Abstract
The objective of this study was to determine the effect of time on the clinical efficacy of topical anesthetic in reducing pain from needle insertion alone as well as injection of anesthetic. This was a randomized, double-blind, placebo-controlled, split-mouth, clinical trial which enrolled 90 subjects, equally divided into 3 groups based upon time (2, 5, or 10 minutes) of topical anesthetic (5% lidocaine) application. Each group was further subdivided into 2: needle insertion only in the palate or needle insertion with deposition of anesthetic (0.5 mL 3% mepivacaine plain). Each subject received drug on one side and placebo on the other. Subjects recorded pain on a 100-mm visual analog scale (VAS). The results showed that for needle insertion only, 5% lidocaine reduced pain as determined by a significant difference in mean VAS after 2 minutes (20.1 mm, P < .002), 5 minutes (15.7 mm, P < .022), and 10 minutes (13.7 mm, P < .04), as analyzed by paired t tests. For needle insertion plus injection of local anesthetic, a significant difference in mean VAS was noted only after 10 minutes (14.9 mm, P < .031), yet pain scores for both topical anesthetic and placebo were elevated at this time point resulting in no reduction in actual pain. Time of application did not result in a significant difference in effect for either needle insertion only or needle insertion plus injection of local anesthetic, as analyzed by 1-way analysis of variance (ANOVA). In conclusion, topical anesthetic reduces pain of needle insertion if left on palatal mucosa for 2, 5, or 10 minutes, but has no clinical pain relief for anesthetic injection.
Keywords: Topical anesthesia, Local anesthesia, Pain
Effective intraoral anesthesia without the need for needles would be a major advancement for dentistry. The reality today, however, is that needles are required to achieve reliable local anesthesia. Topical anesthetics are used primarily with the intention of reducing the associated discomfort.
The literature shows that there is little pain relief from topical anesthesia, and one reason for failure may be that dentists do not wait long enough to allow them to take effect.1,2 Studies in the past have looked more into the clinical effectiveness of various topical anesthetics than on the effect of application time. There is no consensus regarding the most appropriate time for a topical anesthetic to be left on intraoral tissue prior to injection. A review of the literature reveals that in most of the studies, the local anesthetic was not actually injected into the tissues but the needle was only inserted.1,2 The deposition of any local anesthetic solution would have acted as a confounding factor, as that in itself is painful. This makes these studies less clinically applicable because in the real world there would be deposition of local anesthetic solution. It is worth attempting to differentiate between the two to see the potential difference in pain scores on needle insertion only and the actual deposition of the local anesthetic solution. Therefore, the objective of this study was to test the following null hypothesis: regardless of the time of application over a 10-minute period, there is no difference in the clinical effectiveness of the topical anesthetic 5% lidocaine on (a) the pain of needle stick insertion and (b) the pain of local anesthetic administration.
METHODS
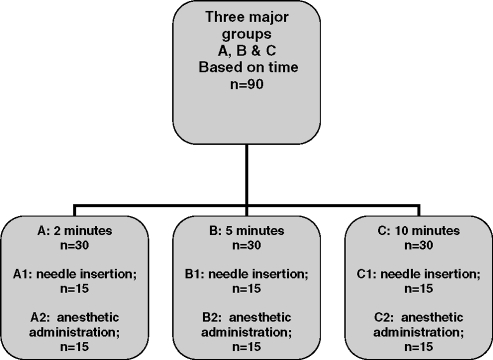
The study was a double blind, randomized, controlled, split-mouth, clinical trial, which received approval by the University of Toronto Health Sciences Research Ethics Board. There were 3 major groups with each subdivided into 2. Groups A1 and A2 were those receiving topical anesthetic and placebo for 2 minutes; groups B1 and B2 were those receiving them for 5 minutes; and Groups C1 and C2 were those receiving them for 10 minutes. The groups' subdivisions were based on needle insertion alone (A1, B1, C1) or needle insertion with injection of anesthetic (A2, B2, C2). This study design is summarized in Figure 1.
Figure 1.
Study design.
The topical anesthetic paste used was 5% lidocaine (Dentsply Inc, Canada). The site tested was the palatal soft tissue adjacent to the first premolar, chosen because the perception of pain on injection in the palate is usually perceived to be the highest in the oral cavity. The contralateral side received placebo.
At the first visit, each prospective subject had a medical history taken and underwent a brief oral exam to verify the health of the periodontium, especially in the region of maxillary first premolars. The subjects were either tentatively accepted into, or rejected from, the study according to the inclusion and exclusion criteria. On the day of the study, the subjects were evaluated again to see if they still met the inclusion and exclusion criteria, as listed in Table 1.
Table 1.
Inclusion and Exclusion Criteria
A sample size calculation3 was carried out and consisted of an α level of .01 for a 2-tailed test and β level .05 (ie, power equals .95). The σ (standard deviation) of 20 mm was taken from previous topical anesthetic studies, which also used a visual analog scale (VAS) for scoring pain.4 For this study a difference of 25 mm on a 100-mm VAS scale was considered clinically significant. Thus “n” was calculated from the following formula:
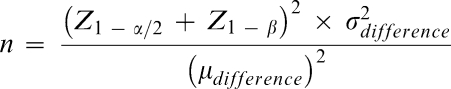 |
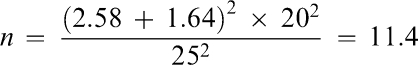 |
Zα and Zβ are values taken from standard statistical tables of the normal distribution. Assuming a “no-show” rate of 10%, it was proposed to have a minimum of 15 subjects per group for a total “n” of 90.
Subjects were seated and monitored continuously with pulse oximetry (Vital care 506 DXN, Criticare Systems Inc, Waukesha, Wisconsin) to record SpO2 and heart rate. All data were recorded on paper throughout the study. The sites of application of the needle and the agent were dried with 2 × 2 inch gauze. Additionally, the tongue and buccal surfaces of the lips were isolated with gauze and cotton rolls, respectively, to prevent the anesthetic agent from contacting these tissues. This was done to prevent the subject from interpreting a numb lip or a numb tongue as a numb palate, thereby possibly affecting the true pain score.5
The group assignment and selection of order of placebo and lidocaine administration for each patient was determined by tables of random numbers. Placebo and lidocaine topical were drawn onto cotton swab applicators from preweighed drug boxes to standardize the amount of drug applied. Each increment was 0.2 mg. It was then handed to the principal investigator in a blinded manner, who then applied it to the palatal mucosa 10 mm below the gingival margin of the first bicuspid, first on the right side of the palate. Baseline heart rate readings were gathered before needle insertion or anesthetic administration for each subject in each group. A research assistant used a stop-watch to accurately time the placement of the agents and to record heart rate.
After the designated wait, needle insertion or anesthetic administration was carried out, depending upon the subgroup to which the subject belonged. The needle (25 gauge, Dentsply Inc, Canada) was inserted until it contacted the periosteum, then withdrawn 1 mm; either 0.5 mL of 3% plain mepivacaine solution (Scandonest 3% plain, Septodont, Canada) was deposited over a 30-second time period, or the needle was left in place for 30 seconds. Needle insertion or anesthetic administration was standardized for the groups at this time. A new needle was used for each insertion. Immediately after each insertion, the subjects indicated the pain intensity they perceived on the 100-mm VAS. Following this, the procedure was repeated in a standardized manner for the contralateral side. The clinical trial for each participant was completed in a single appointment.
Data Analysis
Comparisons of proportions for gender distribution in the study groups were done using the chi-square test. One-way analysis of variance (ANOVA) was used to compare the means of ages and weights among the 3 groups. Independent samples t test was carried out to look for any significant differences in VAS scoring among male and female subjects. The paired t test was used to compare the means of the VAS scores between the placebo and the topical anesthetic within groups. To test the null hypothesis that the means of the 3 time groups are the same, the within-subject difference in VAS measurement between split-mouths was compared using a 1-way ANOVA. Univariate analysis of variance and analysis of covariance were applied to the clinical trial results to look for possible confounding by the covariates. Multiple regression was used to model the effect of time on VAS difference adjusting for age, gender, needle insertion, anesthetic administration, weight, and heart rate. Statistical tests were 2-tailed and interpreted at the 5% level.
RESULTS
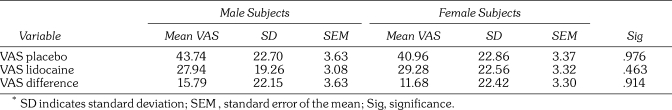
Ninety patients were enrolled in the study, and data from 85 (39 male and 46 female) patients were analyzed. Data from 5 subjects were rejected because they were not able to maintain isolation of the tongue. The weight of the subjects ranged from 41 kg to 100 kg with a mean of 68.2 kg. The age range was 18 to 67 years with a mean of 32.7 years. There were no significant differences in the mean ages and weights of the 3 groups. There were no significant gender differences in pain, when looking at all VAS scores, as seen in Table 2.
Table 2.
Mean Visual Analog Scale (VAS) Scores*
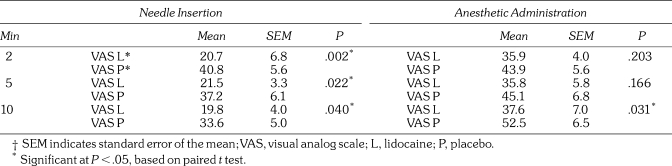
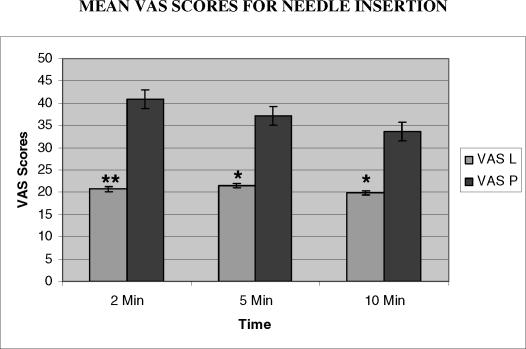
The results for topical anesthetic efficacy are shown in Table 3. Statistically significant values were obtained for all groups that received needle insertions only. For the subjects who also received the anesthetic injection, significant pain relief was obtained only after 10 minutes. These effects are shown graphically in Figures 2 and 3.
Table 3.
Topical Anesthetic Efficacy on Pain From Needle Insertion and Anesthetic Administration†
Figure 2.
The mean visual analog scale (VAS) scores for needle insertion over time are shown above. ** P < .005, * P < .05. Analyzed by paired t test.
Figure 3.
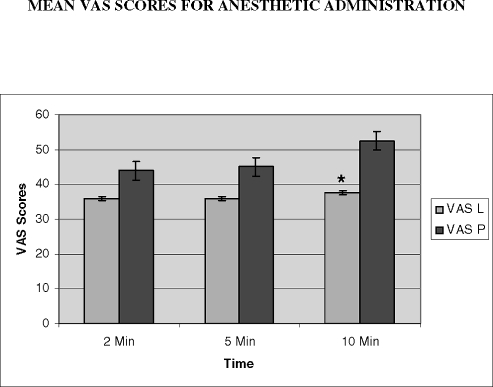
The mean visual analog scale (VAS) scores for anesthetic administration over time are shown above. * P < .05. Analyzed by paired t test.
To see the effect of time itself, the mean VAS score differences were assessed globally by 1-way ANOVA. Table 4 reveals that statistical analysis showed that time of application did not result in any significant difference in effect for either needle insertion or anesthetic administration. This can also be seen in Figures 4 and 5.
Table 4.
Effect of Time on Topical Anesthetic Efficacy
Figure 4.
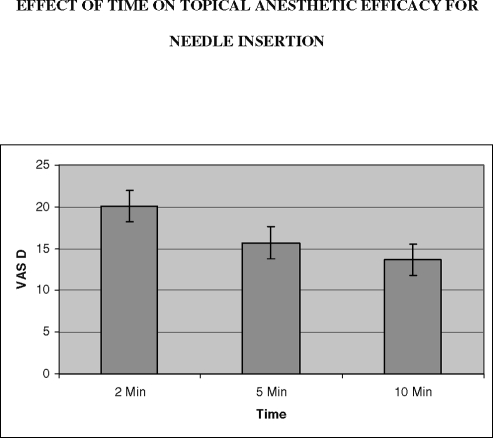
The mean visual analog scale (VAS) differences for needle insertion over time are shown above. These were not significant as analyzed by 1-way ANOVA (P > .05).
Figure 5.
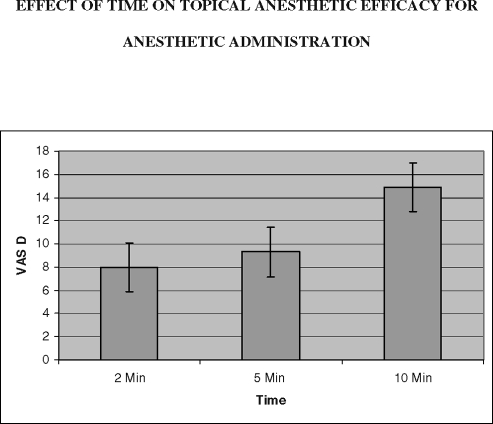
The mean visual analog scale (VAS) differences for anesthetic administration over time are shown above. These were not significant as analyzed by 1-way ANOVA (P > .05).
Based on the multiple regression analysis, no statistically significant correlation was detected among any one of age, gender, weight, or heart rate with pain relief in this study (data not shown).
DISCUSSION
This study showed that topical anesthetic reduced the pain of needle insertion if left on the palatal mucosa for 2, 5, or 10 minutes, but had no clinical benefit for the actual anesthetic injection. This study used a within-subject design, which has 2 fundamental advantages: power and reduction in error variance associated with individual difference. In this study, placebo and active drug were compared in the same subject who scored 2 similar painful stimuli, reducing variance which otherwise would have been apparent had 2 different subjects been used.
There are 2 possible interpretations on the effect on anesthetic administration. It may take 10 minutes for lidocaine to become effective in reducing pain. The alternative explanation is that the pain increased in the controls, so that lidocaine reduced this relative increase. The basis for this supposition may be found in looking at the mean VAS values for lidocaine, which were similar in all 3 groups (35.9, 35.8, and 37.6 mm), consistent with no change in effect. As can be seen in Figure 3, the VAS values for placebo increased with application time (45.1, 43.9, and 52.5 mm). Statistical significance resulted as relief from pain was interpreted as the difference between the VAS scores of placebo and lidocaine.
A new needle was used for each insertion or anesthetic administration. Higher gauge needles such as 27 and 30 are sometimes used in the belief by some that they cause less discomfort of intraoral needle penetrations.6 Studies done on the subject all point to the fact that needle gauge did not affect pain upon insertion.7–9
This study used the VAS, which is the most commonly used pain-measuring tool. Studies by Revill and McCormack10 found the VAS to be a reproducible method for measuring pain. Seymour12 in a clinical trial on postoperative dental pain found the VAS to be more sensitive than other pain scales and one that could discriminate between small changes in pain intensity. Most of the subjects in the study were comfortable in its use after receiving instructions and did not need any further directions.
This is the first study to show that there is no benefit in keeping the topical anesthetic beyond 2 minutes in the belief of increasing its efficacy.
The secondary hypothesis tested was that topical anesthetic would be effective equally in reducing the pain from both needle insertion and anesthetic administration. Confirming previous studies, this one showed that topical anesthetic was very effective in reducing the pain from needle insertion only.
In conclusion, the study showed that the topical anesthetic 5% lidocaine could relieve pain from needle insertion but not pain from actual injection, unless a 10-minute period has passed. Yet after 10 minutes, patients appear to perceive more pain than earlier time points, which negates the benefit of waiting. Therefore, 2 minutes of topical anesthetic application appears to be a reasonable recommendation in dentistry, in order to diminish pain from needle insertion. There is no evidence from this study that topical anesthetic will diminish pain from the actual anesthetic insertion at that time point.
Acknowledgments
This study was funded by the Anesthesia Research Foundation (ARF) of the American Dental Society of Anesthesiology and the Ontario Dental Society of Anaesthesiology. The results of this study were originally presented at the International Association of Dental Research Meeting in New Orleans, LA in March 2007. This report is based, in part, on a thesis submitted by Dr Bhalla in conformity with the requirements for the Master of Science in dental anesthesia at the University of Toronto.
REFERENCES
- Meechan J.G. Intra-oral topical anesthetics: a review. J Dent. 2000;28:3–14. doi: 10.1016/s0300-5712(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Meechan J.G. Effective topical anesthetic agents and techniques. Dent Clin North Am. 2002;46:759–766. doi: 10.1016/s0011-8532(02)00035-6. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Haas D.A, Meechan J.G, May S. Injection pain: comparison of the three mandibular block techniques and modulation by nitrous oxide. J Am Dent Assoc. 2003;134:869–876. doi: 10.14219/jada.archive.2003.0285. [DOI] [PubMed] [Google Scholar]
- Meechan J.G, Thomason J.M. A comparison of 2 topical anesthetics on the discomfort of intraligamentary injections: a double-blind, split-mouth volunteer clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:362–365. doi: 10.1016/s1079-2104(99)70224-6. [DOI] [PubMed] [Google Scholar]
- Gill C.J, Orr D.L., 2nd A double-blind crossover comparison of topical anesthetics. J Am Dent Assoc. 1979;98:213–214. doi: 10.14219/jada.archive.1979.0476. [DOI] [PubMed] [Google Scholar]
- Meechan J.G, Howlett P.C, Smith B.D. Factors influencing the discomfort of intraoral needle penetration. Anesth Prog. 2005;52:91–94. doi: 10.2344/0003-3006(2005)52[91:FITDOI]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller N.P, Menke R.A, Meyers W.J. Perception of pain to three different intraoral penetrations of needles. J Am Dent Assoc. 1979;99:822–824. doi: 10.14219/jada.archive.1979.0384. [DOI] [PubMed] [Google Scholar]
- Lehtinen R. Penetration of 27- and 30-gauge dental needles. Int J Oral Surg. 1983;12:444–445. doi: 10.1016/s0300-9785(83)80036-2. [DOI] [PubMed] [Google Scholar]
- Mollen A.J, Ficara A.J, Provant D.R. Needles—25 gauge versus 27 gauge—can patients really tell. Gen Dent. 1981;29:417–418. [PubMed] [Google Scholar]
- Revill S.I, Robinson J.O, Rosen M, Hogg M.I. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976;31:1191–1198. doi: 10.1111/j.1365-2044.1976.tb11971.x. [DOI] [PubMed] [Google Scholar]
- McCormack H.M, Horne D.J.L, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychological Medicine. 1988;18:1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- Seymour R.A. The use of pain scales in assessing the efficacy of analgesics in post-operative dental pain. Eur J Clin Pharmacol. 1982;23:441–444. doi: 10.1007/BF00605995. [DOI] [PubMed] [Google Scholar]