Abstract
Negative pressure pulmonary edema (NPPE) following upper airway obstruction (UAO) has been reported in several clinical situations. The main cause of NPPE is reported to be increased negative intrathoracic pressure. We present a case of NPPE that occurred after general anesthesia for plate removal after jaw deformity surgery. After completion of the surgery, administration of inhaled anesthetics was stopped and the patient opened his eyes on verbal command. Immediately after extubation, the patient stopped breathing and became cyanotic. Acute UAO following laryngospasm was suspected. Soon after reintubation, pink, frothy fluid came out of the endotracheal tube, and a tentative diagnosis of NPPE was made. Continuous positive airway pressure was applied. In addition, furosemide and dexamethasone were administered. By the next day, the symptoms had almost disappeared.
Keywords: Negative pressure pulmonary edema, Upper airway obstruction, Continuous positive airway pressure
Negative pressure pulmonary edema (NPPE) following upper airway obstruction (UAO) has been reported in several clinical situations including postanesthetic state.1–3 It has been reported that NPPE occurred at a rate less than 1 ∶ 1000 (0.094%) in surgical patients.4 After oral and maxillofacial surgery, the risk of UAO is considered to be much higher than that after other types of surgery because its surgical field overlaps the airway. We present a case of NPPE that occurred in an adult patient after general anesthesia for plate removal after jaw deformity surgery.
CASE REPORT
A 27-year-old male patient was admitted for plate removal after jaw deformity surgery and extraction of the mandibular third molars. He was 181 cm tall and weighed 66.6 kg. He had undergone Le Fort I and sagittal split mandibular ramus osteotomy under general anesthesia uneventfully 1 year before this surgery. His medical history included depression, which had been well controlled with bromazepam, clomipramine hydrochloride, and sulpiride. His Mallampati grade was assessed as Class 1. Preoperative antidepressant medicines were continued to prevent restlessness just after his recovery from general anesthesia—the same strategy used previously. Atropine sulfate and midazolam were administered IM for premedication. General anesthesia was induced with propofol, butorphanol tartrate, and vecuronium bromide. Nasotracheal intubation was facilitated easily with a 7.5-mm cuffed tube. Nasogastric suction was not placed in this case because this type of surgery usually is associated with minimal bleeding. Anesthesia was maintained with 66% nitrous oxide in oxygen and 1.0% sevoflurane. Six milliliters of 1% lidocaine solution containing 5 µg/ml epinephrine was used twice around the surgical fields without cardiorespiratory disturbances. Systolic blood pressure was maintained between 95 and 110 mmHg, heart rate was between 80 and 90 bpm, and oxygen saturation was around 98% during surgery. Urine output was not monitored.
The planned surgical procedures were completed uneventfully in 1 hour 25 minutes. Estimated blood loss was 50 ml and total infusion of lactated Ringer's solution was 900 ml. After the anesthetic gases were discontinued and 100% oxygen was provided, spontaneous breathing emerged and oxygen saturation reached 99%. The patient seemed to breathe smoothly. An antagonist of neuromuscular blockade was not used because the blockade's effect was considered fully dispelled. When the patient opened his eyes quickly on verbal command, the endotracheal tube was removed after oral suctioning. Endotracheal suctioning was not performed because breath sounds in both lungs were clear. Immediately after extubation, the patient seemed to be a little drowsy. Since his respiratory rate was normal, he was transferred to a stretcher.
On the way to the postanesthesia care unit in the operating area, he became unconscious. Supplemental oxygen was not administered during transport from the operating room because the distance was very short, nor was SpO2 monitoring used. Dyspnea with marked inspiratory efforts suggested UAO. Paradoxical respiration did not improve with the aid of an oral airway. It was too difficult to maintain enough oxygenation using artificial ventilation with a bag valve mask. As cyanosis rapidly developed on his skin, we decided to reintubate. We observed that, by direct laryngoscopy, his pharynx seemed edematous. After intubation with a 7.5-mm oral endotracheal tube, we ventilated several times using a bag-valve device. At this moment, large amounts of foamy, pink fluid emerged from the endotrachal tube, prompting a tentative diagnosis of acute pulmonary edema. Ten milligrams of furosemide was used twice and a urinary catheter was inserted. Eight milligrams of dexamethasone and 0.4 mg of digoxin were also administered IV. We repeated manual ventilation and tracheal suction. Bilateral moist rales were confirmed on auscultation. Arterial blood gas analysis revealed partial pressure of arterial oxygen (PaO2) at 57.4 mm Hg and partial pressure of arterial carbon dioxide (PaCO2) at 98 mm Hg.
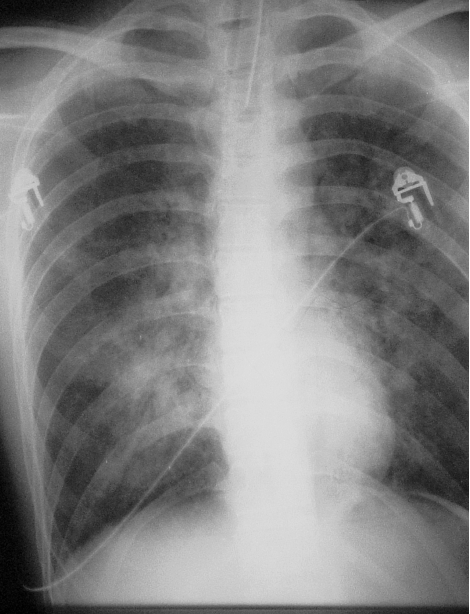
About 20 minutes later, spontaneous respiration was observed. However, there was no reply to verbal stimuli. We again transferred the patient to the operating room to provide positive end-expiratory pressure breathing treatments with 100% oxygen, and endotracheal suction was repeated. At first, positive end-expiratory pressure was set to 10 cm H2O, and was decreased to 3 cm H2O about 30 minutes later. Secretions from the trachea began to decrease, and he opened his eyes and moved his foot on verbal command. A chest radiogram showed a typical pattern of pulmonary edema with symmetric, bilateral middle-lobe infiltrates with a normal heart size and a wide vascular pedicle (Figure). Continuous positive airway pressure at 5 cm H2O under propofol sedation was started. In the ward, the fluid from the endotracheal tube almost disappeared. The next day, after confirming improvement in blood-gas analysis and taking a chest radiogram, we removed the endotracheal tube. He was discharged on the 10th hospital day without any mental damage.
A typical pattern of pulmonary edema roentgenogram showing bilateral middle-lobe infiltrates without cardiomegaly.
DISCUSSION
This patient was suspected of having NPPE after acute UAO occurred following laryngospasm, which is the main reported cause of NPPE.4–7 Another reported cause was a patient's biting the endotracheal tube.8 The pathogenesis of NPPE is complicated. Inspiratory effort against airway obstruction can result in extremely negative intrathoracic pressure, causing decreased pulmonary capillary perivascular pressure favoring hydrostatic transudation of fluid into the interstitial tissue. Normal pleural inspiratory pressure of −2 to −5 cm H2O may drop to −50 cm H2O, and negative pressures as of −100 cm H2O have been reported during severe episodes of obstructive sleep apnea.9,10 Therefore, many NPPEs occur in young and muscular patients because of the strong inspiratory effort they can generate against the closed airway.8,9 When the obstruction is suddenly relieved, there is an abrupt recovery of airway pressure, resulting in a sudden increase in venous return and a redistribution of blood volume from peripheral to central circulation. The increased pulmonary hydrostatic pressure causes pulmonary edema.3 Another potential pathogenesis, capillary leak, due to either disruption of capillary walls by extremely high negative pressure or the effect of extreme hypoxia, has been proposed.9,11
Other factors that could have augmented the airway obstruction in this case were the prolonged effect of antidepressant medicines, residual effect of premedications, early extubation when the patient did not fully awaken, or oral edema following surgery. Prior to extubation, we must ensure that the patient is completely awake, thus regaining optimal upper airway muscle tone. In several cases, development of pulmonary edema is delayed for several hours. Because of this delayed onset, patients who experience postanesthetic laryngospasm should be observed for longer than the usual 60 to 90 minutes.12 The patient with underlying risk factors for delayed recovery of anesthesia should be recognized to have an increased risk of developing pulmonary edema. In addition, preparation for reintubation after extubation is always necessary. This patient had no symptoms of sleep apnea or other risk factors for hypopharyngeal airway obstruction except for taking antidepressant medicines. Central nervous system depression and upper airway muscle relaxation associated with anesthesia and antidepressant medicine could have precipitated UAO after extubation in this predisposed group. He was thought to have been extubated prior to full awakening. Finally, we must always be aware of the possibility of NPPE as a complication of UAO.
Orthognathic surgery is considered to have more risk of NPPE than other types of oral surgeries because, in cases of prognathia, it makes patient's upper airway space narrower by moving the lower jaw backward. Other risk factors regarding oral surgery are bleeding and swelling in the oral cavity which also makes the airway space narrower. In addition, oozing from the wound sometimes irritates the patient's larynx and might initiate laryngospasm. Maxillomandibular fixation after the operation might disturb patients' smooth respiration and narrow the oral cavity. NPPE usually resolves rapidly without aggressive therapy or invasive monitoring.7 Treatment consists of variable regimens of diuretics, digoxin, corticosteroids, morphine, and fluid restriction.1,13 Willms reported that mechanical ventilation, frequently with the use of positive end expiratory pressure, was provided in 65% of the whole series and in 55% of those with laryngospasm.1 Continuous positive airway pressure prevents the development of pulmonary edema by decreasing venous return and decreasing the preload in the pulmonary circulation. It also prevents the closure of airways on expiration.3 The use of steroids is controversial; however, they have been shown to lessen the pulmonary edema lesion resulting from trauma.14 Since there might be physical damage to the alveoli and capillaries caused by high negative pressures, steroid administration might be useful. Many reports suggest that symptoms in NPPE improve gradually even if no aggressive treatment is done. Our patient was provided aggressive therapies because his symptoms were so drastic, and he recovered rapidly.
Epinephrine has been known to have undesirable interactions with antidepressants. Because epinephrine in local anesthetic solutions was used safely during his previous surgery, we felt that it could be safely used again. To decrease the total amount of epinephrine, we diluted it to half the routine concentration, from 1 ∶ 100,000 to 1 ∶ 200,000.
Because the surgical field overlaps the patient's airway in oral surgery, the risk of postoperative UAO is considered high, and once it has occurred and been relieved, NPPE might occur. Aggressive respiratory therapies, including CPAP, provide the patient's rapid recovery from NPPE.
REFERENCES
- Willms D, Shure D. Pulmonary edema due to upper airway obstruction in adults. Chest. 1988;94:1090–1092. doi: 10.1378/chest.94.5.1090. [DOI] [PubMed] [Google Scholar]
- Weissman C, Damask M.C, Yang J.Noncardiogenic pulmonary edema following laryngeal obstruction Anesthesiology 198460( 2) 163–165. [DOI] [PubMed] [Google Scholar]
- Kamal R.S, Agha S. Acute pulmonary oedema—a complication of upper airway obstruction. Anaesthesia. 1984;39:464–467. doi: 10.1111/j.1365-2044.1984.tb07316.x. [DOI] [PubMed] [Google Scholar]
- Deepika K, Kenaan C.A, Barrocas A.M, Fonseca J.J, Bikazi G.B. Negative pressure pulmonary edema after acute upper airway obstruction. Clin Anesth. 1997;9:403–408. doi: 10.1016/s0952-8180(97)00070-6. [DOI] [PubMed] [Google Scholar]
- Lang S.A, Duncan P.G, Shephard D.A.E, Ha H.C.Pulmonary oedema associated with airway obstruction Can J Anaesth 199037( 2) 210–218. [DOI] [PubMed] [Google Scholar]
- Cozanitis D.A, Leijala M, Pesonen E, Zaki H.A. Acute pulmonary oedema due to laryngeal spasm. Anaesthesia. 1982;37:1198–1199. doi: 10.1111/j.1365-2044.1982.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Lorch D.G, Sahn S.A.Post-extubation pulmonary edema following anesthesia induced by upper airway—are certain patients at increased risk Chest 198690( 6) 802–805. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Chaffee T.L, Goto H.Pulmonary edema induced by endotracheal tube obstruction: a case report Respir Care 199338( 5) 479–481. [Google Scholar]
- Cascade P.N, Alexander G.D, Mackie D.S. Negative-pressure pulmonary edema after endotracheal intubation. Radiology. 1993;186:671–675. doi: 10.1148/radiology.186.3.8430172. [DOI] [PubMed] [Google Scholar]
- Timby J, Reed C, Zeilender S, Glauser F.L. “Mechanical” causes of pulmonary edema. Chest. 1990;98:973–979. doi: 10.1378/chest.98.4.973. [DOI] [PubMed] [Google Scholar]
- Louis P.J, Fernandes R. Negative pressure pulmonary edema. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:4–6. doi: 10.1067/moe.2002.119909. [DOI] [PubMed] [Google Scholar]
- Glasser S.A, Siler J.N. Delayed onset of laryngospasm-induced pulmonary edema in an adult outpatient. Anesthesiology. 1985;62:370–371. doi: 10.1097/00000542-198503000-00034. [DOI] [PubMed] [Google Scholar]
- Barin E.S, Stevenson I.F, Donnelly G.L. Pulmonary odedema following acute upper airway obstruction. Anaesth Intensive Care. 1986;14:54–57. doi: 10.1177/0310057X8601400112. [DOI] [PubMed] [Google Scholar]
- Oswalt C.E, Gates G.A, Holmstrom F.M.G.Pulmonary edema as a complication of acute airway obstruction JAMA 1977238( 17) 1833–1835. [PubMed] [Google Scholar]