity in normal tissues, as shown by FDG uptake in PET scans performed after treatment, was correlated with dietary and quality of life outcomes in a cohort of patients treated with chemoradiotherapy for head and neck cancer.
METHODS
Patients
This work was performed with approval from the University of Iowa Institutional Review Board IRB-01. The patients reviewed for this report are a subset of 27 patients with stage III and IV squamous cell cancer of the head and neck, previously reported by our group (1). The subset of 18 patients reported here are those patients that had FDG PET scans performed at approximately 12 months post-therapy. Seventeen of the 18 patients also had an FDG PET scan performed at approximately 3 months after therapy. Patient characteristics are provided (Table 1). These patients received cisplatin-based chemotherapy concurrently with radiotherapy delivered via intensity modulated radiation therapy (IMRT) (1, 24). The details of the IMRT have been reported previously (24). Briefly, areas of gross disease were treated using 2 Gy daily fractions to 66–74 Gy. Adjacent regions at high risk of harboring microscopic disease were treated using 1.8 Gy fractions to 63 Gy. Anatomic regions with standard risk were treated to 50.4 to 54 Gy in 1.8 Gy fractions. Doses were prescribed to ensure that 95% of the target volume received the indicated doses, therefore some areas could receive higher doses.
Table 1.
Characteristics for patients reviewed for this study
| Characteristic | Number (%) |
|---|---|
| Age* | 35–75 |
| Post-treatment PET† | |
| First | 2.0 – 6.5 |
| Second | 6.2 – 16.0 |
| Sex | |
| Male | 16 (88.9) |
| Female | 2 (11.1) |
| Clinical stage | |
| I | 0 (0) |
| II | 0 (0) |
| III | 6 (33.3) |
| IV | 12 (66.7) |
| Subsite | |
| Nasopharynx | 1 (5.6) |
| Oropharynx | 9 (50.0) |
| Hypopharynx | 1 (5.6) |
| Larynx | 6 (33.2) |
| Unknown primary | 1 (5.6) |
| Treatment | |
| RT only | 0 (0) |
| RT + Chemo | 18 (100.0) |
measured in years; mean age was 54 years
measured in months post-treatment; mean was 3.5 months for PET 1 and 12.3 months for PET 2
These patients have been followed after completing therapy for at least one year without recurrence. All patients were followed radiographically using PET and CT imaging. In addition, patients were also followed with clinical assessments, which included at a minimum a directed history and physical examination, including fiberoptic nasopharyngoscopy. One patient with concerning findings on physical exam underwent direct laryngoscopy and biopsy showing no evidence of cancer. Finally, all patients were followed by clinical exam by both the responsible Radiation Oncologist and Otolaryngologist for an additional minimum of 6 months after the one year PET and toxicity assessment. No locoregional recurrences have been seen in this group.
PET Imaging
The dose of FDG, given intravenously, was 10–15 mCi. After an uptake period of 90 min, patients were imaged with a CTI Biograph duo PET/CT tomograph (Siemens LSO Biograph duo PET/CT tomograph; Siemens Medical Systems, Hoffman Estates, IL) from skull base to umbilicus. Images were reconstructed using ordered subset expectation maximization at a resolution of approximately 8 mm. These images were interpreted with a display station showing orthogonal attenuation corrected and noncorrected images. For images with increased focal FDG uptake, a maximum standardized uptake value (SUV) was calculated in manually placed regions of interest using built-in software based on the conventional formula for SUV using body weight normalization. All PET scans utilized in this study were obtained in a systematic fashion, with the use of a uniform length of time between FDG injection and imaging, pre-imaging determination of blood glucose levels, routine use of benzodiazapines to limit muscle activity and FDG uptake and close observation and instruction to rest quietly and not talk between FDG injection and imaging.
Functional Outcome
Twelve months after treatment, all subjects completed the Head and Neck Cancer Inventory (HNCI), a 31-point validated health related quality of life questionnaire addressing eating, speaking, aesthetics and social function domains in quality of life (5, 25). Scores in each domain are normalized to a total possible 100 points. The type of diet tolerated by the patient was also determined at 12 months post-treatment and scored from 1 to 6, with no oral intake (NPO) scored as 1, and unrestricted diet scored as 6. The degree of weight loss at 12 months and the use of percutaneous endoscopic gastrostomy (PEG) tubes were also determined for each patient.
Imaging Outcome
Subjects also underwent serial FDG PET imaging at approximately 3 and 12 months post-treatment. Maximum SUV were determined in normal tissues at five levels, corresponding to the superior border of the tongue, the mid- tongue base, the vallecula, the supraglottic larynx and the glottic larynx. At each of these levels, a region of interest including mucosal surfaces and adjacent tissue was defined on an axial image. The maximum SUV within this region of interest was determined and used for further analysis (Fig. 1). Correlations between maximum SUV levels and outcome measures were determined using the Pearson product moment correlation (Pearson’s r).
Figure 1. Determining FDG SUV in irradiated tissues.
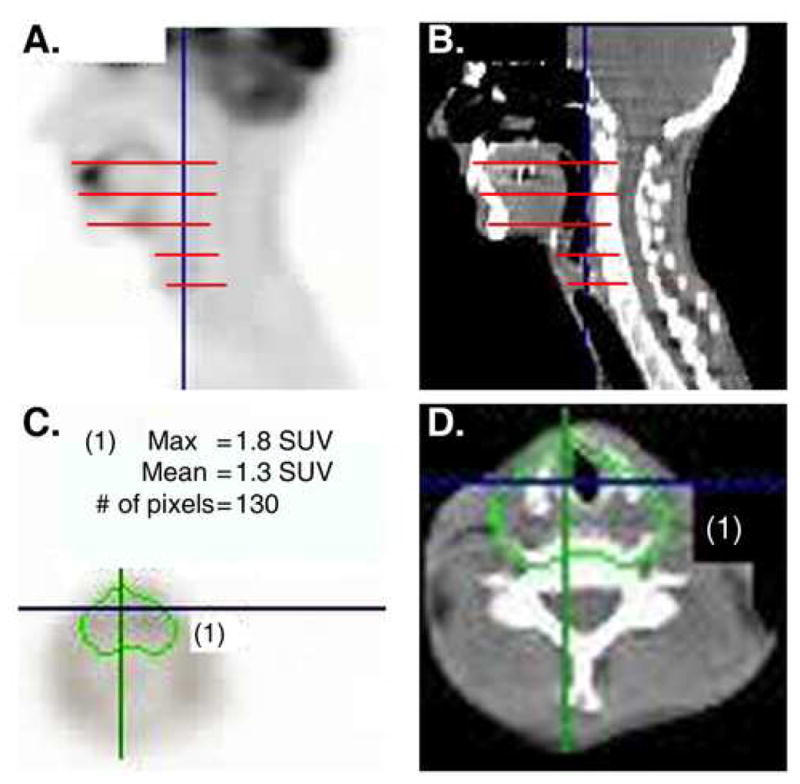
Sagittal views of PET(a) and CT (b) showing the 5 axial planes for which FDG SUVs were determined. At each level, a region of interest was defined to include all mucosal surfaces and surrounding soft tissue. An example is shown for the laryngeal axial plane with both PET(c) and CT (d) images.
RESULTS
Eighteen patients with locoregionally advanced head and neck cancer undergoing definitive chemoradiotherapy at the University of Iowa form the patient population (Table 1). These patients had a minimum of one year follow-up without recurrence and had also completed a validated health related quality of life questionnaire, the HNCI. All were treated with IMRT and details of the radiotherapy have been described (24). The patients studied for the current report are a subset of 18 from a larger group of 27 patients also treated with chemoradiotherapy and followed using the HNCI. These 18 were chosen because FDG PET scans were performed at approximaately 3 and 12 months post-treatment. Previous analysis of the 27 patients showed higher doses of radiation to various structures in the upper aerodigestive tract had an inverse dose response between radiation dose and late term toxicities (1). Specifically, higher doses of radiation in the region of the supraglottic larynx are correlated with increased toxicity 12 months after treatment. The specific doses of radiation delivered to a series of points along the upper aerodigestive tract were presented previously (1), and ranged from 25 to 90 Gy.
Toxicity was assessed by a variety of measures for these patients as previously described. Briefly, the HNCI was used to determine the health-related QOL 12 months following treatment. Objective measures of outcome including the amount of weight loss, presence or absence of a PEG tube and type of diet tolerated, ranging from NPO to unrestricted, were also assessed one year after treatment. The outcome measures assessed for this group are summarized (Table 2).
Table 2.
Outcome Measures. A variety of outcome measures were used. Health-related quality of life for various domains was determined using the Head and Neck Cancer Inventory (5,25). Data regarding weight and PEG tube use and duration were determined from reviewing the patient medical record. Type of diet tolerated data was obtained prospectively with the HNCI data, but was not available for two subjects
| Score |
||
|---|---|---|
| Measure | Average (SD) | Range |
| Quality of Life | ||
| Speech | 62.3 (31.2) | 3–100 |
| Diet | 59.6 (24.7) | 2.5–88 |
| Aesthetics | 79.2 (24.2) | 25–100 |
| Social disruption | 75.1 (22.8) | 25–100 |
| Diet tolerated | ||
| 1 (NPO) | 3 | — |
| 2 | 3 | — |
| 3 | 0 | — |
| 4 | 0 | — |
| 5 | 2 | — |
| 6 (unrestricted) | 8 | — |
| PEG tube | ||
| At any time* | 15 | — |
| At 12 months | 9 | |
| Weight loss† | 12.4 | 0.5–29.4 (8.9) |
at any time during or in the first 12 months after treatment.
measured in kilograms
FDG PET uptake by head and neck tissues was assessed at 5 axial levels, corresponding to the most superior extent of the tongue, the mid base of tongue, the vallecula, the suprglottic larynx approximately at the AE folds, and the glottic larynx. At each of these levels, a region of interest was defined including essentially all soft tissues anterior to the vertebral bodies. The location of the axial planes chosen are shown (Fig. 1); examples of the regions of interest within the axial planes are also provided (Fig. 2). The maximum SUV within each region of interest was determined and used for subsequent analysis. Each PET scan was therefore condensed to 5 SUV numbers, representing the highest SUV for the neck tissues at each of 5 axial levels (Table 3). A number of patients showed increases in normal tissue FDG uptake between the 3 and 12 month scan. At each of the 5 planes measured, 17% to 40% of the patients demonstrated a minimum increase of 12% in the SUV between the 3 and 12 month scan. The threshold of 12% was chosen based on previous studies showing a variation of between 10% to 12% in SUV levels on subjects imaged serially at close intervals without receiving any therapy (26, 27). Again, no recurrences have been found for these patients, suggesting these rising SUV levels in normal tissue may be a relatively common finding and not necessarily a harbinger of tumor recurrence.
Figure 2. Higher FDG SUVs in the glottic larynx 12 months after treatment are correlated with a worse functional outcome.
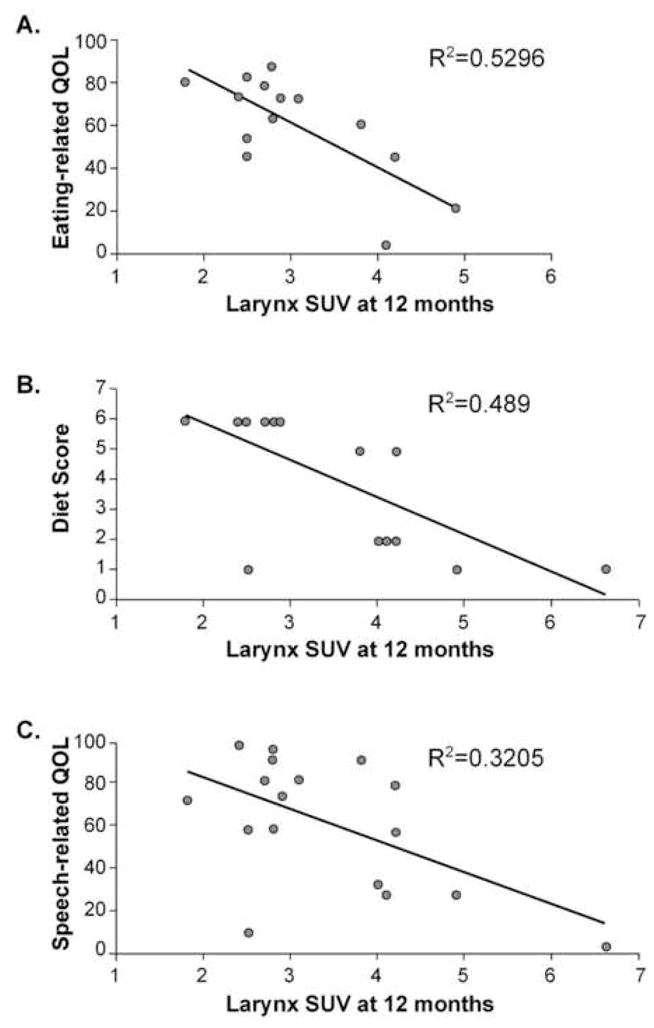
A) Normalized eating-related quality of life scores measured at 12 months post-treatment from the HNCI are shown as a function of normal tissue FDG uptake at the level of the glottic larynx (see figure 1) from a PET scan also obtained 12 months after therapy. B) Diet scores ranging from 1 for NPO to 6 for full, unrestricted diet determined 12 months after treatment are shown as a function of normal tissue FDG uptake at the level of the glottic larynx (see figure 1) from a PET scan also obtained 12 months after therapy. C) Normalized speech-related quality of life scores measured at 12 months post-treatment using the HNCI are shown as a function of normal tissue FDG uptake at the level of the glottic larynx (see figure 1) from a PET scan also obtained 12 months after therapy. In each graph, there is a significant association showing higher FDG uptake is associated with increased toxicity 12 months after therapy.
Table 3.
SUV Data for FDG PET. The average and range in FDG SUV levels for PET scans performed approximately 3 months (PET1) and 12 months (PET2). SUV levels were determined at the 5 levels shown in fig. 1. The number of patients displaying a change in SUV of greater than 12% (26,27) likely representing a significant change are shown in the right hand columns for either increasing or decreasing SUV levels between PET1 and PET2. These data show changes in FDG uptake in irradiated tissues between 3 and 12 months post-treatment are common
| PET 1 | PET 2 | SUV change* | ||||
|---|---|---|---|---|---|---|
| Average | Range | Average | Range | increase† | decrease† | |
| Top of tongue | 3.0 | 1.4–4.7 | 2.8 | 1.6–3.5 | 4 | 7 |
| Mid tongue base | 3.1 | 2.3–4.7 | 3.0 | 1.7–5.9 | 3 | 8 |
| Vallecula | 2.8 | 1.4–5.0 | 3.0 | 1.8–5.7 | 7 | 5 |
| Supraglottic larynx | 2.8 | 1.9–5.1 | 2.7 | 1.6–4.2 | 4 | 5 |
| Glottic larynx | 3.4 | 2.2–5.4 | 3.4 | 1.8–6.6 | 5 | 9 |
Changes in SUV, either increasing or decreasing, were defined as greater than 12%.
Number of patients with noted change (increase or decrease) between the 2 PET scans.
To determine if a significant correlation existed between the degree of FDG uptake and quality of life outcomes, the SUV numbers were compared to the HNCI scores. Using a Pearson correlation test, correlation between FDG uptake and outcome was examined. No significant relationships were found between any outcome measure and the FDG PET uptake at the five levels examined for the 3 month scan. However, significant correlations were found between quality of life outcomes and FDG uptake at the level of the supraglottic and glottic larynx for the 12 month post-treatment PET scan. Figure 2 shows the correlations between FDG SUV at the level of the glottic larynx and eating QOL (2A), type of diet (2B) and speech related quality of life (2C). Figure 3 shows the correlation between FDG uptake in the plane of the supraglottic larynx 12 months after treatment and eating QOL (3A) and type of diet tolerated (3B) also assessed at 12 months.
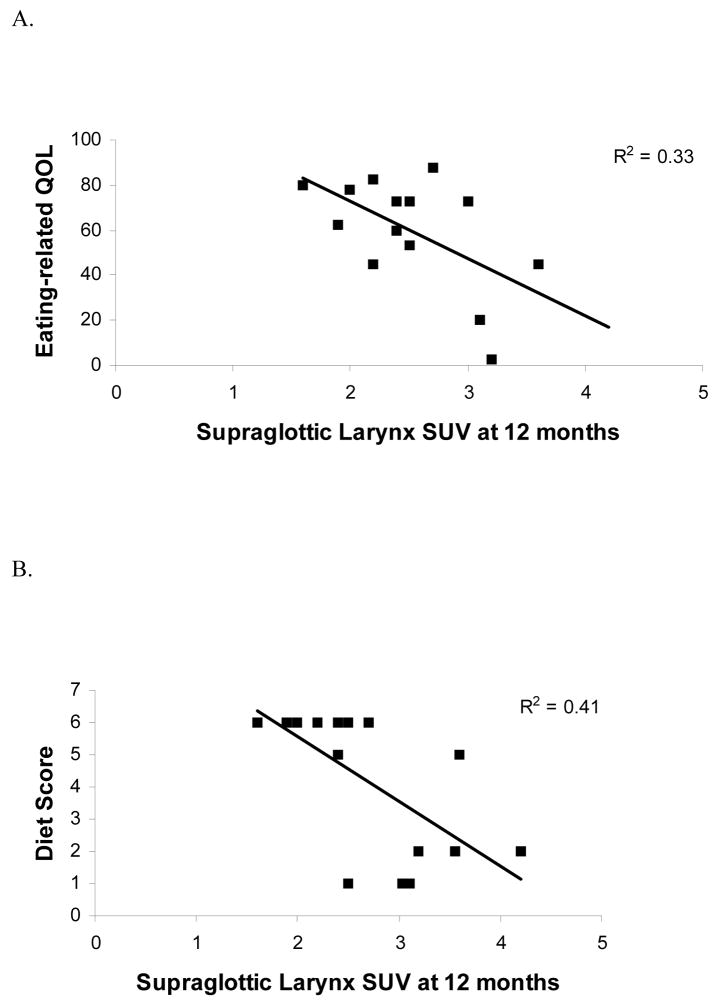
Figure 3. Higher FDG SUVs in the supraglottic larynx 12 months after treatment are correlated with a worse functional outcome.
A) Normalized eating-related quality of life scores measured at 12 months post-treatment from the HNCI are shown as a function of normal tissue FDG uptake at the level of the supraglottic larynx (see figure 1) from a PET scan also obtained 12 months after therapy. B) Diet scores ranging from 1 for NPO to 6 for full, unrestricted diet determined 12 months after treatment are shown as a function of normal tissue FDG uptake at the level of the supraglottic larynx (see figure 1) from a PET scan also obtained 12 months after therapy. In each graph, there is a significant association showing higher FDG uptake is associated with increased toxicity 12 months after therapy.
Table 4 summarizes the significant correlations between post-treatment FDG uptake at 12 months in the glottic and supraglottic larynx and functional outcomes 12 months after treatment. The type of diet tolerated was given a numeric score, ranging from 1 for NPO to 6 for full unrestricted diet. Higher FDG SUV in the supraglottic and glottic larynx is inversely correlated with this diet score. No significant correlations were seen at the superior three levels where SUV was measured, nor was there any correlation between FDG SUV levels and persistence of PEG tubes or degree of weight loss. For each correlation, increased FDG uptake was associated with increased toxicity. Higher FDG uptake and worse functional outcome is consistent with our hypothesis that increased metabolic activity, such as inflammation, in irradiated tissues corresponds to increased toxicity.
Table 4.
Summary of correlations between FDG SUV and functional outcomes. Significant correlations between the 12 month PET and outcome. The negative values again show higher SUV levels are associated with lower quality of life scores and functional outcome at 12 monhts
| Quality of Life |
|||||
|---|---|---|---|---|---|
| Maximum SUV | Diet | Eating | Speech | Aesthetic | Social function |
| Level of supraglottic larynx | −0.64* | −0.57† | n.s. | −0.62* | −0.44† |
| Level of glottic larynx | −0.70* | −0.73* | −0.57* | −0.53† | −0.45† |
Significant at p < 0.05;
Significant at p < 0.01; n.s. = not significant
DISCUSSION
This retrospective study analyzed quality of life and dietary outcome one year after definitive chemoradiotherapy for head and neck cancer. Elevated SUV levels in and around the larynx one year after chemoradiotherapy for head and neck cancer is associated with a decreased functional outcome. This finding suggests FDG PET scans may be used to assess normal tissue damage as well as tumor response following chemoradiotherapy for head and neck cancers. This association is consistent with our hypothesis and supports investigating inflammation or other hypermetabolic conditions associated with oxidative stress as causal agents for radiation induced normal tissue damage.
Increased FDG uptake in normal tissues following irradiation has also been described for the lungs (23, 28) and heart (29). Data from lung treatment suggests not only is there a dose response between radiation delivered to normal lung and subsequent FDG uptake (23), but the degree of FDG uptake in adjacent normal lung is also directly correlated with local control. Jingu et al.(29) have recently reported that increased FDG uptake in myocardium after radiotherapy may be correlated with myocardial injury. These authors reported that 13% of patients who received significant radiation dose to the heart had increased FDG uptake and retention several months after treatment. The majority of patients with increased FDG uptake also showed altered 123I methyl-iodophenyl pentadecanoic acid (123I BMIPP) and/or thallium 201 SPECT scans, suggesting abnormalities in either cardiac perfusion, fatty acid metabolism, or both. Thus, the findings of Jingu and colleagues are consistent with the data presented in the current study and suggest metabolic changes after radiotherapy are associated with radiation induced toxicity. Since biopsies were not routinely obtained in any of these studies, it is not possible to define a mechanism for the increased FDG uptake in patients with greater toxicity. The findings of our study are consistent with either a prolonged inflammatory state producing both increased FDG uptake and tissue injury, or radiation induced metabolic damage producing both increased glucose consumption and tissue damage. The limitations of our study do not enable us to define a mechanism.
Inflammation has long been appreciated as a cause for increased FDG uptake and retention during FDG PET scanning (30). An alternative possibility is suggested by the recent appreciation of mitochondrial injury following radiotherapy. Kim et al. (31) have found changes in mitochondrial DNA and function following irradiation that appear to result in chronic nuclear genotoxic stress. If radiation exposure can lead to prolonged mitochondrial injury, cellular uptake of glucose (and therefore FDG) may increase as a compensatory mechanism. Kim et al. (31) also demonstrated that irradiated cells produced larger amounts of superoxide. Furthermore, Slane et al. (22) demonstrated that mutations in genes coding for mitochondrial electron transport chain proteins lead to increased superoxide production and oxidative stress that is accompanied by increased glucose consumption and genomic instability. If this process occurs in normal irradiated tissues, the persistent increased production of superoxide following irradiation could contribute to increased oxidative stress and normal tissue toxicity, as well.
Although our results cannot distinguish between these, or other potential, mechanisms, the correlation between increased FDG uptake and normal tissue toxicity appears robust. Given the expanding role of FDG PET in cancer surveillance after chemoradiotherapy, the opportunity to simultaneously examine the effects of treatment on adjacent normal tissues will also increase. Further efforts to explore the clinical connection between increased FDG uptake and toxicity based on preclinical models of tissue injury resulting from prolonged inflammation or mitochondrial injury leading to oxidative stress may provide the insight necessary to develop treatments able to decrease toxicity.
FDG uptake in the larynx has been described as a potential artifact in PET imaging. Speaking during the FDG uptake phase prior to imaging is thought to result in higher accumulation of FDG in laryngeal tissues. Several maneuvers listed in the Methods section, including avoiding speech and use of benzodiazapines, were used to minimize potential artificial uptake in the larynx. Therefore, since all reasonable measures to limit the known causes of spurious laryngeal FDG uptake have been taken, the association between laryngeal FDG uptake and late term toxicity is likely an actual association.
The data presented in the current study also suggest rising SUV levels in previously uninvolved normal tissues are common and do not necessarily imply recurrent cancer. The results described here provide a rationale for using FDG PET scans not only for cancer restaging and surveillance, but also for assessing chronic normal tissue toxicity. These results also provide further justification for examining the role of increased metabolic activity, resulting from either inflammatory reactions within irradiated tissues or from metabolic or mitochondrial damage of the irradiated tissue itself, in creating radiation induced toxicity via chronic oxidative stress.
Acknowledgments
The authors would like to thank Kellie Bodeker for her editorial assistance. This work was funded in part by grants from the National Institutes of Health to G Funk (R01 CA106908-01) and Ken Dornfeld (1K08CA111404-01A1) and by support from the Holden Comprehensive Cancer Center, University of Iowa.
Footnotes
Conflicts of Interest: Neither actual nor potential conflicts of interest exist for any of the authors of this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007 Jul 1;68(3):750–7.2. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 4.El-Deiry M, Funk GF, Nalwa S, et al. Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131:879–885. doi: 10.1001/archotol.131.10.879. [DOI] [PubMed] [Google Scholar]
- 5.Funk GF, Karnell LH, Smith RB, et al. Clinical significance of health status assessment measures in head and neck cancer: what do quality-of-life scores mean? Arch Otolaryngol Head Neck Surg. 2004;130:825–829. doi: 10.1001/archotol.130.7.825. [DOI] [PubMed] [Google Scholar]
- 6.Kotz T, Costello R, Li Y, et al. Swallowing dysfunction after chemoradiation for advanced squamous cell carcinoma of the head and neck. Head Neck. 2004;26:365–372. doi: 10.1002/hed.10385. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus CL, Logemann JA, Pauloski BR, et al. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope. 1996;106:1157–1166. doi: 10.1097/00005537-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 9.Shiley SG, Hargunani CA, Skoner JM, et al. Swallowing function after chemoradiation for advanced stage oropharyngeal cancer. Otolaryngol Head Neck Surg. 2006;134:455–459. doi: 10.1016/j.otohns.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 10.Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47:1–12. doi: 10.1016/s0360-3016(99)00558-1. [DOI] [PubMed] [Google Scholar]
- 11.Parsons JT, Mendenhall WM, Stringer SP, et al. Twice-a-day radiotherapy for squamous cell carcinoma of the head and neck: the University of Florida experience. Head Neck. 1993;15:87–96. doi: 10.1002/hed.2880150202. [DOI] [PubMed] [Google Scholar]
- 12.Fung K, Yoo J, Leeper HA, et al. Vocal function following radiation for non-laryngeal versus laryngeal tumors of the head and neck. Laryngoscope. 2001;111:1920–1924. doi: 10.1097/00005537-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex ‘wound’. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 14.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 15.Evans ES, Kocak Z, Zhou SM, et al. Does transforming growth factor-beta1 predict for radiation-induced pneumonitis in patients treated for lung cancer? Cytokine. 2006;35:186–192. doi: 10.1016/j.cyto.2006.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 17.Averill-Bates DA, Przybytkowski E. The role of glucose in cellular defences against cytotoxicity of hydrogen peroxide in Chinese hamster ovary cells. Arch Biochem Biophys. 1994;312:52–58. doi: 10.1006/abbi.1994.1279. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle SW, Varnes ME, Mitchell JB, et al. Sensitivity to chemical oxidants and radiation in CHO cell lines deficient in oxidative pentose cycle activity. Int J Radiat Oncol Biol Phys. 1992;22:671–675. doi: 10.1016/0360-3016(92)90500-h. [DOI] [PubMed] [Google Scholar]
- 19.Nath KA, Ngo EO, Hebbel RP, et al. alpha-Ketoacids scavenge H2O2 in vitro and in vivo and reduce menadione-induced DNA injury and cytotoxicity. Am J Physiol. 1995;268:C227–236. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- 20.Berggren MI, Husbeck B, Samulitis B, et al. Thioredoxin peroxidase-1 (peroxiredoxin-1) is increased in thioredoxin-1 transfected cells and results in enhanced protection against apoptosis caused by hydrogen peroxide but not by other agents including dexamethasone, etoposide, and doxorubicin. Arch Biochem Biophys. 2001;392:103–109. doi: 10.1006/abbi.2001.2435. [DOI] [PubMed] [Google Scholar]
- 21.Nomura K, Imai H, Koumura T, et al. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J Biol Chem. 1999;274:29294–29302. doi: 10.1074/jbc.274.41.29294. [DOI] [PubMed] [Google Scholar]
- 22.Slane BG, Aykin-Burns N, Smith BJ, et al. Mutation of succinate dehydrogenase subunit C results in increased O2. -, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero T, Johnson V, Hart J, et al. Radiation pneumonitis: Local dose versus [(18)F]-fluorodeoxyglucose uptake response in irradiated lung. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63:410–421. doi: 10.1016/j.ijrobp.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Funk GF, Karnell LH, Christensen AJ, et al. Comprehensive head and neck oncology health status assessment. Head Neck. 2003;25:561–575. doi: 10.1002/hed.10245. [DOI] [PubMed] [Google Scholar]
- 26.Nakamoto Y, Zasadny KR, Minn H, et al. Reproducibility of common semi-quantitative parameters for evaluating lung cancer glucose metabolism with positron emission tomography using 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol. 2002;4:171–178. doi: 10.1016/s1536-1632(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 27.Weber WA, Ziegler SI, Thodtmann R, et al. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–1777. [PubMed] [Google Scholar]
- 28.Hicks RJ, Mac Manus MP, Matthews JP, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys. 2004;60:412–418. doi: 10.1016/j.ijrobp.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Jingu K, Kaneta T, Nemoto K, et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int J Radiat Oncol Biol Phys. 2006;66:845–851. doi: 10.1016/j.ijrobp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Love C, Tomas MB, Tronco GG, et al. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 31.Kim GJ, Fiskum GM, Morgan WF. A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res. 2006;66:10377–10383. doi: 10.1158/0008-5472.CAN-05-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]