Abstract
Activity-dependent dendrite elaboration influences the pattern of interneuronal connectivity and network function. In the present study, we examined the mechanism by which the GluR1 subunit of AMPA receptors controls dendrite morphogenesis. GluR1 binds to SAP97, a scaffolding protein that is a component of the postsynaptic density, via its C-terminal 7 aa. We find that elimination of this interaction in vitro or in vivo (by deleting the C-terminal 7 aa of GluR1, GluR1Δ7) does not influence trafficking, processing, or cell surface GluR1 expression but does prevent translocation of SAP97 from the cytosol to membranes. GluR1 and SAP97 together at the plasma membrane promotes dendrite branching in an activity-dependent manner, although this does not require physical association. Our findings suggest that the C-terminal 7 aa of GluR1 are essential for bringing SAP97 to the plasma membrane, where it acts to translate the activity of AMPA receptors into dendrite growth.
Keywords: motor neurons, spinal cord, synaptic activity, postsynaptic density, trafficking, scaffold protein
Introduction
AMPA-type glutamate receptors (AMPARs) subserve moment-to-moment interneuronal communication and are also critical for the regulation of neuronal function on a time scale of minutes to years. By virtue of their subunit composition and posttranslational modifications, AMPARs will directly influence the exchange of signals between neurons (i.e., synaptic efficacy), as well as trigger intracellular events leading to enduring alterations in neuronal architecture and patterned connectivity. One means by which this is achieved is through the assembly of AMPARs with distinctive electrophysiological properties. For example, the degree of calcium permeability of AMPARs can control whether neuronal dendrites grow or wither (Inglis et al., 2002; Jeong et al., 2006).
AMPA receptor subunits bind to a variety of proteins through their C-terminal intracellular domain (Sheng and Sala, 2001; Song and Huganir, 2002; Shepherd and Huganir, 2007). Subunits with long intracellular tails (GluR1, GluR2L, and GluR4) can bind Band 4.1, CaMKII, PKA, and SAP97, whereas short-tailed receptors (GluR2, GluR3, and GluR4c) can bind AP2, NSF, PKC, and GRIP/ABP/PICK1 (Bredt and Nicoll, 2003; Shepherd and Huganir, 2007). The juxtamembrane portion of GluR1 interacts with the Band 4.1 protein, and the extreme C-terminal portion of GluR1 interacts with SAP97 (Leonard et al., 1998; Shen et al., 2000).
SAP97 is the mammalian homolog of the Drosophila discs-large tumor suppressor and is a member of the MAGUK (membrane-associated guanylate kinase) family of proteins (Sheng and Sala, 2001). It has a modular organization with several protein–protein interaction domains (i.e., PDZx3, GUK, L27, SH3, and an I3 domain). A consistent picture of SAP97 function in neurons has yet to emerge. In organotypic hippocampal slice cultures, long-term potential-evoking stimuli induce GluR1 delivery into synapses in a manner requiring the integrity of the extreme C terminus of GluR1, a region that is required for binding SAP97 (Hayashi et al., 2000). On the other hand, long-term potentiation is normal in GluR1Δ7 mice [animals in which the wild-type (WT) GluR1 allele has been replaced with a version lacking the C-terminal 7 aa] (Kim et al., 2005). Biochemically, SAP97 appears to predominantly associate with GluR1 early in the secretory pathway (as opposed to synaptic domains) and has been suggested to function during receptor maturation, not anchoring, of GluR1 at synapses (Sans et al., 2001). However, other studies localize SAP97 to excitatory synapses, and overexpression of SAP97 can enhance synaptic AMPA receptor function and promote dendritic spine growth (Rumbaugh et al., 2003). To make matters more confusing, none of these effects on cell surface AMPA receptors or synaptic transmission are detected in GluR1Δ7 mice (Kim et al., 2005). To account, in part, for these disparities, it has been suggested that another PDZ domain-containing protein (in addition to SAP97) binds the extreme C terminus of GluR1, although this protein, thus far, has not been identified (Boehm et al., 2006). Using a combination of in vitro and in vivo approaches, we show that the interaction of SAP97 with GluR1 is crucial for neuronal dendrite growth and branching in the spinal cord. We suggest that GluR1 plays a significant role in the recruitment SAP97 to the cell surface, where it functions to promote dendrite elaboration.
Materials and Methods
Plasmids.
YFP SAP97 expression plasmid was obtained from Dr. Morgan Sheng (Massachusetts Institute of Technology, Cambridge, MA). Two forms of SAP97, which differ in their N-terminal domains, are known to exist (Schlüter et al., 2006), and all SAP97 constructs used in this study are β-isoforms. This form of SAP97 contains an L27 domain, which confers the capacity for homo-oligomerization or hetero-oligomerization; the α-isoform of SAP97 lacks the L27 domain and is normally palmitoylated. All the GluR1 expression constructs are the flip version of GluR1 and contain a hexamyc tag between signal peptide and the N-terminal, extracellular portion of the mature protein. For neuronal expression, all the target genes were subcloned into pGW expression vector except the YFP SAP97, which employs a β-actin promoter.
Antibodies.
The antibodies used in this study were as follows: anti-c-myc, monoclonal (M-5546; Sigma), anti-β-galactosidase, monoclonal (Z-378A; Promega); anti-GFP, monoclonal (G-6539; Sigma) and polyclonal (632376; Clontech); anti-GluR1 C terminus (06-306; Millipore); anti-GluR1 N terminus (07-660; Millipore); anti-SAP97, polyclonal (PA1-741; Affinity BioReagents); anti-heat-shock protein 90 (HSP90) (SPA-830; Stressgen); PSD-95, monoclonal (05-306; Millipore); and anti-actin (A2066; Sigma). Antibody to the N terminus of GluR1 for immunoblotting was generated in the Huganir laboratory. Secondary antibody for cell surface labeling was a biotinylated goat anti-rabbit IgG (Vector Laboratories), and the fluorescent secondary reagents were Cy3-conjugated streptavidin (Sigma), Alexa Fluor 488-conjugated anti-rabbit IgG, and Alexa Fluor 594-conjugated anti-mouse IgG (Molecular Probes).
Animals.
The generation of the GluR1Δ7 mouse has been described previously (Kim et al., 2005). They have been backcrossed into a C57BL/6 background for >10 generations.
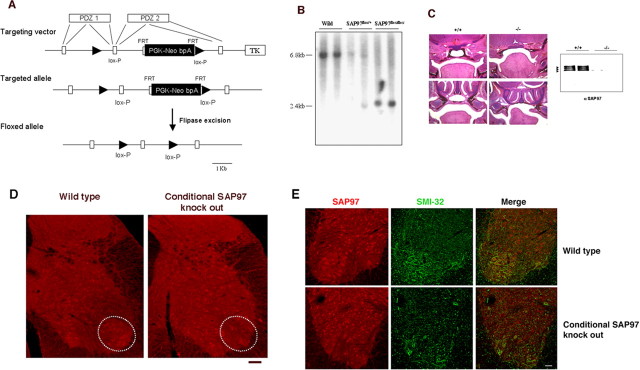
The Cre-loxP system was used to generate a SAP97 conditional mouse as reported previously (Gardner et al., 2005). The design of the targeting vector is shown in Figure 9 A; exons encoding PDZ domains of SAP97 are indicated. The Neor cassette (PGK-Neo bpA) with two FRT sequences at both sides (boxes) was inserted into the intron, and one lox-P site (triangle) was inserted into the intron downstream of the exon encoding part of both PDZ1 and PDZ2, and another loxP site was located upstream of the exon. The targeted allele is indicated in Figure 9 A. After electroporation of the linearized targeting vector into R1 embryonic stem (ES) cells (Dr. A. Nagy, Mount Sinai Hospital, Toronto, Canada) (Nagy et al., 1993), homologous recombinants were isolated by positive and negative selection. Correct ES clones were confirmed by Southern blot analysis and injected into C57BL/6 blastocyst, and chimeric mice were produced in the Transgenic Facility of Johns Hopkins University School of Medicine. After germ line transmission, the neor cassette was deleted using flipase-FRT system by breeding the chimeric mice to flipaseE transgenic mice. The complete SAP97flox allele is shown in the bottom of Figure 9 A. Mutant mice were backcrossed into C57BL/6 twice and then bred to the HB9-Cre mice (gift from Thomas Jessell, Columbia University, New York, NY).
Figure 9.
Generation and characterization of SAP97 conditional knock-out mice. A, Schematic representation of SAP97 conditional targeting strategy. B, Southern blot analysis of mouse genomic DNA from mutant mice. EcoRI digestion of wild-type genomic DNA generates a 6.8 kb fragment, and mutant genomic DNA with/without neor cassette produces a 2.4 kb fragment. C, Left, Coronal sections stained with hematoxylin and eosin of E18.5 wild-type (+/+) and CMV-Cre:SAP97LoxP/LoxP (−/−) embryos at two levels of the nasopharynx. The top row shows that the −/− mice have a cleft secondary palate as previously described in the null mice (Caruana and Bernstein, 2001). The bottom row shows that the palatal shelves are unfused in the −/− mice. Right, An immunoblot for SAP97 using brain from wild-type and conditional SAP knock-out mice. No SAP97 immunoreactivity is found in the −/− mice. D, Immunohistology of the spinal cord from wild-type and conditional SAP97 knock-out mice. SAP97 is expressed by motor neurons in wild-type but not the conditional knock-out mice (see regions denoted by ovals). There were no other discernible differences in SAP97-expressing cells between the two genotypes. Scale bar, 45 μm. E, The ventral horn of wild-type and conditional SAP97 knock-out mice stained for SAP97 (in red) and SMI32 (green). Colocalization of SAP97 in motor neurons is evident in the wild-type animals (see merged image). The conditional SAP97 motor neurons do not display SAP97 immunoreactivity. Scale bar, 55 μm.
Immunoprecipitation.
Protein samples, regardless of source, were finally suspended in immunoprecipitation buffer (20 mm HEPES, pH 7.4, 150 mm NaCl, and 5 mm EDTA) containing protease inhibitor mixture. Triton X-100 was gradually added into the buffer until the final concentration reached 1%. Solubilization was performed at 37°C for 45 min, with gentle pipetting every 5 min. Appropriate amount of antibody (typically ∼4 μg/ml) was added to 0.5–1 ml of protein extract. The samples were rocked gently for 2 h at 4°C, and then protein-A Sepharose was added to the mix. After incubation at 4°C overnight on a rocking platform, the samples were washed five times with washing buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 5 mm EGTA, and 0.1% Triton X-100). The proteins were eluted in 2× protein sample buffer and subject to SDS-PAGE electrophoresis and immunoblotting.
Membrane protein preparation.
Cells were washed twice using ice-cold PBS and resuspended in lysis buffer (10 mm KCl, 1.5 mm MgCl2, and 10 mm Tris-Cl, pH 7.4; 1.5 ml/60 mm plate) After incubation on ice for 10 min, cell lysis was finalized using a homogenizer (Dounce). The cell lysate was centrifuged at 2000 × g for 2 min (to remove nuclei and unlysed cells), and the supernatant was centrifuged a second time at 100,000 × g for 30 min at 4°C to pellet cell membranes.
Synaptosome preparation.
Subcellular fractionation and synaptic plasma membranes were prepared according to Gurd et al. (1974) and Blackstone et al. (1992) with modification. Briefly, tissue was homogenized in buffered sucrose (0.32 m sucrose and 10 mm HEPES, pH 7.4, w/v 10%). The homogenate was centrifuged at 800 × g for 10 min, and the supernatant was further centrifuged at 9000 × g for 15 min. The supernatant (S2) was saved. The pellet was resuspended in 10 volumes of buffered sucrose and centrifuged at 10,200 × g for 15 min. The pellet was resuspended in water, and HEPES, pH 7.4, was added rapidly to a final concentration of 1 mm. The cell suspension was stirred on ice for 30 min and then centrifuged at 25,000 × g for 20 min. The pellet was resuspended in 0.25 m buffered sucrose, layered onto a discontinuous sucrose gradient containing 0.8 m/1.0 m/1.2 m sucrose, and then centrifuged for 2 h at 65,000 × g. The visible interface between 1.0 m/1.2 m sucrose was collected as synaptosomal plasma membrane (SPM). S2 was centrifuged at 12,000 × g for 30 min, and the supernatant was centrifuged at 140,000 × g for 2 h. Pellet was collected as microsomes (P3) and the supernatant (S3) as the cytosolic fraction. The distribution of a cytosolic protein, HSP90, between S3:P3 is 50:1 (data not shown).
Deglycosylation.
For deglycosylation experiments, protein samples were resuspended in denaturing buffer (10 mm NaH2PO4, pH 6, 0.5% SDS, 2% glycerol, and 1% β-mercaptoethanol), incubated for 3 min at 100°C, and diluted with 1% NP-40 in 10 mm NaH2PO4, pH 6, and incubated with endoglycosidase-H (Roche) for 4 h at 37°C.
Surface biotinylation.
Steady-state biotinylation assays were performed as described previously (Chung et al., 2000) with minor modifications. Briefly, cells were washed with modified PBS (2.5 mm CaCl2 and 1 mm MgCl2 in PBS) and incubated with 1.5 mg/ml Sulfo-NHS-SS-biotin (Pierce) in modified PBS for 20 min at 4°C to label surface proteins with biotin. Remaining reactive biotin reagent was quenched by incubating cells with 50 mm glycine in modified PBS twice each for 20 min at 4°C. Cells were washed with modified PBS and lysed in modified RIPA buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mm Tris-HCl, 100 mm NaCl, 1 mm EDTA, 2 mm EGTA, 50 mm NaF, 10 mm sodium pyrophosphate, and 1 mm sodium orthovanadate, pH 7.4, with protease inhibitor mixture) at 4°C for 30 min. The lysate was centrifuged at 16,000 × g for 15 min at 4°C, and the resulting supernatant was incubated with 40 μl of 50% NeutrAvidin agarose beads (Pierce) for 3 h at 4°C. The beads were then washed three times with modified RIPA buffer, and the bound proteins were eluted with SDS sample buffer containing dithiothreitol (50 mm final concentration) at 65°C for 10 min. The amounts of the total and the biotinylated proteins were analyzed by quantitative immunoblotting using anti-GluR1 polyclonal antibody. The immunoblots were visualized using SuperSignal substrate (Pierce), and the images were acquired using a ChemiDoc camera (Bio-Rad).
Immunocytochemical live labeling of cell surface GluR1 and quantification.
Mixed cultured spinal cord neurons on coverslips were cotransfected with myc-tagged GluR1 or GluR1Δ7 plus a GFP expression vector on the fifth day in vitro (DIV 5). On DIV 9, anti-myc antibody (9E10) was added to the medium to achieve a final concentration of 2 μg/ml, and the cells were kept on ice for 45 min. Cells were then washed twice with PBS and fixed with 4% paraformaldehyde. Anti-GFP antibody (Clontech; catalog #632376) was diluted 100-fold in DMEM + 5% FBS containing 1% Triton X-100 and added onto coverslips. After incubation at 4°C for 48–72 h, the primary antibody was removed, and the cells were washed three times with PBS and then incubated with fluorescently tagged secondary antibodies (Alexa Fluor 488 anti-rabbit and Alexa Fluor 594 anti-mouse) for 4 h. Washed coverslips were mounted, and images were acquired using Olympus Fluoview laser confocal microscope. All image acquisition was performed at the same settings (laser density, offset, etc.), and images were analyzed using ImagePro (Media Cybernetics) software. Quantified myc signal and GFP signal for each individual cell were obtained for soma and dendrites separately. The ratio between myc signal and GFP signals was used as measurement for the surface GluR1 or GluR1Δ7.
Western blot.
Western blot was performed according to standard procedures (David and Kalb, 2005; Kim et al., 2005; Jeong et al., 2006; Mojsilovic-Petrovic et al., 2006).
Cell culture, transfection, and immunocytochemistry/immunohistochemistry.
Mixed spinal cord cell cultures were prepared on coverslips as described previously (Mojsilovic-Petrovic et al., 2006). Cells were transfected on day 5 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions (Jeong et al., 2006). Cells were fixed on day 9 and incubated in primary antibodies diluted in screening buffer (DMEM + 5% BSA) containing 1% Triton X-100 at 4°C for 2 d. After removing primary antibodies, the coverslips were washed three times using PBS and then incubated in species-specific Alexa secondary antibodies diluted in screening buffer at room temperature for 2–6 h. The coverslips were washed three times using PBS and mounted on slides.
Immunohistologic staining was performed on 50-μm-thick tissue sections from animals perfusion fixed with 4% paraformaldehyde, as previously described (Inglis et al., 1998). No tissue staining was evident when primary antibody was omitted.
Image acquisition, neuron trace, and statistical analysis.
Cell images were taken using Olympus Fluoview system. Cells were chosen according to the following criteria: the maximum diameter may not be smaller than 20 μm; the cell must have an axon; and the cell must have at least three primary dendrites, which must be at least 40 μm in length. Cells were traced using Neurolucida program (Jeong et al., 2006). Only branches longer than 10 μm were included. Neuron data were obtained using NeuroExplorer program, in which soma area, number of primary dendrites, number of bifurcation nodes, total arbitration, and length of longest dendrites were collected. ANOVA one-way analysis was performed using Prism program.
Results
For a number of reasons, the GluR1 subunit of AMPAR is of special interest. In the spinal cord, motor neurons express GluR1 at high abundance exclusively in early postnatal life when their dendrites undergo very large-scale growth (Kalb, 1994; Jakowec et al., 1995a,b). This activity-dependent growth of dendrites is limited in the absence of GluR1, and forcing expression of GluR1 in mature motor neurons leads to remodeling of their dendritic tree (Inglis et al., 2002; Zhang et al., 2005). Thus GluR1 is both necessary and sufficient to control motor neuron dendrite geometry. The role of the GluR1 partner SAP97 in activity-dependent dendrite growth is unknown, but the large and diverse set of binding partners of this scaffolding protein is intriguing.
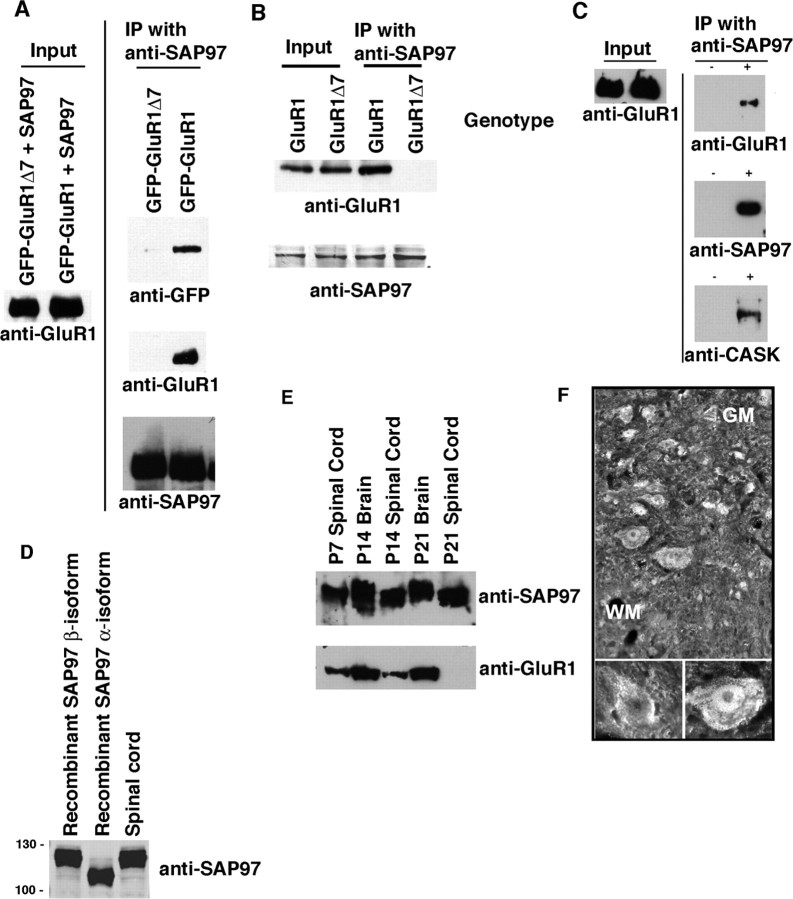
To investigate the role of SAP97 in the GluR1-dependent growth of motor neuron dendrites (Inglis et al., 2002; Jeong et al., 2006), we needed the capacity to manipulate the interaction of these two proteins. In glutathione S-transferase pull-down experiments, SAP97 interacts with the extreme C-terminal 4 aa of GluR1 (ATGL-COOH) as well as the SSG sequence located 9–11 residues upstream of the C terminus (Cai et al., 2002). Thus, theoretically GluR1Δ7 might retain the capacity to interact with SAP97 in cells. To explore this possibility, we cotransfected WT GluR1 or GluR1Δ7 (both GFP tagged at their N termini) with SAP97 into HEK 293 cells, immunoprecipitated with anti-SAP97 and blotted for GluR1 or GFP. Both GluR1 constructs expressed at equivalent levels, but only WT GluR1 was coimmunoprecipitated with SAP97 (Fig. 1 A). To study the endogenous interaction between GluR1 and SAP97, we made lysates from postnatal day 7 (P7) spinal cord of WT and GluR1Δ7 mice, immunoprecipitated with anti-SAP97 and blotted for GluR1 (using an antibody directed to the N terminus of the protein). The abundance of the WT and GluR1Δ7 proteins in the lysates was the same, but only WT GluR1 coimmunoprecipitated with SAP97 (Fig. 1 B). As a control for nonspecific adherence of either SAP97 or GluR1 to the beads, we repeated this analysis with and without primary antibody. Coimmunoprecipitation only occurred when anti-SAP97 was added to the lysates (Fig. 1 C). In addition to GluR1, anti-SAP97 immunoprecipitates also brought down the SAP97 binding partner calcium/calmodulin-dependent serine kinase (CASK) (Fig. 1 C). Thus the C-terminal 7 aa of GluR1 are required for WT GluR1 to interact with SAP97, and GluR1 and SAP97 are components of a multiprotein intracellular complex.
Figure 1.
The C-terminal 7 aa of GluR1 are required for its association with SAP97; both proteins are found in the neonatal spinal cord. A, HEK 293 cells were cotransfected with expression vectors for SAP97 + GFP-tagged GluR1 or GFP-tagged GluR1Δ7, and lysates were immunoprecipitated with anti-SAP97 and probed with anti-GFP, anti-GluR1, or anti-SAP97. Full-length GluR1 (detected with either anti-GFP or anti-GluR1), but not GluR1Δ7, could be pulled down with anti-SAP97. B, Spinal cord lysates from WT or GluR1Δ7 P7 mouse pups were immunoprecipitated with anti-SAP97 and blotted with anti-GluR1 or anti-SAP97. Endogenous full-length GluR1, but not GluR1Δ7, is pulled down with anti-SAP97. Levels of expression of GluR1, GluR1Δ7, and SAP97 are equivalent in the mice of either genotype. C, Spinal cord lysates from WT P7 pups were immunoprecipitated with anti-SAP97 or no primary antibody and probed with anti-SAP97, anti-GluR1, or anti-CASK. Protein complexes containing GluR1, SAP97, and CASK were pulled down only when immunoprecipitating with anti-GluR1. The level of GluR1 in the starting material (input) was equivalent. D, Lysates from HEK 293 cells heterologously expressing the α-isoform or β-isoform of SAP97 were run along with P7 spinal cord lysates and blotted for SAP97. Spinal cord SAP97 migrates at the same molecular weight as the β-isoform, indicating that this is the predominantly expressed endogenous species. E, SAP97 is present in lysates from WT mouse spinal cord at P7, P14, and P21 and mouse brain at P14 and P21. GluR1 is expressed at a lower level in the spinal cord at P7–P14 in comparison with the brain. F, Immunostaining of the P7 spinal cord with anti-SAP97 reveals staining of motor neurons in the ventral horn. (Photomicrograph printed with permission from Dr. Maria Rubio, University of Connecticut, Storrs, CT.) IP, Immunoprecipitation; GM, gray matter; WM, white matter.
Alternative splicing leads to the generation of two isoforms of SAP97 that differ at their N terminus (Schlüter et al., 2006). The 815 aa α-isoform contains a double cysteine motif that can undergo palmitoylation and has a deduced molecular weight of ∼89 kDa. The 911 aa β-isoform contains an L27 domain instead of the double cysteine motif and has a deduced molecular weight of ∼100 kDa. The N-terminally directed antibody we used has the capacity to recognize both isoforms, and both run slower on SDS-PAGE gels than would be predicted by deduced weights. On Western blot of spinal cord lysates, we see a single band that migrates at the molecular weight of recombinantly expressed β-isoform (Fig. 1 D). Thus, in contrast with several forebrain structures (Schlüter et al., 2006), in the spinal cord only the β-isoform of SAP97 is detectable. All of the studies described in this study were based on the β-isoform of SAP97.
Although GluR1 expression is developmentally regulated in the postnatal rodent spinal cord (Jakowec et al., 1995a,b), the pattern of expression of SAP97 is undefined. We found that SAP97 is abundantly expressed in the P7, P14, and P21 spinal cord at levels comparable to its expression in the brain (Fig. 1 E). At P7 and P14, GluR1 is expressed at lower levels in the spinal cord in comparison with the brain, and by P21, its level is quite low. Immunohistology for SAP97 in the P7 spinal cord reveals that immunoreactivity is present in neurons scattered throughout the gray matter (Fig. 1 F), including putative motor neurons. These experiments show that the β-isoform of SAP97 is likely to be expressed in spinal motor neurons along with GluR1 in early postnatal life, when active dendrite elaboration is taking place.
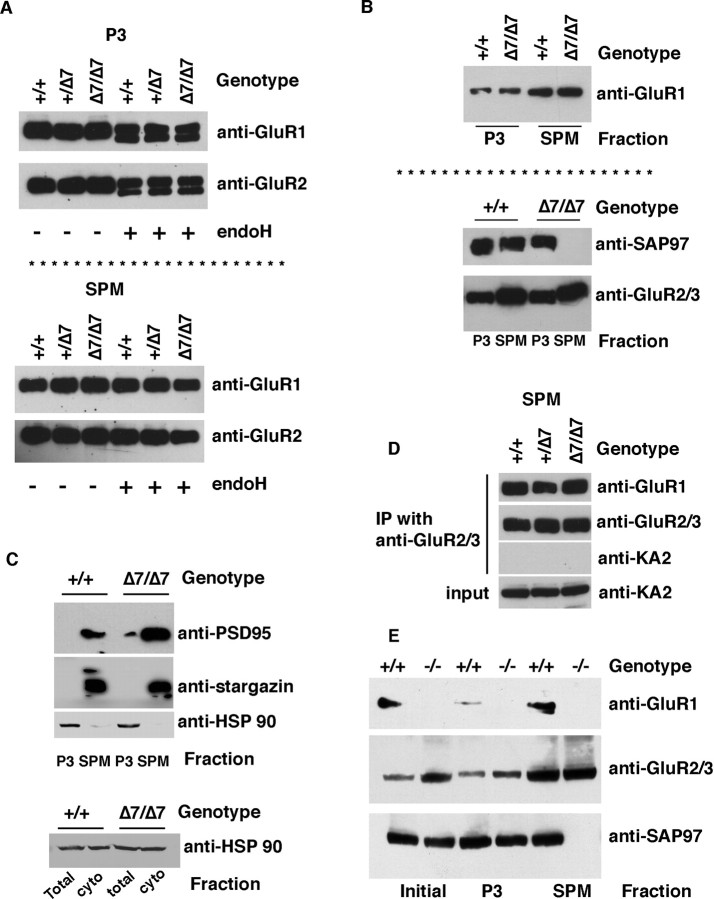
AMPAR subunits are glycoproteins, and previous work indicates that SAP97 associates with GluR1 and GluR2 in the endoplasmic reticulum-cis-Golgi (ER-CG), where they contain N-linked high mannose carbohydrates that are sensitive to endoglycosidase H (endoH) (Sans et al., 2001). If the association of SAP97 with GluR1 in the ER-CG was important for subunit trafficking and assembly into tetrameric AMPA receptors, then abrogation of the SAP97-GluR1 interaction would be expected to perturb GluR1 processing and maturation. To examine this issue, we fractionated P7 spinal cord lysates from animals to generate P3 (microsomes; a preparation enriched in ER and other intracellular membranes) and synaptosomes (SPM). The abundance of GluR1 in the P3 fraction is the same in WT and animals heterozygous (+/Δ7) or homozygous (Δ7/Δ7) for the GluR1Δ7 allele (Fig. 2 A). The same is true for the abundance of GluR2. Similarly, the abundance of GluR1 (and GluR2) in the SPM fraction is the same in WT and GluR1Δ7 lysates. Equivalent amounts of endoH-sensitive GluR1 (and GluR2) are seen in lysates from WT and GluR1Δ7 spinal cords, and as expected, there is no endoH-sensitive GluR1 or GluR2 in the SPM (Fig. 2 A). By this measure, posttranslational processing of GluR1Δ7 is normal, suggesting that its association with SAP97 is dispensable for this process.
Figure 2.
GluR1Δ7 traffics normally to the plasma membrane, where it properly oligomerizes with other AMPA receptor subunits, whereas SAP97 is not detectable in synaptic membranes of GluR1Δ7 mice. A, An EndoH-sensitive pool of GluR1 and other GluR subunits is found in the P3 fraction but not SPM fraction of P7 spinal cord lysates from WT animals and animals bearing the GluR1Δ7 allele (1 or 2 copies; +/Δ7 or Δ7/Δ7, respectively). B, Whereas GluR1, GluR1Δ7, and GluR2/3 are found in the P3 and, to a greater extent, SPM fractions regardless of genotype, SAP97 is not detected in the SPM fraction from the GluR1Δ7 mice. C, Two other components of the postsynaptic density, PSD-95 and stargazin, are enriched in the SPM fraction regardless of genotype. The cytosolic protein HSP90 was excluded from the SPM fraction and is a minor contaminant of the P3 fraction. HSP90 is equivalently abundant in the total lysate and the cytosolic fraction. D, Using SPM as the material source, immunoprecipitation with anti-GluR2/3 reveals that GluR1 is in association with AMPA receptor subunits GluR2 and/or GluR3 but not the kainate receptor subunit KA2. E, Lysates of P7 spinal cord from WT and GluR1 null mice were fractionated and subjected to Western blot analysis. No GluR1 is detected in any fraction from the null mice, whereas in the WT mouse, GluR1 is found in the initial lysate, SPM, and, to a lesser extent, the P3 fraction. GluR2/3 immunoreactivity is present in all three fractions of WT and null mice. SAP97 is found in all fractions of WT mice but not in the SPM fraction of the null mice. cyto, Cytosolic; IP, immunoprecipitation.
We next investigated the distribution and associations of GluR1 binding partners in fractionated spinal cord lysates from WT GluR1 and GluR1Δ7 mice (Fig. 2 B). In WT animals, SAP97 is found in both the P3 and SPM fractions, but in GluR1Δ7 lysates, SAP97 is only found in the P3 fraction. In contrast, regardless of genotype, GluR1 and GluR2/3 are found in the P3 and in greater abundance in the SPM fraction. Because SAP97 does not traffic to the SPM fraction in the GluR1Δ7, we wondered whether the subcellular distribution of other postsynaptic density proteins was affected. We found that PSD-95 and Stargazin (components of the postsynaptic density) were absent from the P3 fraction but abundantly present in SPM fractions in both WT and GluR1Δ7 (Fig. 2 C). A cytosolic protein, HSP90, was found in total spinal cord lysates and cytosol fractions but excluded from the SPM fraction. The P3 fraction is contaminated with a small amount of HSP90. Thus deletion of the C-terminal 7 aa of GluR1 prevents trafficking of SAP97 into synaptic membranes.
The presence of GluR1 and GluR2 in the SPM fraction of both WT and GluR1Δ7 mice suggests that AMPA receptors assemble normally in both strains of mice and populate synapses. In support of this, we find that GluR1 (WT or GluR1Δ7) can be immunoprecipitated using anti-GluR2 from the SPM fraction of WT and GluR1Δ7 mice (Fig. 2 D). This interaction was specific to the extent that GluR2 immunoprecipitates did not contain the kainate receptor subunit, KA2.
In summary, these experiments show that WT GluR1 and GluR1Δ7 are normally processed in ER-CG and assemble into heteromeric plasma membrane complexes. The major defect associated with the deletion of the C-terminal 7 aa of GluR1 is the failure to traffic SAP97 to the plasma membrane, whereas other postsynaptic density proteins are unaffected by the trafficking defect of SAP97.
The above results led to the prediction that SAP97 would not be found in the SPM of GluR1 null mice. To test this idea, we probed subfractions of spinal cord lysates from WT and GluR1 null mice for glutamate receptor subunits and SAP97 (Fig. 2 E). GluR1 was found in the total lysate, the P3 fraction, and SPM fraction of WT but not GluR1 null mice, as expected. GluR2 was found in all three fractions of both the WT and GluR1 null mice, and it was enriched in the SPM fraction. SAP97 was found in all three fractions in the WT animals but found only in the total and P3 fraction of the GluR1 null mice. Thus in the absence of GluR1, SAP97 is expressed at normal levels but does not partition into SPMs.
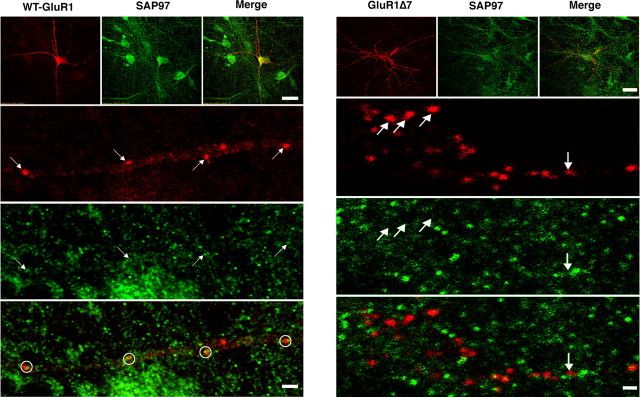
We complemented these biochemical experiments with immunolocalization studies of neurons in vitro. Dissociated embryonic spinal cord neurons were plated on confluent cortical astrocytes, transfected with expression plasmids for N-terminally myc-tagged versions of WT GluR1 or GluR1Δ7 plus SAP97 at DIV 5. On DIV 10, cell surface GluR1 was identified by live labeling with anti-myc antibody and after fixation, combined with SAP97 immunocytochemistry (Fig. 3). GluR1-positive puncta were seen on the cell body and dendrites of neurons expressing either WT GluR1 or GluR1Δ7. Immunostaining for SAP97 was also punctate, and there was frequent colocalization of WT GluR1 with SAP97. In contrast, in cultures expressing GluR1Δ7 and SAP97, these two proteins rarely colocalized. These findings suggest that clustered, cell surface WT GluR1 (presumably at synapses) is associated with SAP97. GluR1Δ7 forms cell surface clusters similar to WT GluR1, but SAP97 does not colocalize with them. These observations are in agreement with the biochemical fractionation studies in Figure 2. We conclude that SAP97 does not stably associate with synaptosomal membranes or putative synapses when it cannot physically associate with GluR1.
Figure 3.
Immunocytological localization of GluR1 or GluR1Δ7 with SAP97. Spinal cord cultures were transfected with expression vectors for N-terminally myc-tagged GluR1 or GluR1Δ7 and SAP97. Five days later, live labeling with anti-myc was performed to identify cell surface GluR1 or GluR1Δ7, and after fixation, this was combined with anti-SAP97 staining. Left, Representative laser confocal images (at low and high power) of the localization of WT GluR1 and SAP97. At low power (top triptych), puncta of cell surface GluR1 (red) decorate neurons, and SAP97 has a similar punctate distribution (green). Scale bar, 40 μm. At higher magnification (bottom triptych), accumulation of cell surface GluR1 into discrete puncta (arrows) along a dendrite can be seen. The SAP97 (green) images reveal numerous puncta of immunoreactivity, and some coregister with the GluR1 puncta (arrows). The bottom image (merge of GluR1 and SAP97 immunocytology) shows that many GluR1 puncta have close association with SAP97 (circles). Scale bar, 3 μm. Right, Representative laser confocal images (at low and high power) of the localization of GluR1Δ7 and SAP97. At low power (top triptych), puncta of cell surface GluR1 (red) decorate neurons, and SAP97 similarly has a punctate distribution (green). Scale bar, 40 μm. At higher magnification (lower triptych), accumulation of cell surface GluR1 into discrete puncta (arrows) along a dendrite can be seen. The SAP97 (green) images reveal numerous puncta of immunoreactivity, but in contrast with WT GluR1, there is no coregistration of the GluR1 puncta with the SAP97 puncta (arrows). The bottom image (merge of GluR1 and SAP97 immunocytology) shows that most GluR1 puncta are independent of SAP97 puncta. A single potential colocalization of GluR1Δ7 with SAP97 (merge; arrow) is different in appearance from colocalization of WT GluR1 and SAP97 and is likely to have occurred by chance. Scale bar, 3 μm.
The respective roles of GluR1 and SAP97 in dendrite morphogenesis were investigated in a mixed spinal cord neuron culture system. Spinal cord cultures were transfected with various expression vectors plus a GFP or LacZ reporter plasmid on DIV 5 and analyzed (after fixation) 4 d later. Coexpression of the proteins was confirmed by two-color immunocytology (data not shown). By Western blot analysis and immunocytochemistry, both GluR1 and SAP97 are endogenously expressed (data not shown) under these culture conditions.
We began by determining the effect of overexpressing WT GluR1 versus GluR1Δ7. In comparison with neurons expressing GFP alone, GluR1 led to an ∼100% increase in dendrite branches, whereas GluR1Δ7 had no effect on dendrite branching (F (2,102) = 36.33; p < 0.0001) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Overexpression of WT GluR1, but not GluR1Δ7, also led to a shortening of the longest dendrite, an observation we have reported previously (Inglis et al., 2002; Jeong et al., 2006). Neither WT nor GluR1Δ7 had any morphogenic effect on other dendrite parameters, such as number of primary dendrites, total dendritic tree size, or length of average dendrite.
Because of concerns that GluR1Δ7 might not traffic normally to the cell surface, we used two approaches to quantify the abundance of cell surface GluR1. First, neuronal cultures derived from WT and GluR1Δ7 mice were established and cell surface proteins were labeled with the cell-impermeable biotinylation reagent Sulfo-NHS-SS-biotin, isolated with avidin-coated beads, and immunoblotted with an anti-GluR1 antibody. We detected no differences in the abundance of total GluR1 or cell surface GluR1 in cultures from WT versus GluR1Δ7 mice (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Second, we used quantitative image analysis of cell surface GluR1 immunocytological signal acquired separately from the cell body and dendrites and then normalized to the signal of cotransfected GFP (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). WT GluR1- and GluR1Δ7-expressing cells had the same level of cell surface GluR1 immunoreactivity at the cell body (0.88 ± 0.09 vs 0.87 ± 0.08 arbitrary units; p = 0.62); this was also true when the comparison was made in dendrites (0.86 ± 0.12 vs 0.88 ± 0.14 arbitrary units; p = 0.57). These results provide evidence that both WT GluR1 and GluR1Δ7 are expressed at equivalent levels on the plasma membrane of neurons in vitro. The same conclusion was reached by Kim et al. (2005) in the original description of the GluR1Δ7: there were no differences in cell surface GluR1 between the WT and GluR1Δ7 mice using quantitative immunogold electron microscopy, or by electrophysiological characterization of basal spontaneous and evoked synaptic transmission. Thus the inability of GluR1Δ7 to induce changes in dendritic architecture is likely not tied to its abundance on the cell surface, but instead to its capacity to bring/maintain SAP97 at the cell surface.
Next we looked at the effects on dendrite morphology of expressing SAP97 or a truncated version of the protein that contains PDZ2 (through which it binds GluR1) and all other C-terminal sequences (SAP97C). In comparison with neurons expressing GFP alone, SAP97 led to a statistically significant 30% increase in dendrite branches (F (2,100) = 31.67; p < 0.0001) and cell body area (F (2,100) = 10.36; p = 0.001) but had no effects on the number of primary dendrites, total dendrites, average length of dendrites, or longest dendrite (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). SAP97C had an even more robust effect on dendrites. The number of branches more than doubled (26.9 vs 12.1; p < 0.001), and there were statistically significant increases in total dendritic tree size (F (2,100) = 11.46; p < 0.001) and length of average dendrite (F (2,100) = 3.34; p < 0.01) in comparison with GFP-expressing cells. The level of expression of full-length SAP97 and SAP97C appeared to be the same by Western blot or immunocytochemistry (data not shown). Thus, like WT GluR1, SAP97 stimulates dendrite branching (without changing overall tree size), and this effect requires sequences C-terminal to PDZ2. The more robust effect of SAP97C versus full-length SAP97 suggests that a dendrite growth inhibitory domain exists N-terminal to PDZ2. These observations contrast with the effects of a different postsynaptic density protein, PSD-95, which, when overexpressed in immature hippocampal neurons, inhibits dendrite branching (Charych et al., 2006).
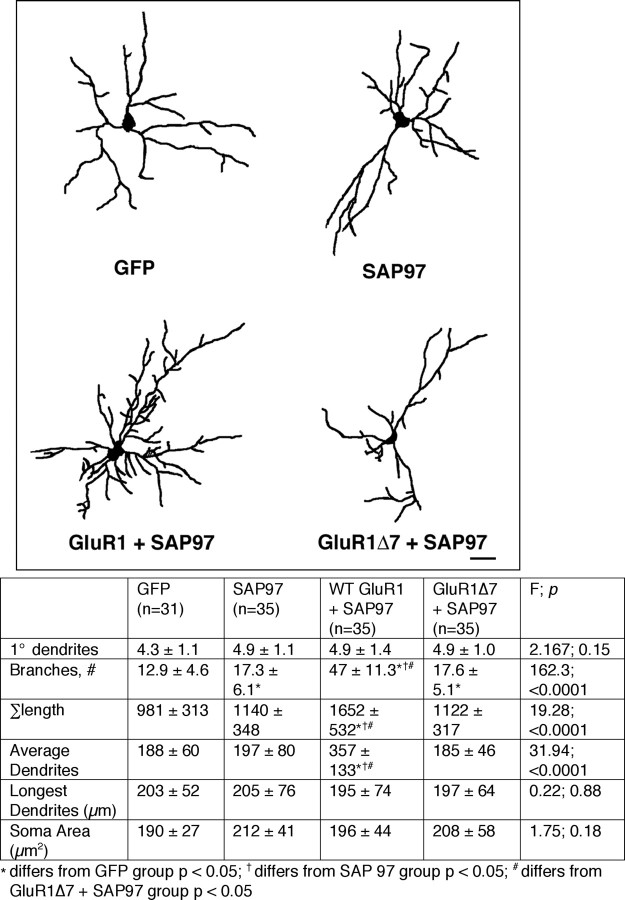
Because WT GluR1 and SAP97 physically interact and both promote growth, they may have an epistatic relationship. A number of approaches were undertaken to examine this possibility. First we compared the effects on dendrite morphology in four experimental groups: (1) GFP alone, (2) SAP97 alone, (3) WT GluR1 + SAP97, and (4) GluR1Δ7 + SAP97 (Fig. 4). As seen previously, SAP97 leads to an ∼30% increase in the number of branches in comparison with GFP alone (F (3,132) = 162.3; p < 0.0001). Coexpression of WT GluR1 + SAP97 leads to an even more robust effect on growth, with a fourfold increase in branches (compared with GFP alone) and a further increase in overall tree size (F (3,132) = 19.28; p < 0.001). Next, we looked at the effects on dendrite morphology of expressing GluR1Δ7 with SAP97. Coexpression of GluR1Δ7 with SAP97 added nothing to the ability of SAP97 alone to promote dendrite branching (p = 0.5) or any of the other measured features of dendrites. Thus the effects of WT GluR1 and SAP97 on dendrite branch elaboration are not merely additive, they are synergistic. Moreover, these multiplicative effects of GluR1 and SAP97 require the C-terminal 7 aa of GluR1 that enable the two proteins to physically interact.
Figure 4.
Overexpression of SAP97 with WT GluR1 (but not GluR1Δ7) leads to synergistic dendrite growth-promoting effects. Top, Representative camera lucida images of neurons expressing GFP alone, SAP97 alone, WT GluR1 + SAP97, and GluR1Δ7 + SAP97. Scale bar, 35 μm. Bottom, The chart provides quantitative analysis of dendrites as well as statistical analysis using ANOVA. The number of neurons drawn is noted in parentheses next to the column title. There is a statistically significant increase in branching when SAP97 is expressed in neurons compared with GFP-expressing neurons. Coexpression of WT GluR1 + SAP97 leads to a marked increase in dendrite branching as well as increase in overall arbor size and average length of dendrites. This growth-promoting effect was not seen when GluR1Δ7 was coexpressed with SAP97.
In hippocampal slice cultures, knockdown of PSD-95 results in a 50% reduction in EPSCs. This effect can be ameliorated by expression of SAP97, and this effect requires activity (Schlüter et al., 2006). In light of the activity dependence of SAP97 action on synapses, we wondered whether the dendrite growth-promoting effect of GluR1 + SAP97 was also activity dependent. To examine this issue, we administered the competitive AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; final concentration, 10 μm) to cultures every other day from DIV 5 when transfection occurred to DIV 10. We compared three experimental groups: (1) GFP alone, (2) WT GluR1 + SAP97; vehicle and (3) WT GluR1 + SAP97; CNQX. As observed before, coexpression of WT GluR1 + SAP97 led to a threefold increase in dendritic branches in comparison with the neurons expressing GFP alone (F (2,94) = 85.92; p < 0.0001) as well as an increase in overall tree size (F (2,94) = 17.41; p < 0.0001) and average dendrite length (F (2,94) = 5.07; p < 0.008) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). The robust dendrite growth-promoting action of WT GluR1 + SAP97 was completely eliminated by CNQX. In fact the only statistically significant difference between the GFP alone and the WT GluR1 + SAP97; CNQX-expressing neurons was an ∼15% decrease in soma area (F (2,94) = 5.53; p < 0.005). These results suggest that SAP97-mediated dendrite growth is initiated by the activity of GluR1-containing AMPARs. This finding is an interesting complement to the observation that the inhibitory effect of PSD-95 on immature dendrite growth occurs in an activity-independent manner (Charych et al., 2006).
The contribution of endogenously expressed SAP97 to GluR1-dependent dendrite growth was examined using a short hairpin RNA (shRNA) knockdown of SAP97. It is worth noting that neurons cultured from SAP97 mutant mice (lacking the SH3 and GK domains) develop normal synapses with normal levels of AMPARs (Klöcker et al., 2002). When expressed in HEK 293 cells along with SAP97, the active shRNA (but not a control, scrambled sequence shRNA) led to a pronounced reduction in the abundance of SAP97. The effect was specific for SAP97, because the active shRNA did not reduce the abundance of other synapse-associated proteins PSD-93, PSD-95, or SAP102 in cotransfection experiments (Fig. 5, top). Armed with this tool, we next analyzed the dendrites from four groups of transfected neurons: (1) GFP + scrambled (control) shRNA, (2) GFP + active shRNA, (3) WT GluR1 + scrambled shRNA, and (4) WT GluR1 + active shRNA. The active shRNA led to a statistically significant reduction in dendrite branches (F (3,138) = 30.44; p < 0.0001) and overall tree size (F (3,138) = 7.20; p < 0.001) in comparison with neurons expressing GFP + the inactive shRNA (Fig. 5, bottom). This implies that endogenously expressed SAP97 promotes dendrite growth. The expression of WT GluR1 with the scrambled shRNA led to increased dendrite branching and a reduction in the longest dendrite, as seen previously (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Remarkably, the growth-promoting effect of WT GluR1 on dendrites was completely eliminated when it was expressed with the active shRNA, and there was no statistical difference between the dendritic trees when WT GluR1 + active shRNA neurons were compared with GFP + inactive shRNA neurons (Fig. 5, bottom). Thus, SAP97 is necessary for the dendrite growth-promoting effects of endogenous GluR1 containing AMPAR as well as when WT GluR1 is overexpressed.
Figure 5.
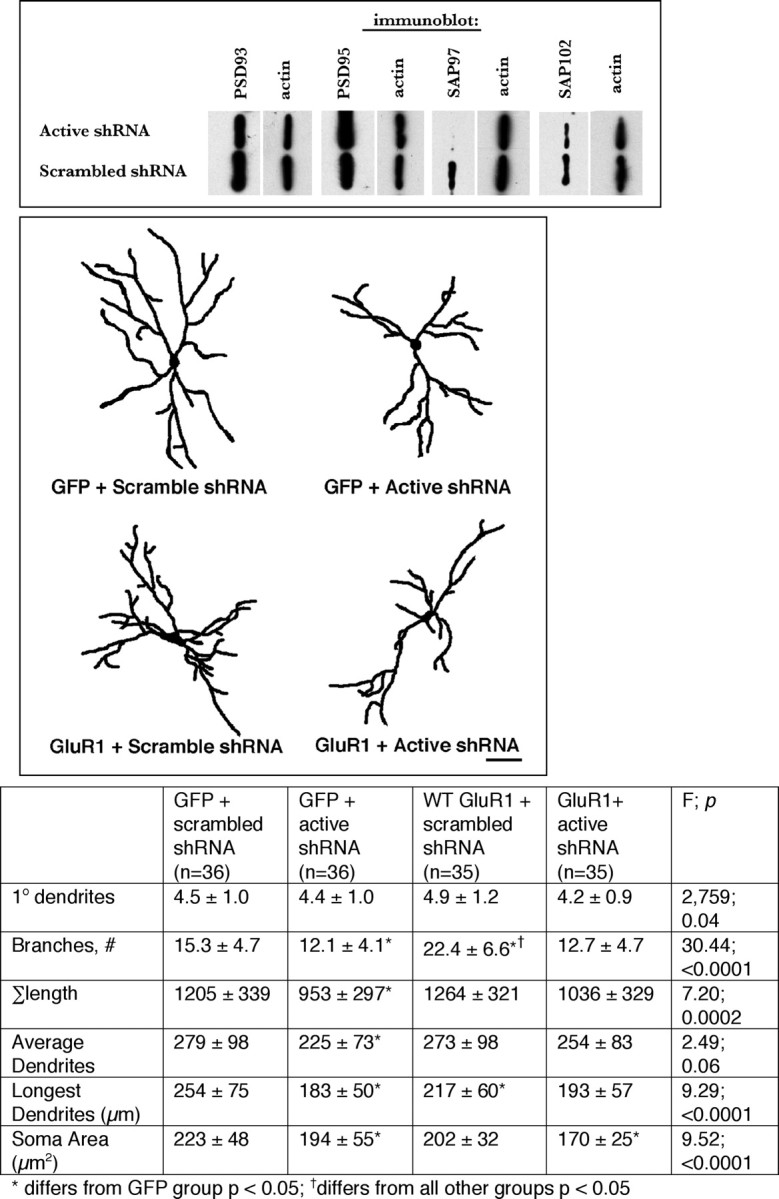
Selective knockdown of SAP97 causes dendritic tree atrophy and prevents GluR1-dependent dendrite growth. Top, Western blots from HEK 293 cells transfected with expression vectors for PSD-93, PSD-95, SAP97, or SAP102 and either the active shRNA to SAP97 or a scrambled sequence shRNA. The active shRNA led to the selective knockdown of SAP97 and did not affect the expression of other members of the postsynaptic density family of proteins. Middle, Representative camera lucida images of neurons expressing GFP + scrambled shRNA, GFP + active shRNA, WT GluR1 + scrambled shRNA, or WT GluR1 + active shRNA. Scale bar, 40 μm. Bottom, The chart provides quantitative analysis of dendrites as well as statistical analysis using ANOVA. The number of neurons drawn is noted in parentheses next to the column title. There is a statistically significant decrease in branching, overall arbor, and average dendrite size in the GFP + active shRNA-expressing neurons in comparison with neurons expressing GFP + scrambled shRNA. There was a statistically significant increase in dendrite branching in neurons expressing WT GluR1 + scrambled shRNA in comparison with neurons expressing GFP + scrambled shRNA as seen previously. This growth-promoting effect was eliminated when WT GluR1 was coexpressed with the active shRNA. There were no statistically significant differences in the dendritic tree of neurons expressing GFP + active shRNA versus WT GluR1 + active shRNA.
In the absence of unambiguous evidence that SAP97 functions to traffic GluR1 to the cell surface, we examined the converse: the role of GluR1 is to guide SAP97 to the cell surface. To study this, we introduced the palmitoylation sequence from paralemmin (DLDMKKHRCKCCSIM) onto C terminus of the β-isoform of SAP97. When HEK 293 cells were transfected with this construct, lysed in the absence of detergent, fractionated into membrane/soluble pools, and subjected to Western blot analysis, the majority of palmitoylated SAP97 was found in the membrane fraction, and little is detected in the soluble fraction [supplemental Fig. 4 (top), available at www.jneurosci.org as supplemental material]. In this assay system, unpalmitoylated SAP97 is predominantly cytosolic. These results confirm that modified versions of SAP97 constitutively associate with membranes as expected, and therefore this construct was used in all subsequent experiments.
We next analyzed the dendrites from three groups of transfected neurons: (1) GFP alone, (2) palmitoylated SAP97, and (3) WT SAP97. Both palmitoylated SAP97 and WT SAP97 led to an ∼30% increase in dendrite branches (F (2,102) = 10.48; p < 0.0001) in comparison with GFP alone, and post hoc analysis revealed no statistical difference between palmitoylated SAP97 and WT SAP97 (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). No other measured dendritic parameter differed between the three experimental groups. Thus, both versions of SAP97 behave (in terms on dendrite morphogenesis) equivalently.
Next we asked whether membrane-targeted SAP97 rescued the dendrite growth-promoting defect associated with GluR1Δ7. To this end, we compared four groups of transfected neurons: (1) GFP alone, (2) palmitoylated SAP97 alone, (3) WT GluR1 + palmitoylated SAP97, and (4) GluR1Δ7 + palmitoylated SAP97. Palmitoylated SAP97 leads to an ∼30% increase in dendrite branching in comparison with GFP expression only (F (3,136) = 42.41; p < 0.0001) (Fig. 6). Coexpression of WT GluR1 + palmitoylated SAP97 led to a further, statistically significant increase in dendrite branching and overall growth of the dendritic tree (F (3,136) = 14.32; p < 0.0001). These results phenocopy what we saw with WT SAP97 + WT GluR1 (Fig. 4; supplemental Fig. 3, available at www.jneurosci.org as supplemental material) and confirm that addition of palmitate to SAP97 does not result in loss of its biological activity. Coexpression of GluR1Δ7 + palmitoylated SAP97 also led to a statistically significant increase in branching and an increase in overall dendritic tree growth in comparison with palmitoylated SAP97 alone or GFP. There were no differences in dendrite morphology of neurons expressing WT GluR1 + palmitoylated SAP97 versus GluR1Δ7 + palmitoylated SAP97. This is noteworthy because it markedly contrasts with the effects of coexpressing GluR1Δ7 + WT SAP97 (Fig. 4), namely, that GluR1Δ7 had no growth-promoting action. These findings provide evidence that only when GluR1Δ7 and SAP97 are both associated with membranes do their multiplicative effects on dendrite branching become evident.
Figure 6.
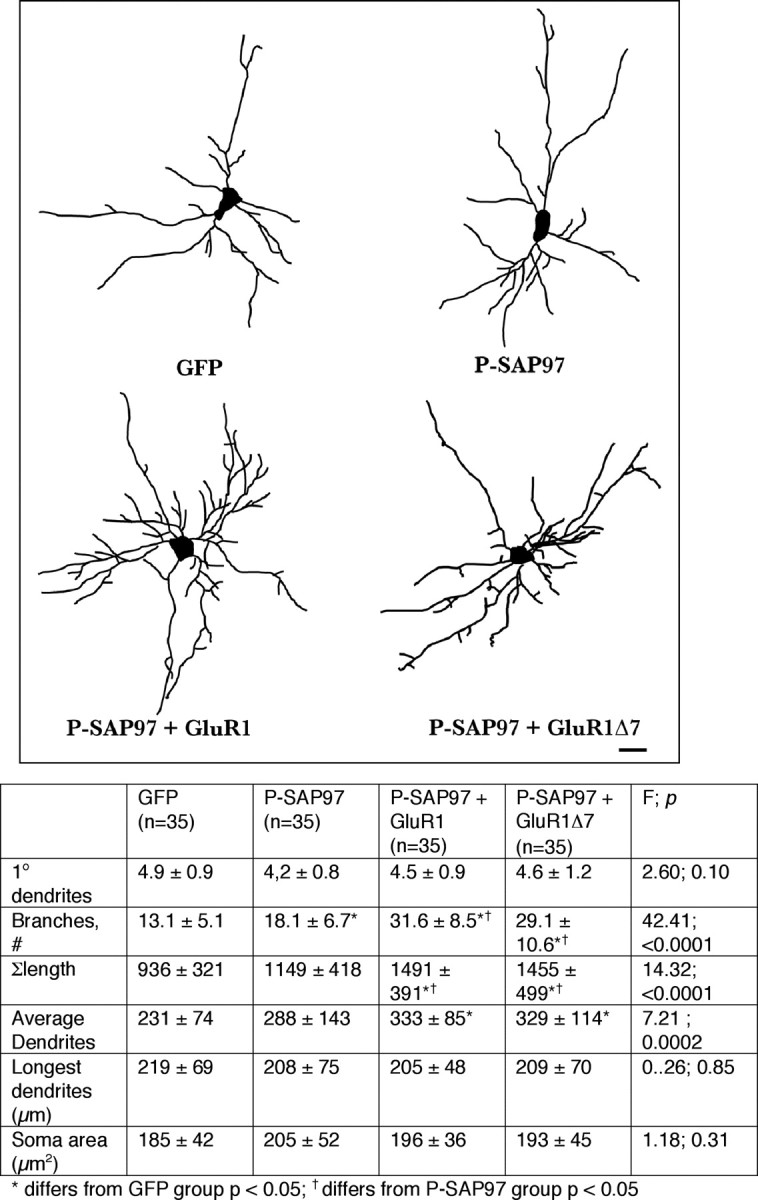
Synergistic dendrite growth when P-SAP97 is coexpressed with WT GluR1 or GluR1Δ7. Top, Representative camera lucida images of neurons expressing GFP alone, P-SAP97 alone, WT GluR1 + P-SAP97, or GluR1Δ7 + P-SAP97. Scale bar, 22 μm. Bottom, The chart provides quantitative analysis of dendrites as well as statistical analysis using ANOVA. The number of neurons drawn is noted in parentheses next to the column title. There is a statistically significant increase in branching when P-SAP97 is expressed in neurons compared with GFP-expressing neurons. Coexpression of P-SAP97 + WT GluR1 leads to a synergistic increase in branching as well as overall arbor and average dendrite growth. In contrast with observations in Figure 4, coexpression of P-SAP97 + GluR1Δ7 also leads to a synergistic increase in branching as well as overall arbor and average dendrite growth. There are no statistically significant differences between the dendritic arbors of P-SAP97 + WT GluR1- and P-SAP97 + GluR1Δ7-expressing neurons.
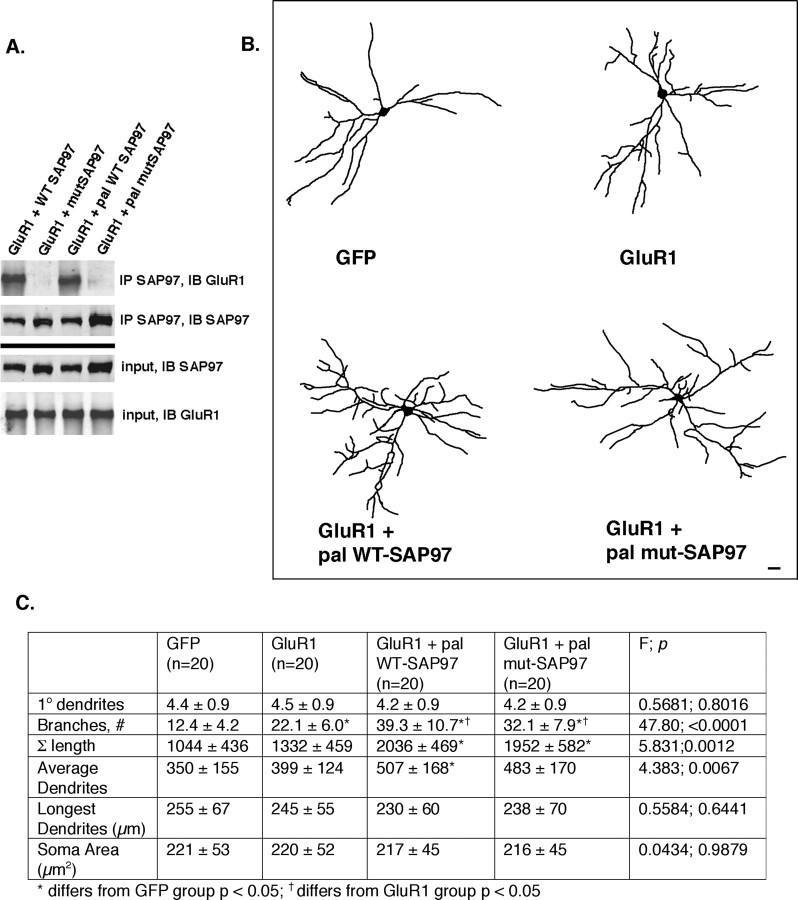
The observation that expressing palmitoylated SAP97 with GluR1Δ7 rescues the dendrite growth-promoting phenotype implies that a physical interaction between these proteins is not required for their actions. One way to test this idea is to disrupt the physical interaction of WT GluR1 with SAP97. To this end, we engineered mutations in the second PDZ domain of SAP97 that are predicted to affect the interaction of the GluR1 C terminus with this domain. Structural studies indicate that the ligand-binding site of PDZ domains are highly conserved and form a hydrophobic pocket that contains a positively charged residue (arginine or lysine) critical for the ligand binding (Doyle et al., 1996; Morais Cabral et al., 1996; Xia et al., 1999). We identified this region within PDZ2 of SAP97 and generated a GluR1 binding-mutant form of SAP97 (designated “mutant SAP97”) that contains the amino acid substitutions K323A and K326A. We cotransfected this construct or WT SAP97 into HEK 293 cells along with WT GluR1 and immunoprecipitated lysates with anti-SAP97. Mutant SAP97 did not bring down WT GluR1, whereas WT SAP97 robustly immunoprecipitated WT GluR1 (Fig. 7 A). Both WT and mutant SAP97 expressed at equivalent levels in transfected cells. We next generated a palmitoylated version of mutant SAP97, so that SAP97 would traffic to the plasma membrane (supplemental Fig. 4, available at www.jneurosci.org as supplemental material) but not physically associate with WT GluR1. We cotransfected this palmitoylated mutant SAP97 or WT SAP97 into HEK 293 cells along with WT GluR1 and immunoprecipitated lysates with anti-SAP97. The palmitoylated version of mutant SAP97 did not bring down WT GluR1, whereas WT SAP97 robustly immunoprecipitated WT GluR1 (Fig. 7 A). Both WT and palmitoylated mutant SAP97 expressed at equivalent levels in transfected cells. These experiments demonstrate that neither the palmitoylated nor the nonpalmitoylated version of mutated SAP97 can bind to WT GluR1.
Figure 7.
Mutations in PDZ2 of SAP97 disrupt its interaction with GluR1 but do not prevent the synergistic effects on growth if mutant SAP97 is targeted to the plasma membrane. A, Western blots of HEK 293 cells cotransfected with expression vectors for wild-type GluR1 + one of the following constructs: WT SAP97, mutant SAP97 (mutSAP97), palmitoylated (pal) wild-type SAP97, or palmitoylated mutant SAP97. Immunoprecipitation (IP) using anti-SAP97 and immunoblotting (IB) for GluR1 reveal that only WT SAP97 and palmitoylated SAP97 physically associate with GluR1. IP for SAP97 and IB for SAP97 demonstrate that all constructs immunoprecipitate equivalently and the input controls of all constructs express equivalently. B, Representative camera lucida images of neurons expressing GFP alone, GluR1 alone, GluR1 + palmitoylated WT SAP97, or GluR1 + palmitoylated mutant SAP97. Scale bar, 25 μm. C, The chart provides quantitative analysis of dendrites as well as statistical analysis using ANOVA. The number of neurons drawn is noted in parentheses next to the column title. GluR1 leads to enhancement of dendrite branching and overall tree growth, in comparison with neurons expressing GFP alone. Both palmitoylated WT SAP97 and palmitoylated mutant SAP97, when coexpressed with GluR1, cause synergistic dendrite growth. There are no statistically significant differences between the GluR1 + palmitoylated WT SAP97 and GluR1 + palmitoylated mutant SAP97.
Armed with these tools, we undertook two sets of comparisons. First, we asked whether WT or palmitoylated mutant SAP97 can synergistically promote GluR1-dependent dendrite growth (Fig. 7). To this end, we compared four groups of transfected neurons: (1) GFP alone, (2) WT GluR1, (3) WT GluR1 + palmitoylated wild-type SAP97, and (4) WT GluR1 + palmitoylated mutant SAP97. Expression of GluR1 leads to ∼80% increase in dendrite branching in comparison with GFP expression alone (F (3,76) = 47.80; p < 0.0001). Coexpression of palmitoylated wild-type SAP97 led to a further statistically significant increase in dendrite branching and overall growth of the dendritic tree (F (3,76) = 5.831; p = 0.0012). These results are entirely consonant with our observations in supplemental Figure 4 (available at www.jneurosci.org as supplemental material) and Figure 6. Interestingly, coexpression of palmitoylated mutant SAP97 is also capable of synergistically promoting dendrite branching when coexpressed with wild-type GluR1. There were no statistically significant differences between the experimental group 3 (WT GluR1 + palmitoylated wild-type SAP97) and group 4 (GluR1 + palmitoylated mutant SAP97) in terms of dendrite branching or overall tree size. These results provide further evidence that the promotion of dendrite growth by GluR1 and SAP97 is dependent on colocalization to the plasma membrane, but does not require the two proteins to physically interact.
To formally test the requirement for colocalization to the plasma membrane, we performed a final comparison, namely, the effects of mutant SAP97, with or without the palmitoylation sequence, on GluR1-dependent growth (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). To this end, we compared four groups of transfected neurons: (1) GFP alone, (2) WT GluR1, (3) WT GluR1 + wild-type SAP97, and (4) GluR1 + mutant SAP97. As seen above, expression of GluR1 causes a significant increase in branching (in comparison with GFP-expressing neurons), and this is further enhanced by coexpression of WT SAP97 (F (3,76) = 57.51; p < 0.001). Similarly, GluR1 causes a significant increase in overall tree size, and this is further enhanced by coexpression of WT SAP97 (F (3,76) = 23.44; p < 0.001). Substitution of mutant SAP97 for WT SAP97 eliminated the synergistic effect, and the dendrites of neurons expressing GluR1 + mutant SAP97 were not statistically significantly different from the dendrites of neurons expressing GluR1 alone. Thus, the ability of WT GluR1 to physically interact with SAP97 is not strictly required for their synergistic, dendrite branch-promoting action. This is apparently only true if SAP97 is colocalized with GluR1 at the plasma membrane. This conclusion holds if the physical interaction is disrupted by mutation of GluR1 (e.g., GluR1Δ7) or mutating SAP97 such that it cannot bind WT GluR1. Because the biological effects of palmitoylated mutant SAP97 differ from palmitoylated SAP97 [compare group 4 from Fig. 7 with supplemental Fig. 5 (available at www.jneurosci.org as supplemental material)], the mere introduction of the palmitoylation sequence to the β-isoform of SAP97 itself [as suggested by Gauthier-Campbell et al. (2004)] cannot explain our observations.
Finally, we studied motor neuron dendritic tree architecture in two different strains of mice to complement the in vitro anatomical observations. We began by characterizing the motor neuron dendrite tree from WT and GluR1Δ7 mice. Application of DiI to the ventral roots of the lumbar spinal cord of P25 perfusion fixed tissue leads to extensive definition of motor neuron dendrites (Inglis et al., 2000). GluR1Δ7 motor neurons had an ∼25% reduction in branches (Student's t test, p = 0.0003), shortening of the longest dendrite (p = 0.04), and reduction in overall tree size (p = 0.0009) in comparison with WT (Fig. 8, top). Other measured dendritic parameters did not differ between groups. These results are similar to previous characterizations of the motor neuron dendrites from GluR1 knock-out animals (Zhang et al., 2005). Because the major defect associated with replacement of WT GluR1 with GluR1Δ7 is a failure of SAP97 to traffic to synaptic membranes, these in vivo results suggest that the GluR1–SAP97 partnership is required for proper dendrite elaboration.
Figure 8.
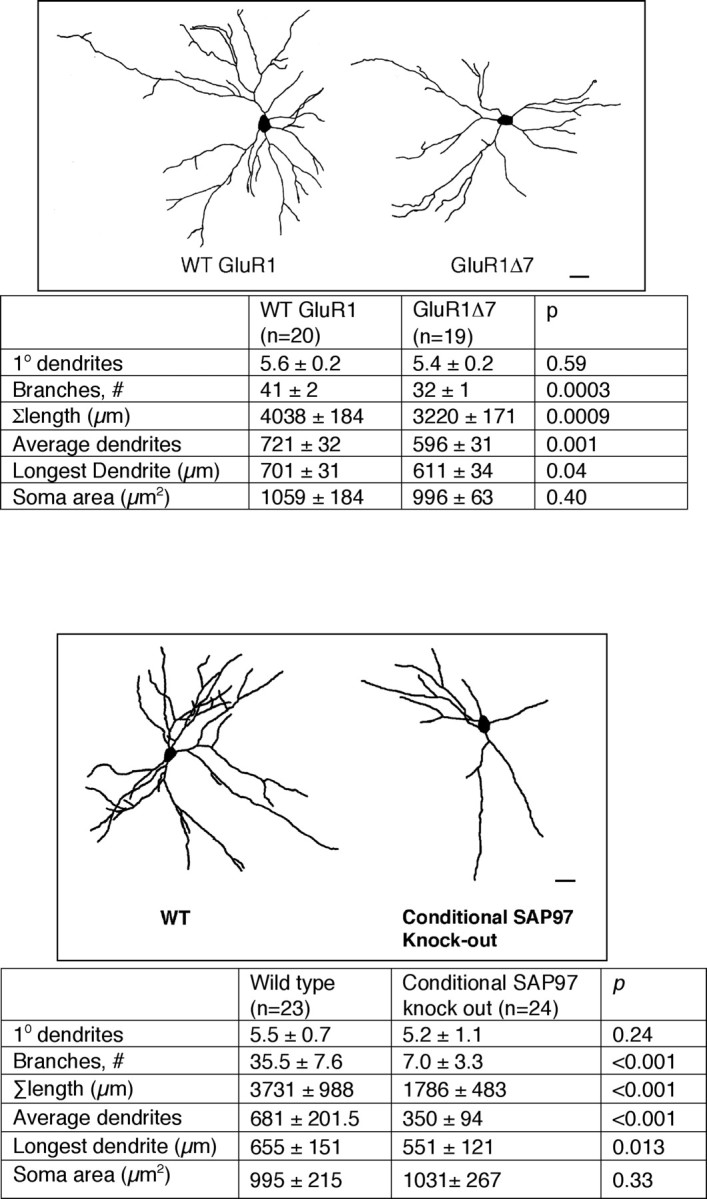
Comparison of motor neuron dendrites of WT mice versus homozygous GluR1Δ7 knock-in mice and versus conditional SAP97 knock-out mice. Top, Representative camera lucida images of motor neurons from WT mice and GluR1Δ7 knock-in mice. Scale bar, 22 μm. The chart below provides quantitative analysis of dendrites as well as statistical analysis using Student's t test. The number of neurons drawn is noted in parentheses next to the column title. In comparison with the WT animals, the motor neuron dendrites from GluR1Δ7 knock-in mice had fewer branches and a reduction in the overall arbor, the average dendrite length and the longest dendrite were also shortened. Bottom, Representative camera lucida images of motor neurons from WT mice and conditional SAP97 knock-out mice. Scale bar, 30 μm. The chart below provides quantitative analysis of dendrites as well as statistical analysis using Student's t test. The number of neurons drawn is noted in parentheses next to the column title. In comparison with the WT animals, the motor neuron dendrites from conditional SAP97 mice had fewer branches, smaller overall arbor size, reduction in average dendrite length, and shortening of the longest dendrite.
If SAP97 is the major binding partner of the extreme C terminus of GluR1, then elimination of SAP97 should phenocopy the effects of GluR1Δ7 on dendrite development. Unfortunately the SAP97 null mice were not suitable for our studies, because they die in the neonatal period as a result of craniofacial abnormalities (Caruana and Bernstein, 2001). To overcome this problem, we used the loxP-Cre system to conditionally knock out SAP97 in motor neurons.
A targeting vector with loxP sites flanking the exon encoding part of PDZ1 and PDZ2 was used to generate a floxed allele of SAP97 (SAP97flox), and a Southern blot confirmed the generation of the appropriate allele (Fig. 9 A,B). To demonstrate that the Cre recombinase could delete this allele in vivo, SAP97flox mice were bred to the ubiquitously expressing Cre transgenic mouse (Nagy et al., 1998). SAP97LoxP/LoxP:Cre mice had the same craniofacial abnormalities and early mortality as the null mice, and Western blots of brain tissue from these mice was devoid of SAP97 immunoreactivity (Fig. 9 C). Thus, the floxed allele of SAP97 was capable of undergoing recombination and deletion in vivo.
To knock out SAP97 in motor neurons, we bred the SAP97flox mice to the Hb9-Cre mice, in which Cre recombinase is expressed in motor neurons. The appropriate F1 animals were crossed to generate motor neuron-specific deletion of SAP97. The mice were born at Mendelian frequencies and appeared grossly normal. Immunohistological staining of spinal cords from these animals confirmed the loss of SAP97 in the ventral horn motor neurons (Fig. 9 D). To determine whether SAP97 expression was lost in motor neurons, tissue slices from these animals were double stained for SAP97 and SMI32 (a marker for motor neurons in the ventral horn). Whereas SAP97 and SMI32 colocalized in the wild-type mice, all SMI32+ motor neurons were devoid of SAP97 immunoreactivity in the conditional knock-out mice (Fig. 9 E).
We next analyzed motor neuron dendrites from P23 conditional knock-out mice versus wild-type mice, and we found large statistically significant differences between groups. The conditional knock-out mice had an ∼80% reduction of dendrite branches, an ∼50% reduction in overall tree size, an ∼50% reduction in average dendrite length, and an ∼15% reduction in the length of the longest dendrite (Fig. 8, bottom). Although not excluding the possibility that other proteins associate with GluR1 via its C terminus, the present observations indicate that SAP97 is likely to be the major binding partner that promotes dendrite growth in vivo.
Discussion
Excitatory neurotransmission during prenatal and early postnatal life sculpts neuronal architecture and patterned interneuronal connectivity (Shatz, 1990). Much of our mechanistic understanding of these processes has come from investigation of the events that follow activation of the NMDA subtype of glutamate receptors (Constantine-Paton et al., 1990). Less is known about the mechanism(s) by which activation of AMPA receptors (exclusive of their role in NMDA receptor-dependent development) impress their effects on neuronal process growth, connectivity, and circuit function. Both of these forms of activity-dependent development depend on agonist-evoked rises in intracellular calcium, but the difference in subunit composition of NMDA versus AMPA receptors (and their intracellular binding partners) raises the possibility that distinct molecular machineries are used. The present results indicate that AMPA receptors containing GluR1 promote dendrite growth of spinal cord neurons in a manner that depends on localization of SAP97 to the plasma membrane. Although under normal circumstances the C-terminal 7 aa of GluR1 drives SAP97 membrane localization, the physical interaction between GluR1 and SAP97 is not required for the collaboration of these agents in promoting dendrite growth. Thus, whereas much work emphasizes the role of postsynaptic density proteins on glutamate receptor localization, the present findings turn this relationship around by showing one function of GluR1 is to target SAP97 to synaptic membranes. Here it acts as an effector of GluR1-containing AMPA receptors in the promotion of activity-dependent dendrite growth.
SAP97 appears to control the gain of GluR1-dependent dendrite growth. In the presence of endogenous levels of GluR1, overexpressing SAP97 moderately promotes dendrite growth, whereas overexpressing both SAP97 and GluR1 robustly increases dendrite growth in a synergistic manner. This suggests that the degree of dendrite growth is a function of the abundance (and/or ratio) of GluR1 to SAP97. Further evidence in support of this view comes from the small interfering RNA knockdown experiments: reducing SAP97 (or its ratio to GluR1) blocks GluR1-dependent dendrite growth. Indeed, these experiments indicate that SAP97 plays an obligatory role in GluR1-dependent dendrite growth. The defects in motor neuron dendrites in the conditional SAP97 knock-out mice provide strong support for this view on the role for SAP97. If abundance of GluR1 and SAP97 (or their ability to associate) was regulated at a local level, it could provide a means for spatial control of dendrite growth. In this regard, it is noteworthy that the mRNA for GluR1 is localized in granules juxtaposed to synapses in dendrites and the local translation of this message is controlled in an activity-dependent manner (Ju et al., 2004; Grooms et al., 2006).
The means by which SAP97 controls GluR1-dependent dendrite growth is likely to be tied to the assembly of a multiprotein complex at the plasma membrane. One mechanistic clue comes from our observation that SAP97 does not need to physically bind GluR1 to regulate dendrite growth; both proteins only need to be colocalized to a common membrane. Based on our biochemical characterization that under normal circumstances GluR1 and SAP97 reside in synaptosomal preparations, it would be parsimonious to anticipate that the synaptic (or juxtasynaptic) membrane is the site of relevant interaction. To account for these observations, we postulate the existence of a locally produced and diffusible messenger that signals from GluR1-containing AMPA receptors to SAP97. The chemical nature of this signal and how it acts on SAP97 to promote dendrite growth remain to be established. In this light, it is noteworthy that synaptic activity can govern the functional effects of SAP97 in a calcium/calmodulin-dependent protein kinase II-dependent manner (Schlüter et al., 2006).
The L27-containing β-isoform of SAP97 is the only version of the protein we find endogenously present in the spinal cord in vitro and in vivo. Several previous investigators have shown that simply overexpressing this protein in neurons has no effect on AMPAR EPSCs (Schnell et al., 2002; Ehrlich and Malinow, 2004; Schlüter et al., 2006) [but see Rumbaugh et al. (2003) and Nakagawa et al. (2004)]. However, if the expression of PSD-95 is reduced on a time scale of days, AMPAR EPSCs are reduced, and overexpression of SAP97 will restore normal synaptic efficacy. The ability of SAP97 to effect this change is activity dependent and only occurs with the β-isoform of SAP97 (Schlüter et al., 2006). The L27 domain mediates homo-oligomerization as well as binding to other L27-containing proteins such as CASK (Lee et al., 2002). These observations complement our observations that the effects of SAP97 and GluR1 we see on neuron dendrites are activity dependent and that CASK is part of a GluR1–SAP97 complex in spinal cord neurons. Whether SAP97 multimerization and/or its ability to bring CASK (or other specific binding partners) to the cell surface are required to promote dendrite growth remains to be explored.
Although most intensively studied in the neocortex, experience-dependent development of neural circuitry occurs throughout the neuraxis, including the spinal cord (Bodnarenko and Chalupa, 1993; McAllister et al., 1995, 1997; Rajan and Cline, 1998). During a critical period in the first few weeks of postnatal life, rearing environment sculpts locomotor behavior, and this has long-term effects on motor function (Walton et al., 2005a,b). Part of the anatomical substrate for this phenomenon is reflected in the experience-dependent elaboration of motor neuron dendrites (Inglis et al., 2000), and this, in turn, is controlled by the AMPA receptor phenotype (Inglis et al., 2002). Under normal circumstances, young motor neurons express calcium-permeable AMPA receptors enriched in GluR1 (Jakowec et al., 1995a; Carriedo et al., 1996; Bar-Peled et al., 1999; Vandenberghe et al., 2000a,b), and there is abundant evidence that GluR1 is dendrite-growth promoting. Here we have begun to identify the molecular machinery by which GluR1-containing AMPA receptors effect changes in neuronal architecture. GluR1 brings SAP97 to synaptic membranes, where it receives progrowth (likely diffusible) signal(s) from active receptors. Future work will aim to define the nature of the signal(s) as well as the downstream effectors of SAP97 action.
Footnotes
This work was supported by Public Health Services Grants NS 29837 and 52325 (R.K.) and the Howard Hughes Medical Institute (R.H.). This project was funded, in part, under a grant from the Pennsylvania Department of Health. The Department specifically disclaims responsibility for analyses, interpretation, or conclusions. We thank Natalie Sans and Robert Wenthold for help, early in the course of these experiments, with the SAP97–GluR1 coimmunoprecipitations; Susumu Tomita for the gift of the anti-stargazin antibody; and Zenta Walther for the anti-CASK antibody. Drs. Rolf Sprengel and Peter Seeburg generously provided the GluR-A knock-out mice. Dr. Maria Rubio (University of Connecticut, Storrs, CT) generously provided the photomicrograph in Figure 1 F.
References
- Bar-Peled O, O'Brien RJ, Morrison JH, Rothstein JD. Cultured motor neurons possess calcium-permeable AMPA/kainate receptors. Neuroreport. 1999;10:855–859. doi: 10.1097/00001756-199903170-00034. [DOI] [PubMed] [Google Scholar]
- Blackstone CD, Levey AI, Martin LJ, Price DL, Huganir RL. Immunological detection of glutamate receptor subtypes in human central nervous system. Ann Neurol. 1992;31:680–683. doi: 10.1002/ana.410310620. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Boehm J, Ehrlich I, Hsieh H, Malinow R. Two mutations preventing PDZ-protein interactions of GluR1 have opposite effects on synaptic plasticity. Learn Mem. 2006;13:562–565. doi: 10.1101/lm.253506. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Cai C, Coleman SK, Niemi K, Keinänen K. Selective binding of synapse-associated protein 97 to GluR-A alpha-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. J Biol Chem. 2002;277:31484–31490. doi: 10.1074/jbc.M204354200. [DOI] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/KA receptor-mediated injury in vitro . J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol. 2001;21:1475–1483. doi: 10.1128/MCB.21.5.1475-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Akum BF, Goldberg JS, Jörnsten RJ, Rongo C, Zheng JQ, Firestein BL. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J Neurosci. 2006;26:10164–10176. doi: 10.1523/JNEUROSCI.2379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- David S, Kalb RG. Serum/glucocorticoid-inducible kinase can phosphorylate the cyclic AMP response element binding protein, CREB. FEBS Lett. 2005;579:1534–1538. doi: 10.1016/j.febslet.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gauthier-Campbell C, Bredt DS, Murphy TH, El-Husseini AE-D. Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs. Mol Biol Cell. 2004;15:2205–2217. doi: 10.1091/mbc.E03-07-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26:8339–8351. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd JW, Jones LR, Mahler HR, Moore WJ. Isolation and partial characterization of rat brain synaptic plasma membranes. J Neurochem. 1974;22:281–290. doi: 10.1111/j.1471-4159.1974.tb11591.x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Furia F, Zuckerman KE, Strittmatter SM, Kalb RG. The role of nitric oxide and NMDA receptors in the development of motor neuron dendrites. J Neurosci. 1998;18:10493–10501. doi: 10.1523/JNEUROSCI.18-24-10493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Zuckerman KE, Kalb RG. Experience-dependent development of spinal motor neurons. Neuron. 2000;26:299–305. doi: 10.1016/s0896-6273(00)81164-2. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Crockett R, Korada S, Abraham WC, Hollmann M, Kalb RG. The AMPA receptor GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22:8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience. 1995a;67:893–907. doi: 10.1016/0306-4522(95)00026-f. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Yen L, Kalb RG. In situ hybridization analysis of AMPA receptor subunit gene expression in the developing rat spinal cord. Neuroscience. 1995b;67:909–920. doi: 10.1016/0306-4522(95)00094-y. [DOI] [PubMed] [Google Scholar]
- Jeong GB, Werner M, Gazula VR, Itoh T, Roberts M, David S, Pfister B, Cohen A, Neve RL, Hollmann M, Kalb R. Bi-directional control of motor neuron dendrite remodeling by the calcium permeability of AMPA receptors. Mol Cell Neurosci. 2006;32:299–314. doi: 10.1016/j.mcn.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Kim CH, Takamiya K, Petralia RS, Sattler R, Yu S, Zhou W, Kalb R, Wenthold R, Huganir R. Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nat Neurosci. 2005;8:985–987. doi: 10.1038/nn1432. [DOI] [PubMed] [Google Scholar]
- Klöcker N, Bunn RC, Schnell E, Caruana G, Bernstein A, Nicoll RA, Bredt DS. Synaptic glutamate receptor clustering in mice lacking the SH3 and GK domains of SAP97. Eur J Neurosci. 2002;16:1517–1522. doi: 10.1046/j.1460-9568.2002.02228.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Fan S, Makarova O, Straight S, Margolis B. A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol. 2002;22:1778–1791. doi: 10.1128/MCB.22.6.1778-1791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendrite growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais Cabral JH, Petosa C, Sutcliffe MJ, Raza S, Byron O, Poy F, Marfatia SM, Chishti AH, Liddington RC. Crystal structure of a PDZ domain. Nature. 1996;382:649–652. doi: 10.1038/382649a0. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Moens C, Ivanyi E, Pawling J, Gertsenstein M, Hadjantonakis AK, Pirity M, Rossant J. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr Biol. 1998;8:661–664. doi: 10.1016/s0960-9822(98)70254-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo . J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Robberecht W, Brorson JR. AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability. J Neurosci. 2000a;20:123–132. doi: 10.1523/JNEUROSCI.20-01-00123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Ihle EC, Patneau DK, Robberecht W, Brorson JR. AMPA receptor current density, not desensitization, predicts selective motoneuron vulnerability. J Neurosci. 2000b;20:7158–7166. doi: 10.1523/JNEUROSCI.20-19-07158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Harding S, Anschel D, Harris YT, Llinás R. The effects of microgravity on the development of surface righting in rats. J Physiol. 2005a;565:593–608. doi: 10.1113/jphysiol.2004.074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Benavides L, Singh N, Hatoum N. Long-term effects of microgravity on the swimming behaviour of young rats. J Physiol. 2005b;565:609–626. doi: 10.1113/jphysiol.2004.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kalb JA, Xiong G, Tennyson EE, Perez CA, Kalb RG. The role of GluR1 during the development of the spinal motor neurons: synaptic connectivity, dendritic morphology, and locomotor function. Soc Neurosci Abstr. 2005;31 483.11. [Google Scholar]