Abstract
The intracellular circadian clock consists of a series of transcriptional modulators that together allow the cell to perceive the time of day. Circadian clocks have been identified within various components of the cardiovascular system (e.g. cardiomyocytes, vascular smooth muscle cells), and possess the potential to regulate numerous aspects of cardiovascular physiology and pathophysiology. The present study tested the hypothesis that ischemia/reperfusion (I/R; 30 minute occlusion of the rat left main coronary artery in vivo) alters the circadian clock within the ischemic, versus non-ischemic, region of the heart. Left ventricular anterior (ischemic) and posterior (non-ischemic) regions were isolated from I/R, sham-operated, and naïve rats over a 24 hour period, after which mRNAs encoding for both circadian clock components and known clock-controlled genes were quantified. Circadian clock gene oscillations (i.e. peak-to-trough fold differences) were rapidly attenuated in the I/R, versus the non-ischemic, region. Consistent with decreased circadian clock output, we observe a rapid induction of E4BP4 in the ischemic region of the heart at both the mRNA and protein levels. In contrast with I/R, chronic (1 week) hypobaric chamber-induced hypoxia did not attenuate oscillations in circadian clock genes in either the left or right ventricle of the rat heart. In conclusion, these data show that in a rodent model of myocardial I/R, circadian clocks within the ischemic region become rapidly impaired, through a mechanism that appears to be independent of hypoxia.
Keywords: Chronobiology, Gene Expression, Hypoxia, Ischemia, Metabolism
Introduction
Marked circadian rhythmicities in cardiovascular physiology and pathophysiology exist [1]. For example, heart rate, blood pressure, and cardiac output all exhibit circadian rhythmicity in humans and animal models [2–5]. The onset of myocardial infarctions (MIs) also exhibit a marked circadian rhythm in humans, with increased incidence in the early hours of the morning [6]. Furthermore, patients are at a greater risk for developing heart failure following an MI if it occurs during the evening [7]. These clinical observations have been attributed primarily to extracellular stimuli, such as sympathetic activity [6, 8]. What is becoming increasingly clear is that intrinsic factors, such as circadian clocks, likely mediate some of these cardiovascular observations [1, 9].
Circadian clocks are intracellular molecular mechanisms that allow a cell to sense the time of day, and thus prepare for a particular stimulus prior to its onset (i.e. anticipation). Circadian clocks are transcriptionally-based mechanisms, comprised of a set of positive and negative feedback loops, with a free running period of approximately 24 hours [10, 11]. These clocks have been identified in a vast number of mammalian cells, including various components of the cardiovascular system (e.g. cardiomyocytes, vascular smooth muscle cells) [12, 13]. Circadian clocks therefore have the potential of modulating multiple physiological and pathophysiological cardiovascular parameters. For example, direct regulation of the metabolic genes pdk4 and ucp3 by the circadian clock within the heart suggests that this molecular mechanism mediates diurnal variations in myocardial metabolism [1, 12]. Conversely, direct regulation of pai1 by the circadian clock within vascular endothelial cells may contribute to diurnal variations in the onset of MI [14].
Severe myocardial ischemia followed by reperfusion results in contractile dysfunction and infarct development, involving complex alterations in signaling cascades, gene expression and metabolic fluxes. Given that several components of the mammalian circadian clock have been reported to be responsive to changes in oxygen availability in various cell types [15, 16], we hypothesized that ischemia/reperfusion (I/R) would cause a phase shift in the circadian clock within the heart. We report here that the circadian clock within the ischemic region of the heart is rapidly attenuated, compared to the non-ischemic region. In contrast, hypoxia has little effect on the amplitude of circadian clock gene oscillations in the rat heart, suggesting the effects of I/R are independent of oxygen availability.
Materials and Methods
Animals
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23) and was approved by the Institutional Animal Care and Use Committees at Case Western Reserve University and at the Hatter Institute for Cardiology Research. Male Wistar rats (Harlan; 300–350g initial weight) were housed at the Animal Care Centers of Case Western Reserve University (ischemia/reperfusion studies) and of the Hatter Institute for Cardiology Research (hypoxia studies). Rats were housed under controlled conditions (23±1°C; 12-hr light/12-hr dark cycle), and received standard laboratory chow and water ad libitum. At least ten days prior to experimentation, animals were housed in a separate environment-controlled room, within which a strict 12-hour light/12-hour dark cycle regime was enforced (lights on at zeitgeber time (ZT) 0).
Ischemia/Reperfusion Studies
Rats were divided into three groups: naïve, sham, and ischemia/reperfusion (I/R) rats. I/R rats were subjected to 30 minutes of ischemia in the anterior left ventricle at ZT15, as described previously [17]. Briefly, I/R rats were anesthetized with 1.5–2% isoflurane, intubated, and ventilated on a Harvard ventilator. A lateral thoracotomy was next performed, followed by immediate ligation of the left main coronary artery. The ligation was removed 30 minutes later (i.e. ZT15.5), followed by closure of the chest. Rats were treated with buprenorphrine (0.075mg/Kg s.c.) and allowed to regain consciousness. Sham rats were subjected to the same surgical procedure, in the absence of left main coronary artery ligation. Naïve rats did not undergo surgery. Hearts were isolated from naïve, sham, and I/R rats at 3hr intervals after ZT15, for up to 24hrs. A ZT15.5 group was also included for I/R rats, in which hearts were isolated just prior to reperfusion. Left ventricular anterior (ischemic for I/R rats) and posterior (non-ischemic) regions were separated, frozen in liquid N2, and stored at −80°C prior to RNA and protein preparation. The non-ischemic region served as an internal control for the ischemic region of I/R hearts.
In Vivo Hypoxia Studies
Rats were placed in a hypobaric chamber, wherein they were exposed to 11% O2 (45kPa) for 7 days, as described previously [18]. Age-matched animals exposed to normoxic conditions were used as controls. On the 7th day, left and right ventricles were dissected from control and hypoxic hearts at 3 hour intervals, rapidly frozen in liquid nitrogen, and stored at −80°C prior to RNA isolation.
Isolated Adult Rat Cardiomyocytes
Isolated adult rat cardiomyocytes were prepared using protocols as described previously [12, 19]. Freshly isolated cardiomyocytes were cultured overnight in DMEM-containing laminin-coated plates, supplemented with 2.5% fetal calf serum (FCS). The cells were then challenged with fresh medium and cultured under either normoxic (control) or hypoxic (5% O2) conditions (Sanyo Incubator). In the latter case, media was allowed to equilibrate under hypoxic (5% O2) conditions for at least 3 hours prior to utilization. After a specified period of time, cardiomyocytes were harvested in TriReagent, and stored at −80°C prior to RNA isolation.
RNA Extraction and Quantitative RT-PCR
RNA extraction and quantitative RT-PCR of samples was performed using previously described methods [20, 21]. Specific quantitative assays were designed from rat sequences available in GenBank. Taqman assays for rat mhcβ, serca2a, bmal1, clock, per1, per2, per3, cry1, cry2, rev-erbaα, dbp, hlf, tef, pdk4, and ucp3 have been published previously [22–25]. In contrast, Taqman assays for rat hsp27, npas2, dec1, and e4bp4 are presented in Supplemental Table 1. Standard RNA was made for all assays by the T7 polymerase method (Ambion, Austin, Texas), using total RNA isolated from rat hearts; the use of standard RNA allows absolute quantification of gene expression. The correlation between the Ct (the number of PCR cycles required for the fluorescent signal to reach a detection threshold) and the amount of standard was linear over at least a 5-log range of RNA for all assays (data not shown). Gene expression data are represented as mRNA molecules per ng total RNA.
Western Blotting
DEC1, E4BP4, and CLOCK proteins were detected in heart homogenates, using standard Western Blotting techniques. Myocardial proteins were separated by electrophoresis on a 10% gel, transferred to PVDF membranes, and probed with specific antibodies. Detection was achieved through secondary probing, followed by enhanced chemiluminescence.
Statistical Analysis
Stata version 8.0 (Stata Corp., San Antonio, TX) was used to perform two-way analysis of variance (ANOVA) to investigate the main effects of intervention and time. A full model including second-order interactions was conducted for each experiment. Significant differences were determined using Type III sums of squares. The null hypothesis of no model effects was rejected at p < 0.05.
Results
Expression of Circadian Clock Genes Following Ischemia/Reperfusion
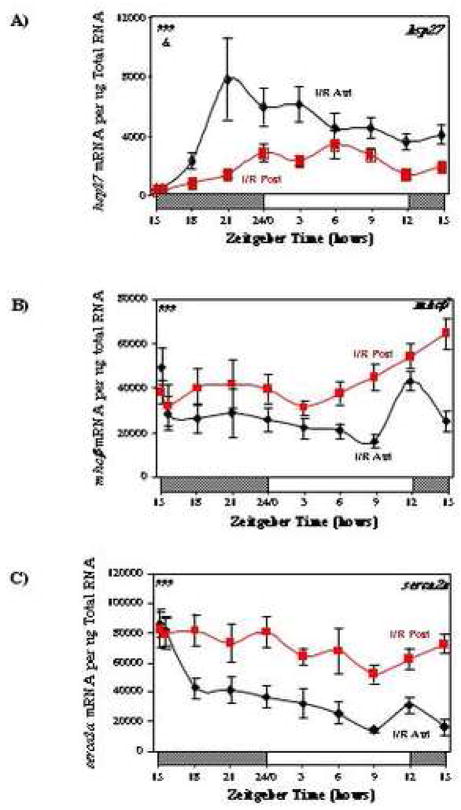
To investigate the efficacy of the coronary artery ligation protocol in the I/R hearts, we measured the expression of hsp27, which has been shown previously to be induced rapidly following ischemia-reperfusion [26]. Figure 1A shows that mRNA encoding for hsp27 increased rapidly in the anterior region of I/R hearts, relative to the posterior region; 6 hours following coronary artery ligation, expression of hsp27 was 6.0-fold higher in the anterior region of I/R hearts, compared to the posterior region. In addition, genes encoding for the contractile protein MHCβ and the calcium channel SERCA2a were investigated in I/R hearts. We report that mhcβ is induced in the posterior region (Figure 1B), while serca2a is repressed rapidly in the anterior region (Figure 1C), of I/R hearts.
Fig. 1.
Effects of ischemia/reperfusion on hsp27 (A), mhcβ (B), and serca2a (C) expression. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA. ###, p<0.001 anterior versus posterior (group); &, p<0.05 anterior versus posterior (group-time interaction).
We next investigated whether ischemia/reperfusion led to significant alterations in circadian oscillations of clock genes in the rat heart. We investigated key components of the clock mechanism (bmal1, clock, npas2, per1/2/3, cry1/2, rev-erbaα), dec1 (a potential modulator of the circadian clock), as well as known clock-controlled genes (dbp, hlf, tef, e4bp4, pdk4, ucp3). Figure 2 shows circadian oscillations in mRNAs encoding bmal1, clock, and npas2. Oscillations in these circadian clock genes were significantly attenuated in the anterior (ischemic) versus posterior (non-ischemic) region of I/R hearts. For example, clock was constitutively induced in the anterior region of I/R hearts, while npas2 was repressed (Figures 2B and C). In the case of bmal1, the trough-to-peak ratio for the posterior region of I/R hearts is 13.5, while this ratio is markedly lower in the anterior region (4.1; Figure 2A and Supplemental Table 2).
Fig. 2.
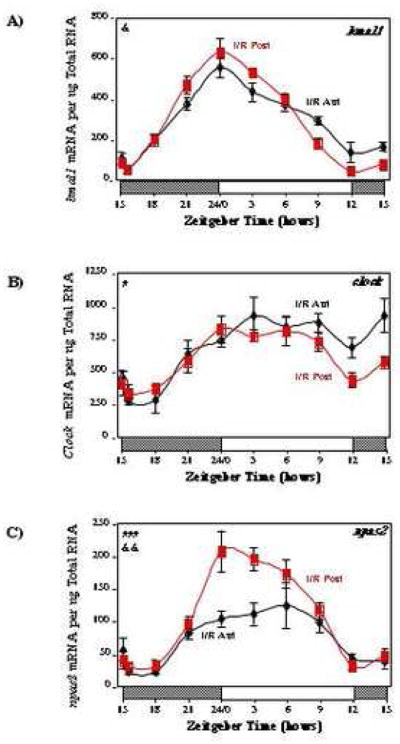
Effects of ischemia/reperfusion on myocardial bmal1 (A), clock (B), and npas2 (C) gene expression. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA. #, p<0.05 and ###, p<0.001 anterior versus posterior (group); &, p<0.05 and &&, p<0.01 anterior versus posterior (group-time interaction).
Circadian clock genes encoding negative components of this molecular mechanism were next investigated. Two known negative loops for the mammalian circadian clock are mediated by: 1) period (PER) and cryptochrome (CRY) proteins; and 2) REV-ERBAα. As observed for npas2 (Figure 2C), expression of per1, per3, cry2, and rev-erbaα was significantly repressed in the anterior region of I/R hearts, versus the posterior region (Figure 3 and Supplemental Table 2). In the case of per3, cry1, and rev-erbaα, the trough-to-peak ratio for the posterior region of I/R hearts is markedly higher than that obtained in the anterior region (Figure 3 and Supplemental Table 2).
Fig. 3.
Effects of ischemia/reperfusion on myocardial per1 (A), per2 (B), per3 (C), cry1 (D), cry2 (E), and rev-erbaα (F) gene expression. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA. #, p<0.05 and ###, p<0.001 anterior versus posterior (group); &, p<0.05 anterior versus posterior (group-time interaction).
A less well characterized potential negative loop of the mammalian circadian clock involves DEC1. Expression of dec1 increased rapidly in the anterior region of I/R hearts, reaching a peak approximately 6 hours following the ischemic insult; this induction was sustained over the time course investigated (Figure 4).
Fig. 4.
Effects of ischemia/reperfusion on myocardial dec1 gene expression. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA. ###, p<0.001 anterior versus posterior (group); &, p<0.05 anterior versus posterior (group-time interaction).
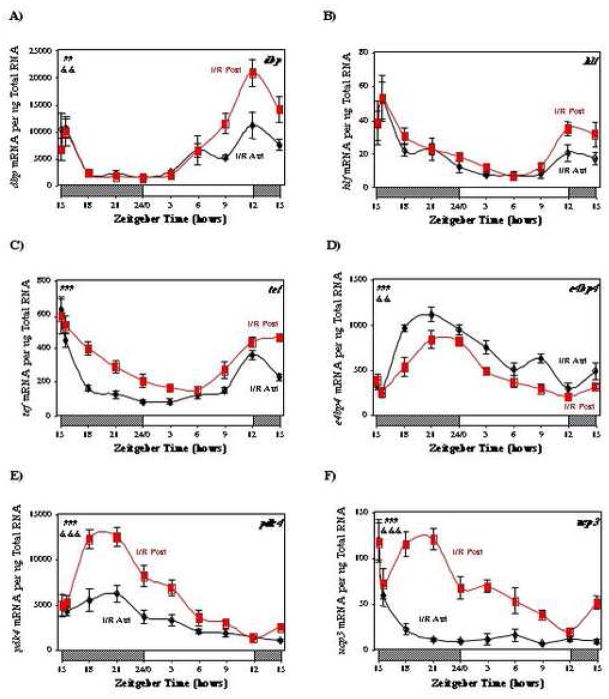
Output from the circadian clock is initially manifested at a transcriptional level. Members of the PAR family of transcription factors (dbp, hlf, tef) are known clock-controlled genes (i.e. genes regulated by the circadian clock, but do not encode for proteins that directly regulate the clock mechanism). In turn, PAR transcription factors have the potential to modulate expression of a host of target genes. In contrast, the basic leucine zipper transcription factor E4BP4, which is also a circadian clock-controlled gene, inhibits the transcriptional activity of PAR transcription factors by competing for the same promoter binding sites in target genes.[27] We therefore investigated whether ischemia/reperfusion influenced myocardial expression of dbp, hlf, tef, or e4bp4. Expression of dbp and tef is significantly repressed in the anterior region, versus posterior region, of I/R hearts (Figure 5A, 5C, and Supplemental Table 2). A marked attenuation in the oscillations of dbp and hlf are observed in the anterior region of I/R hearts relative to the posterior region (Figure 5A, 5B, and Supplemental Table 2). Figure 5D shows that e4bp4, is markedly induced in the anterior region, compared to the posterior region, of I/R hearts.
Fig. 5.
Effects of ischemia/reperfusion on myocardial dbp (A), hlf (B), tef (C), e4bp4 (D), pdk4 (E), and ucp3 (F) gene expression. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA. ##, p<0.01 and ###, p<0.001 anterior versus posterior (group); &&, p<0.01 and &&&, p<0.001 anterior versus posterior (group-time interaction).
We have recently shown that the metabolic genes pdk4 and ucp3 are regulated directly by the circadian clock within the cardiomyocyte [1, 12]. We therefore investigated whether attenuation of the circadian clock within the ischemic region of I/R hearts was associated with alteration in pdk4 and ucp3 gene expression oscillations. Significant oscillations in both pdk4 and ucp3 expression were observed in the posterior region of I/R hearts (Figure 5E and 5F). In contrast, mRNAs encoding for pdk4 and ucp3 exhibited both markedly lower expression levels and oscillations in the anterior region of I/R hearts (Figure 5E and 5F, and Supplemental Table 2).
Circadian Gene Expression Oscillations in Sham and Naïve Hearts
It is possible that the differences noted above were partially a consequence of surgical trauma (i.e. not solely the ischemic stress) and/or regional differences in gene expression (i.e. between anterior and posterior regions). In order to account for these possibilities, sham rats were subjected to anesthesia and surgery, but without LAD ligation, and compared to non-ischemic rats that did not undergo surgery (i.e. naïve rats). For every gene investigated, no significant differences in expression were observed between the anterior and posterior regions of naïve or sham hearts (see Supplemental Figures 1 and 2).
Protein Expression of Circadian Clock Components Following I/R
Given their known functions, we hypothesized that the induction of dec1 caused the attenuation of circadian clock gene oscillations following I/R, while induction of e4bp4 contributed towards repression of circadian clock output following I/R. We therefore investigated the effects of I/R on DEC1 and E4BP4 protein expression. Figure 6A shows that unlike dec1 mRNA, DEC1 protein is markedly repressed in the anterior region of I/R hearts at ZT9 (i.e. 18 hours post I/R). In contrast, consistent with e4bp4 mRNA, E4BP4 protein is induced in the anterior region of I/R hearts at ZT9 (i.e. 18 hours post I/R; Figure 6B). Similarly, CLOCK protein is also induced in the anterior region of I/R hearts at ZT9 (i.e. 18 hours post I/R; Figure 6C).
Fig. 6.
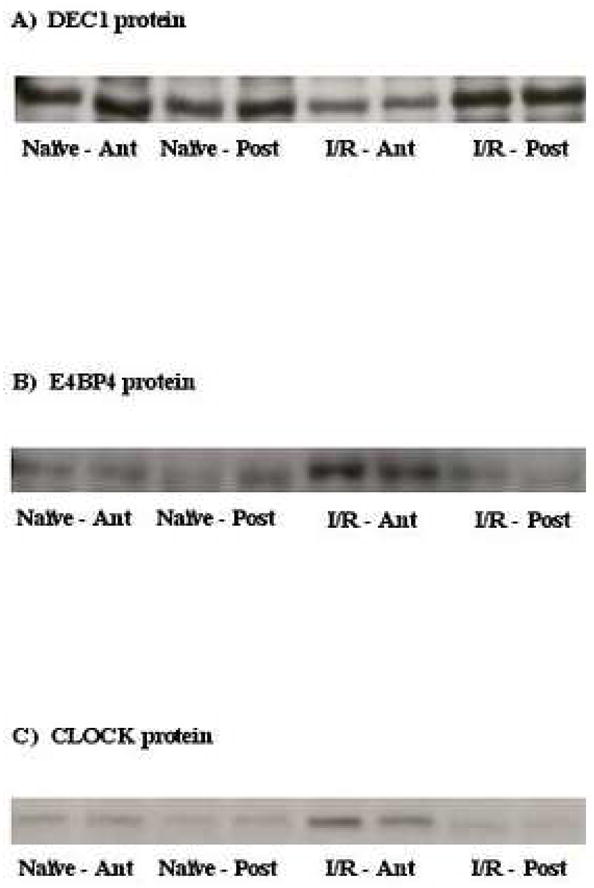
Effects of ischemia/reperfusion on myocardial DEC1 (A), E4BP4 (B), and CLOCK (C) protein expression. Representative Western blots for 4 separate observations in each group.
Expression of Circadian Clock Genes During Hypoxia
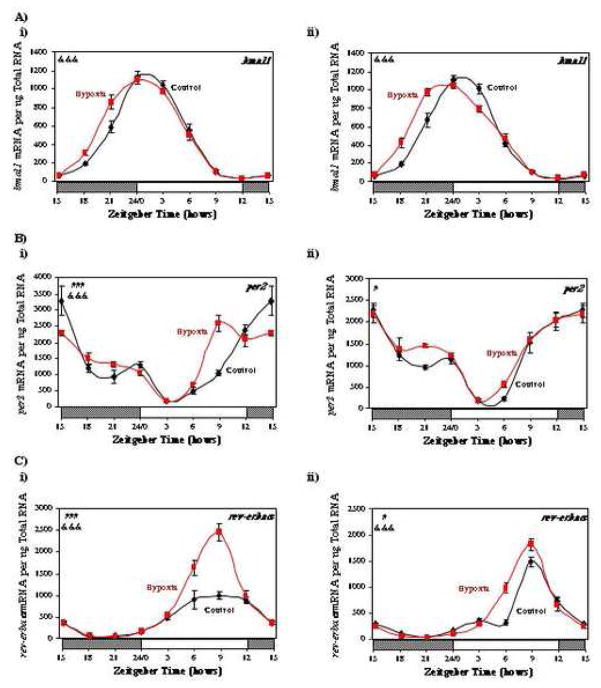
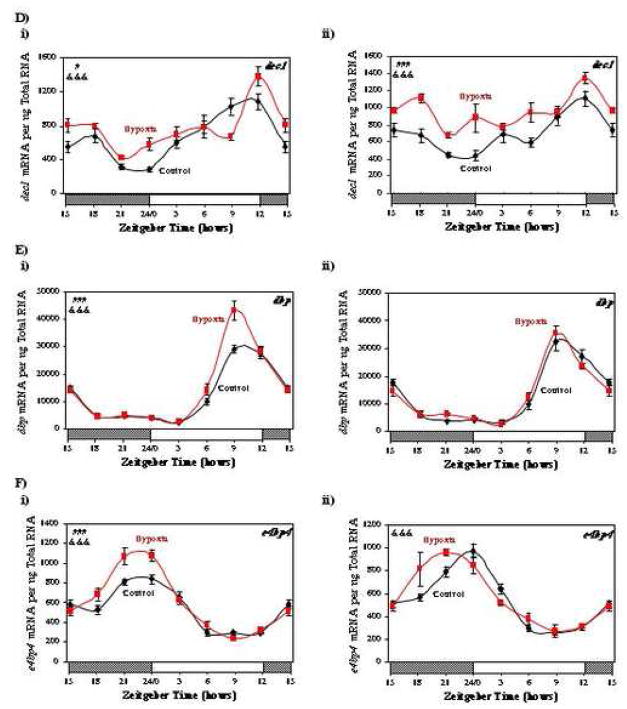
To determine whether oxygen availability mediates attenuation of circadian clock gene oscillations in the heart following I/R, we investigated whether chronic hypoxia caused similar changes in myocardial gene expression. Consistent with previously published reports, one week of hypoxia resulted in a slight (1.5-fold), but significant (p<0.01), increase in right ventricular weight, compared to normoxic hearts (data not shown). In contrast, left ventricular weight was not different between hypoxic and normoxic hearts (data not shown). Figure 7 (and Supplemental Tables 3 and 4) shows that 1 week of hypoxia did not attenuate circadian oscillations in the expression of bmal1, per2, rev-erbaα, dec1, e4bp4, or dbp, in either the left (panels i) or right (panels ii) ventricles. In contrast, hypoxia tended to augment oscillations in several circadian clock genes, particularly in the left ventricle. In addition, a phase advance in bmal1 was observed in both the left and right ventricles for hypoxic versus normoxic hearts (Figure 7A). Similar to observations for the anterior region of I/R hearts, hypoxia induced the expression of both dec1 and e4bp4 (Figure 7D and 7F). As observed for hypoxia in vivo, an acute exposure of isolated adult rat cardiomyocytes to hypoxic conditions in vitro had little effect on the expression either bmal1 or dbp (see supplemental Figure 3).
Fig. 7.
Effects of hypobaric chamber-induced hypoxia on myocardial bmal1 (A), per2 (B), rev-erbaα (C), dec1 (D), dbp (E), and e4bp4 (F) gene expression in the left (i) and right (ii) ventricles. Values are shown as the mean ± SEM for 6 observations in each group. Data are normalized to nanograms total RNA. #, p<0.05 and ###, p<0.001 hypoxia versus control (group); &&&, p<0.001 anterior versus posterior (group-time interaction).
Discussion
The purpose of the present study was to investigate whether myocardial ischemia/reperfusion would rapidly alter circadian clocks in the heart. The results show that ischemia/reperfusion causes a rapid attenuation in circadian clock gene oscillations within the ischemic region of the heart. Furthermore, this is associated with attenuation in oscillations of known clock controlled genes, such as the family of PAR transcription factors, and the metabolic genes pdk4 and ucp3. Consistent with decreased circadian clock output, we observe increased gene and protein expression of E4BP4 in the ischemic region of the heart. Unlike ischemia/reperfusion, hypoxia does not repress the circadian clock in the heart, suggesting that these ischemia-induced alterations are not due solely to oxygen deprivation. These observations reveal that ischemia/reperfusion results not only in loss of synchronization of the heart with the environment, but also loss of synchronization between different regions of the heart. We speculate that such dyssynchronies may contribute to the pathogenesis of reperfusion injury following severe myocardial ischemia.
Impairment of the Circadian Clock Following Ischemia/Reperfusion
When comparing oscillations in the expression of multiple circadian clock genes between the ischemic (anterior) versus the non-ischemic (posterior) region of I/R hearts, we find that amplitudes in the oscillations (i.e. fold change from trough-to-peak) for all core clock components (i.e. bmal1, clock, npas2, per1, per2, per3, cry1, cry2, and rev-erbaα) are decreased (Figures 2 and 3). In the cases of npas2, per1, per2, per3, cry1, cry2, and rev-erbaα, this appears primarily due to attenuation in the peak expression of these genes within the ischemic (anterior) region. In marked contrast, we observe a rapid and persistent induction of clock and dec1 in the ischemic region of I/R hearts (relative to the non-ischemic region). Previous studies have suggested that the latter two genes are responsive to hypoxia. CLOCK, like hypoxia inducible factor-1α (HIF-1α), is a member of the PAS family of transcription factors, and is induced in the brain by hypoxia [15]. Results presented in Figure 2B suggest that induction of clock expression in the anterior region of I/R hearts is due to attenuation in the repression of clock at the end of the light phase. Consistent with observations for clock mRNA, we report that CLOCK protein levels are increased in the anterior region of I/R hearts (Figure 6C). In contrast to clock, dec1 induction is extremely rapid, increasing 2.2-fold within 9 hours of the ischemic episode, during a period of time at which dec1 expression is normally decreasing in the heart (Figure 4). Expression of dec1 has been shown previously to be induced in tumors under hypoxic conditions; the present study also reports that dec1 is also induced in the heart under hypoxic conditions (Figure 7D) [16]. The potential role of DEC1 in the mammalian circadian clock mechanism is to act in a negative manner towards BMAL1 transcriptional activity [28]. As such, a sustained induction of dec1 post-MI would be expected to cause a chronic inhibition of circadian clock oscillations, as observed in the present study. However, Figure 6A shows that DEC1 protein levels are markedly decreased (not increased) in the anterior region of I/R hearts. Furthermore, hypoxia augmented (not attenuated) circadian clock gene oscillations in the heart (Figure 7). Taken together, these data suggest that neither DEC1 nor hypoxia mediate I/R-induced alterations in myocardial circadian clock gene oscillations.
We next investigated whether I/R-induced attenuation of the circadian clock within the heart had a functional consequence on known clock controlled genes. We report that oscillations in known circadian clock controlled genes were markedly attenuated in the anterior region of I/R hearts, compared to the posterior region (Figure 4). Conversely, e4bp4, a known clock-controlled gene that antagonizes the activity of the PAR transcription factors, is markedly induced in the ischemic region of I/R hearts (and the posterior region to a lesser extent; Figure 3B). Increased e4bp4 mRNA is associated with increased E4BP4 protein (Figure 6B). These observations suggest that residual levels of PAR transcription factors in the ischemic region of infarcted hearts would be inactive due to induction of this repressor. As such, circadian clock output would be abolished. Consistent with this idea, oscillations in pdk4 and ucp3, two known clock output genes, are essentially abolished in the ischemic region of I/R hearts (Figure 5). Interestingly, the known consensus sequence for PAR/E4BP4 binding is found within the promoters of both pdk4 and ucp3, providing an attractive potential mechanism linking the circadian clock within the cardiomyocyte to regulation of myocardial metabolism. Induction of e4bp4 during hypoxia (Figure 7E) may also contribute to previously reported decreases in myocardial pdk4 and ucp3 gene expression during these conditions [29]. In contrast to pdk4 and ucp3, mhcβ and serca2a exhibit relatively modest circadian oscillations in expression (Supplemental Figures 1B and 1C), suggesting that repression of these genes following I/R (Figures 1B and 1C) is likely independent of circadian clock impairment.
Potential Clinical Implications
The role of the circadian clock is to confer the selective advantage of anticipation, thereby allowing a cell to respond appropriately to a stimulus at a specific time of the day (e.g. physical activity, food intake). The present study shows that post-MI, circadian clocks located within the ischemic region of the heart become inactivated rapidly, and dyssynchonous not only with the environment, but also with other regions of the heart. We have shown previously that the circadian clock within the heart becomes altered during both pressure overload-induced hypertrophy and diabetes mellitus [22, 30]. Shift workers also exhibit significantly greater risk for the development of cardiovascular disease [31]. We have recently observed that re-entrainment of the circadian clock within the heart requires between 5 and 8 days following reversal of the 12h/12h light/dark cycle [19]. In contrast, circadian rhythms in heart rate and blood pressure completely reset within 1–2 days following reversal of the light/dark cycle, in both rodents and humans [5, 32, 33]. The results of the present study demonstrate that an acute ischemic event rapidly suppresses the circadian clock within the ischemic region of the ventricle, which may contribute to post-ischemic dysfunction and the development of a myocardial infarct. Future studies should further address the causal role of the circadian clock in the pathophysiology of cardiovascular disease using genetically modified mouse models, such as the recently developed cardiomyocyte-specific circadian clock mutant (CCM) mouse [19].
Summary
We report that I/R causes a rapid attenuation in circadian clock gene oscillations within the ischemic region of the heart. This is associated with a marked attenuation in oscillations of known clock-controlled genes, such as the PAR transcription factors, and the metabolic genes pdk4 and ucp3. Consistent with decreased circadian clock output, we observe a rapid induction of E4BP4 in the ischemic region of the heart at both mRNA and protein levels. Impairment of the circadian clock following an ischemic episode is unlikely to be due solely to decreased oxygen availability, as hypoxia augmented (as opposed to attenuated) circadian clock gene oscillations in the heart. We speculate that dyssynchronies between the ischemic region of the heart with non-ischemic regions and/or the environment may contribute to infarct development, tissue remodeling, and ultimately development of heart failure.
Supplementary Material
Supplemental Figure 1. Circadian oscillations in gene expression of hsp27 (A), mhcβ (B), serca2a (C), bmal1 (D), clock (E), npas2 (F), per1 (G), per2 (H), per3 (I), cry1 (J), cry2 (K), rev-erbaα (L), dec1 (M), dbp (N), hlf (O), tef (P), e4bp4 (Q), pdk4 (R), and ucp3 (S) in the anterior and posterior regions of naïve rat hearts. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA.
Supplemental Figure 2. Gene expression of bmal1 (A), dec1 (B), dbp (C), and e4bp4 (D) in the anterior (A) and posterior (P) regions of sham rat hearts. Values are shown as the mean ± SEM for 5 observations in each group. Data are normalized to nanograms total RNA.
Supplemental Figure 3. Gene expression of bmal1 (A) and dbp (B) in isolated adult rat cardiomyocytes cultured under normoxic (control) or hypoxic (5% O2) conditions. Values are shown as the mean ± SEM for 3 observations in each group. Data are normalized to nanograms total RNA.
Acknowledgments
We wish to thank Rodrigo Garcia for help in the preparation of this manuscript. This work was supported by the National Heart, Lung, and Blood Institute (HL-074259, HL-074237, and TW-007344).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 2.Degaute JP, Van Cauter E, van de Borne P, Linkowski P. Twenty-four-hour blood pressure and heart rate profiles in humans. A twin study. Hypertension. 1994;23:244. doi: 10.1161/01.hyp.23.2.244. [DOI] [PubMed] [Google Scholar]
- 3.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang BL, Zannou E, Sannajust F. Effects of photoperiod reduction on rat circadian rhythms of BP, heart rate, and locomotor activity. Am J Physiol Regul Integr Comp Physiol. 2000;279:R169. doi: 10.1152/ajpregu.2000.279.1.R169. [DOI] [PubMed] [Google Scholar]
- 5.van den Buuse M. Circadian rhythms of blood pressure and heart rate in conscious rats: effects of light cycle shift and timed feeding. Physiol Behav. 1999;68:9. doi: 10.1016/s0031-9384(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 6.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 7.Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Increased risk of congestive heart failure among infarctions with nighttime onset. Am Heart J. 2000;140:438. doi: 10.1067/mhj.2000.108830. [DOI] [PubMed] [Google Scholar]
- 8.Turton MB, Deegan T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin Chim Acta. 1974;55:389. doi: 10.1016/0009-8981(74)90014-x. [DOI] [PubMed] [Google Scholar]
- 9.Hu K, Ivanov PC, Hilton MF, Chen Z, Ayers RT, Stanley HE, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Aad Sci USA. 2004;101:18223. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 11.Hardin PE. Transcription regulation within the circadian clock: the E-box and beyond. J Biol Rhythms. 2004;19:348. doi: 10.1177/0748730404268052. [DOI] [PubMed] [Google Scholar]
- 12.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, et al. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 13.McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 14.Maemura K, Layne MD, Watanabe M, Perrell MA, Nagai R, Lee ME. Molecular mechanisms of morning onset of myocardial infarction. Ann N Y Acad Sci. 2001;947:398. doi: 10.1111/j.1749-6632.2001.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 15.Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB J. 2001;15:2613. doi: 10.1096/fj.01-0092com. [DOI] [PubMed] [Google Scholar]
- 16.Turley H, Wykoff CC, Troup S, Watson PH, Gatter KC, Harris AL. The hypoxia-regulated transcription factor DEC1 (Stra13, SHARP-2) and its expression in human tissues and tumors. J Pathol. 2004;203:808. doi: 10.1002/path.1585. [DOI] [PubMed] [Google Scholar]
- 17.Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H2049. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ngumbela KC, Sack MN, Essop MF. Counter-regulatory effects of incremental hypoxia on the transcription of a cardiac fatty acid oxidation enzyme-encoding gene. Mol Cell Biochem. 2003;250:151. doi: 10.1023/a:1024921329885. [DOI] [PubMed] [Google Scholar]
- 19.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, et al. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Gibson UEM, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 22.Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 23.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 24.Stavinoha MA, RaySpellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol. 2004;287:E878. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 25.Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, et al. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000;32:985. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- 26.Das DK, Maulik N, Engelman RM, Rousou JA, Deaton D, Flack JEr. Signal transduction pathway leading to Hsp27 and Hsp70 gene expression during myocardial adaptation to stress. Ann N Y Acad Sci. 1998;851:129. doi: 10.1111/j.1749-6632.1998.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 27.Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. 2001;15:995. doi: 10.1101/gad.873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato F, Kawamoto T, Fujimoto K, Noshiro M, Honda KK, Honma S, et al. Functional analysis of the basic helix-loop-helix transcription factor DEC1 in circadian regulation. Interaction with BMAL1. Eur J Biochem. 2004;271:4409. doi: 10.1111/j.1432-1033.2004.04379.x. [DOI] [PubMed] [Google Scholar]
- 29.Essop MF, Razeghi P, McLeod C, Young ME, Taegtmeyer H, Sack MN. Hypoxia-induced decrease of UCP3 gene expression in rat heart parallels metabolic gene switching but fails to affect mitochondrial respiratory coupling. Biochem Biophys Res Commun. 2004;314:561. doi: 10.1016/j.bbrc.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 30.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the Circadian Clock in the Heart by Streptozotocin-induced Diabetes. J Mol Cell Cardiol. 2002;34:223. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 31.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;12:89. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 32.Chau NP, Mallion JM, de Gaudemaris R, Ruche E, Siche JP, Pelen O, et al. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- 33.Sundberg S, Kohvakka A, Gordin A. Rapid reversal of circadian blood pressure rhythm in shift workers. J Hypertens. 1988;6:393. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Circadian oscillations in gene expression of hsp27 (A), mhcβ (B), serca2a (C), bmal1 (D), clock (E), npas2 (F), per1 (G), per2 (H), per3 (I), cry1 (J), cry2 (K), rev-erbaα (L), dec1 (M), dbp (N), hlf (O), tef (P), e4bp4 (Q), pdk4 (R), and ucp3 (S) in the anterior and posterior regions of naïve rat hearts. Values are shown as the mean ± SEM for between 5 and 7 observations in each group. Data are normalized to nanograms total RNA.
Supplemental Figure 2. Gene expression of bmal1 (A), dec1 (B), dbp (C), and e4bp4 (D) in the anterior (A) and posterior (P) regions of sham rat hearts. Values are shown as the mean ± SEM for 5 observations in each group. Data are normalized to nanograms total RNA.
Supplemental Figure 3. Gene expression of bmal1 (A) and dbp (B) in isolated adult rat cardiomyocytes cultured under normoxic (control) or hypoxic (5% O2) conditions. Values are shown as the mean ± SEM for 3 observations in each group. Data are normalized to nanograms total RNA.