Abstract
OBJECTIVE
To determine the efficacy and safety of liraglutide (a glucagon-like peptide-1 receptor agonist) when added to metformin and rosiglitazone in type 2 diabetes.
RESEARCH DESIGN AND METHODS
This 26-week, double-blind, placebo-controlled, parallel-group trial randomized 533 subjects (1:1:1) to once-daily liraglutide (1.2 or 1.8 mg) or liraglutide placebo in combination with metformin (1 g twice daily) and rosiglitazone (4 mg twice daily). Subjects had type 2 diabetes, A1C 7–11% (previous oral antidiabetes drug [OAD] monotherapy ≥3 months) or 7–10% (previous OAD combination therapy ≥3 months), and BMI ≤45 kg/m2.
RESULTS
Mean A1C values decreased significantly more in the liraglutide groups versus placebo (mean ± SE −1.5 ± 0.1% for both 1.2 and 1.8 mg liraglutide and −0.5 ± 0.1% for placebo). Fasting plasma glucose decreased by 40, 44, and 8 mg/dl for 1.2 and 1.8 mg and placebo, respectively, and 90-min postprandial glucose decreased by 47, 49, and 14 mg/dl, respectively (P < 0.001 for all liraglutide groups vs. placebo). Dose-dependent weight loss occurred with 1.2 and 1.8 mg liraglutide (1.0 ± 0.3 and 2.0 ± 0.3 kg, respectively) (P < 0.0001) compared with weight gain with placebo (0.6 ± 0.3 kg). Systolic blood pressure decreased by 6.7, 5.6, and 1.1 mmHg with 1.2 and 1.8 mg liraglutide and placebo, respectively. Significant increases in C-peptide and homeostasis model assessment of β-cell function and significant decreases in the proinsulin-to-insulin ratio occurred with liraglutide versus placebo. Minor hypoglycemia occurred more frequently with liraglutide, but there was no major hypoglycemia. Gastrointestinal adverse events were more common with liraglutide, but most occurred early and were transient.
CONCLUSIONS
Liraglutide combined with metformin and a thiazolidinedione is a well-tolerated combination therapy for type 2 diabetes, providing significant improvements in glycemic control.
Type 2 diabetes is characterized by insulin resistance and progressive β-cell failure. Treatment often must be intensified over time, usually by a combination of agents that address both insulin resistance and β-cell dysfunction (1,2). However, several available therapies increase the risk for hypoglycemia and weight gain, which may reduce patient adherence and lead to poor glycemic control (3).
Glucagon-like peptide-1 (GLP-1) stimulates insulin secretion and suppression of glucagon secretion in a glucose-dependent manner, delays gastric emptying, and decreases appetite (4). GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (4). Liraglutide is a human GLP-1 analog with 97% homology to native GLP-1 (5). Liraglutide has a half-life in humans of 13 h compared with 1–2 min for native GLP-1, making liraglutide suitable as a once-daily treatment for patients with type 2 diabetes (6).
In previously published phase 3 trials (the Liraglutide Effect and Action in Diabetes [LEAD] Program), treatment with liraglutide produced substantial and clinically significant reductions in A1C and fasting and postprandial glucose (PPG) levels, with a low risk of hypoglycemia, and moderate weight loss (7–10). Liraglutide treatment alone or in combination with oral antidiabetes drugs (OADs) demonstrated significantly larger A1C reductions compared with glimepiride (monotherapy) (7), rosiglitazone (in combination with a sulfonylurea) (8), and insulin glargine (in combination with metformin and sulfonylurea) (10). When initiated as monotherapy in a subgroup of previously treatment-naïve patients with type 2 diabetes, a mean A1C reduction of 1.6% was observed, with mean A1C values sustained below 7.0% over 52 weeks (7). In combination with metformin, liraglutide reduced body weight by 2–3 kg, with the majority of the weight loss being fat (11). In addition, a decrease in systolic blood pressure (SBP) has been previously demonstrated (7–10). No major hypoglycemic events occurred during the randomized treatment period when liraglutide was used as monotherapy or with metformin (7,9). The current study investigated liraglutide treatment in combination with metformin and a thiazolidinedione (TZD) (rosiglitazone) as part of the LEAD program. These three glucose-lowering agents are of particular interest, as they have complementary modes of action and are not generally associated with increased risk of hypoglycemia.
RESEARCH DESIGN AND METHODS
Subjects with type 2 diabetes were screened and enrolled if they were aged 18–80 years, had A1C between 7 and 11% (prestudy OAD monotherapy for ≥3 months) or 7–10% (prestudy combination OAD therapy for ≥3 months), and had BMI ≤45 kg/m2. Subjects who used insulin during the previous 3 months (except short-term treatment) were excluded. The protocol was approved by local institutional review boards, and subjects provided written informed consent before the initiation of any trial-related activities. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines (12).
This 26-week, double-blind, randomized, placebo-control, parallel-group, multicenter (96 sites), two-country (U.S. and Canada) trial randomized subjects (1:1:1) to receive 1.2 or 1.8 mg of once-daily liraglutide (Novo Nordisk, Bagsværd, Denmark) or liraglutide placebo (Novo Nordisk) injected subcutaneously by subjects in combination with metformin and rosiglitazone in all three treatment groups.
Randomization was carried out using a telephone- or Web-based randomization system. Before randomization, eligible subjects underwent a 6- to 9-week metformin and rosiglitazone run-in and dose-titration period. Prior treatment with OADs other than metformin and rosiglitazone were discontinued. Subjects previously treated with pioglitazone underwent rosiglitazone titration (by transferring to the corresponding rosiglitazone dose) or went directly to the maximum dose if they were on the maximum pioglitazone dose. Metformin was started at 500 mg at breakfast and increased weekly by increments of 500 mg to a final dose of 2,000 mg/day (1,000 mg at breakfast and at the evening meal). Rosiglitazone was started at 4 mg in the morning and increased to 8 mg/day (4 mg in the morning and evening, the highest approved dose in the U.S. and Canada). Subjects who tolerated the final OAD doses and had fasting plasma glucose (FPG) values 135–230 mg/dl (7.5–12.8 mmol/l) after 6 weeks' treatment at the titrated doses were eligible for randomization. At randomization, subjects initiated liraglutide or placebo treatment with 100-μl injections corresponding to a 0.6-mg dose and increased to 1.2 mg/day (200 μl injections) after 1 week and then to 1.8 mg/day (300 μl injections) after an additional week for those randomized to the highest dose. Liraglutide (active or placebo) was injected subcutaneously once daily at any time of the day in the upper arm, abdomen, or thigh using a prefilled pen device. Subjects were encouraged to use liraglutide during the same overall time period. The titration period was followed by a 24-week maintenance period during which the doses of study drugs were to be maintained.
The primary outcome measure was change in A1C from randomization to the end of the study. Secondary end points included changes in body weight; FPG; seven-point plasma glucose profiles; β-cell function based on fasting insulin, fasting C-peptide, and fasting proinsulin-to-insulin ratio; the homeostasis model assessment (HOMA) for β-cell function (HOMA-B) and insulin resistance (HOMA-IR) (13); and lipids. Laboratory analyses were performed by a central laboratory (MDS Pharma Services in Canada and Switzerland). A1C was assayed by a method certified by the National Glycohemoglobin Standardization Program. Subjects were provided with MediSense Precision Xtra/MediSense Optium glucose meters (Abbott Laboratories, Abbott Park, IL) calibrated to plasma glucose to determine self-measured plasma glucose (SMPG) and were asked to record values in their diaries. The seven-point SMPG profile measurements were performed before and 90 min after meals and at bedtime for 2 consecutive days at weeks 0 (randomization), 12, and 26. Serum insulin and C-peptide values were determined using a chemiluminescence immunoassay, and proinsulin was measured in serum using an enzyme-linked immunosorbent assay.
Safety variables included adverse events, vital signs, electrocardiogram, biochemical and hematology measures, and subject-reported hypoglycemic episodes (plasma glucose <56 mg/dl [<3.1mmol/l]). A serious adverse event was defined as an adverse event that resulted in death, hospitalization, disability, or a birth defect; was life threatening; or required medical or surgical intervention to prevent one of the other outcomes. Minor hypoglycemic episodes were defined as those that could be self-treated; major episodes were defined as requiring third-party assistance or medical intervention. Nausea was patient reported.
Statistical analysis
The analysis of efficacy end points was based on the intent-to-treat population, defined as subjects who were exposed to at least one dose of trial product and had one postbaseline measurement of the parameter. Each end point was analyzed using an ANCOVA model with treatment, country, and previous antidiabetes treatment as fixed effects and baseline as the covariate. Missing data were imputed as the last observation carried forward. Sample size calculations were based on showing A1C and body weight differences of 0.5 and 3%, respectively. The combined power (calculated as the product of the marginal powers for A1C and weight) was >95%.
Superiority of glycemic control with liraglutide versus comparators was concluded if the upper limit of the two-sided 95% CI for the treatment difference in change in A1C was <0%; equivalence was also tested. The proportion of subjects achieving A1C targets (American Diabetes Association [ADA] target: <7%; American Association of Clinical Endocrinologists [AACE]/International Diabetes Federation [IDF] target: ≤6.5%) was compared between treatments using a logistic regression model with treatment and baseline A1C as covariates. CIs for secondary end points were corrected using Dunnett's test. Hypoglycemic episodes were analyzed using a general linear model including treatment as a fixed effect. The significance level was set at P < 0.05.
RESULTS
A total of 821 subjects were enrolled in the study; 533 subjects were randomly assigned to liraglutide or placebo treatment after the metformin plus rosiglitazone run-in period (288 subjects were run-in failures due to FPG values out of range [135–230 mg/dl; 7.5–12.8 mmol/l] or other reasons). Three subjects were randomized but were withdrawn before receiving the study drug. Baseline characteristics were balanced across treatment groups (Table 1). The majority (83%) of the randomized subjects were treated with two or more OADs before the study.
Table 1.
Characteristics of randomized population and subject disposition
| 1.2 mg Liraglutide | 1.8 mg Liraglutide | Placebo | |
|---|---|---|---|
| Sex (%) (men/women) | 57/43 | 51/49 | 62/38 |
| Age (years) | 55 ± 10 | 55 ± 11 | 55 ± 10 |
| Race (%) (C/B/A/I/O) | 81/15/1/1/2 | 83/10/3/1/3 | 84/10/2/1/3 |
| Ethnicity (Hispanic or Latino/not) | 13/87 | 16/84 | 16/84 |
| BMI (kg/m2) | 33.2 ± 5.4 | 33.5 ± 5.1 | 33.9 ± 5.2 |
| Duration of diabetes (years) | 9 ± 6 | 9 ± 6 | 9 ± 6 |
| Prestudy OAD treatment | |||
| Monotherapy | 29 (16) | 29 (16) | 32 (18) |
| Combination therapy | 149 (84) | 149 (84) | 145 (82) |
| A1C (%) | 8.5 ± 1.2 | 8.6 ± 1.2 | 8.4 ± 1.2 |
| FPG [mg/dl (mmol/l)] | 182 ± 43 (10.1 ± 2.4) | 185 ± 43 (10.3 ± 2.4) | 180 ± 47 (10.0 ± 2.6) |
| SBP (mmHg) | 129 ± 14.8 | 126 ± 14.2 | 128 ± 14.5 |
| DBP (mmHg) | 75.8 ± 9.0 | 75.2 ± 8.4 | 76.2 ± 9.2 |
| Total cholesterol (mmol/l) | 5.01 ± 1.33 | 5.17 ± 1.43 | 4.99 ± 1.34 |
| LDL cholesterol (mmol/l) | 2.82 ± 0.95 | 2.96 ± 1.08 | 2.77 ± 0.95 |
| VLDL cholesterol (mmol/l) | 0.74 ± 0.38 | 0.76 ± 0.38 | 0.71 ± 0.36 |
| HDL cholesterol (mmol/l) | 1.26 ± 0.32 | 1.27 ± 0.31 | 1.25 ± 0.28 |
| Triglycerides (mmol/l) | 2.41 ± 2.24 | 2.39 ± 1.88 | 2.74 ± 2.80 |
| Free fatty acids (mmol/l) | 0.51 ± 0.22 | 0.55 ± 0.27 | 0.52 ± 0.34 |
| Randomized | 178 | 178 | 177 |
| Completers | 153 (86) | 133 (75) | 121 (68) |
| Withdrawals | 25 (14) | 45 (25) | 56 (32) |
| Adverse events* | 11 (6) | 27 (15) | 6 (3) |
| Nausea/vomiting/diarrhea | 5 (3) | 19 (11) | 0 |
| Ineffective therapy | 3 (2) | 3 (2) | 29 (16) |
| Noncompliance | 4 (2) | 4 (2) | 5 (3) |
| Other | 7 (4) | 11 (6) | 16 (9) |
Data are means ± SD or n (%) unless otherwise indicated.
*The adverse events row includes nausea/vomiting/diarrhea. A, Asian; B, black; C, Caucasian; I, American Indian; O, other.
Efficacy
At the end of the study, the mean A1C values for the overall population decreased by (means ± SE) 1.5 ± 0.1% for both 1.2 and 1.8 mg/day liraglutide groups and 0.5 ± 0.1% for the placebo group. Liraglutide-treated subjects had superior glycemic control compared with those in the placebo group (liraglutide 1.2 mg/day vs. placebo: −0.9% [95% CI −1.1 to −0.8] and liraglutide 1.8 mg/day vs. placebo: −1.1% [−1.1 to −0.8]). Within the first 12 weeks of the study, mean A1C values decreased from baseline for the liraglutide-treated groups and thereafter remained steady throughout the trial (Fig. 1A).
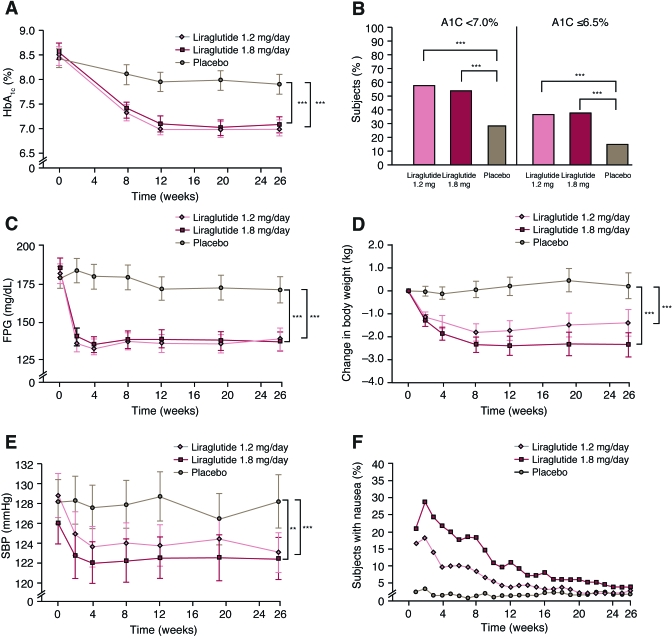
Figure 1.
A: A1C over time for the study population. B: Percentage of subjects achieving ADA and AACE/IDF A1C goals at the end of the study. C: FPG values over time. D: Change in body weight over time. E: SBP over time. F: Percentage of subjects with nausea by week. Data are intent to treat, last observation carried forward for all postbaseline values, with the exception of F, which is data from the safety analysis set. Error bars shown in A, C, D, and E are 2 × SE. **P = 0.0009; ***P < 0.0001.
A logistic regression analysis demonstrated that a significantly greater percentage of subjects in both of the liraglutide groups achieved the ADA and AACE/IDF A1C goals compared with placebo (P < 0.0001 for all comparisons of liraglutide to placebo for both A1C goals) (Fig. 1B). At the end of the study, 57.5 and 53.7% of subjects in the 1.2 and 1.8 mg liraglutide/day groups, respectively, had an A1C <7%, compared with 28.1% in the placebo group, with 37.3 and 36.2%, respectively, reaching ≤6.5% compared with 14.4% with placebo.
FPG values decreased within 2 weeks of randomization with liraglutide, remaining relatively stable thereafter, while with placebo, smaller decreases occurred (Fig. 1C). End-of-study FPG values were 139 ± 49 mg/dl (7.7 ± 2.7 mmol/l), 137 ± 41 mg/dl (7.6 ± 2.3 mmol/l), and 171 ± 54 mg/dl (9.5 ± 3.0 mmol/l) in the 1.2 and 1.8 mg liraglutide/day and placebo groups, respectively. The decreases in FPG from baseline for the liraglutide groups (−40 mg/dl [−2.2 mmol/l] and −44 mg/dl [−2.4 mmol/l] for 1.2 and 1.8 mg liraglutide/day groups, respectively) were significantly greater than the decrease observed in the placebo group (−8 mg/dl [−0.4 mmol/l], P < 0.0001).
Mean 90-min PPG (mean of three meals), from self-monitored seven-point plasma glucose measurements at the end of the study, decreased from baseline in all treatment groups by −47 mg/dl (2.6 mmol/l) for 1.2 mg liraglutide/day, −49 mg/dl (2.7 mmol/l) for 1.8 mg liraglutide/day, and −14 mg/dl (0.8 mmol/l) for placebo (P < 0.001 comparisons of all liraglutide groups to placebo). The postprandial increment (postmeal value minus premeal) was significantly reduced over breakfast with liraglutide treatment (−16, −14, and −5 mg/dl [−0.9, −0.8, −0.3 mmol/l], respectively; P < 0.05 for both liraglutide treatment groups vs. placebo) but not for lunch and dinner.
Mean change in body weight over time is shown in Fig. 1D. Weight loss was observed in the liraglutide-treated groups ([means ± SE] 1.0 ± 0.3 and 2.0 ± 0.3 kg from baseline for 1.2 and 1.8 mg liraglutide/day groups, respectively) and was significantly different (P < 0.0001) from the weight gain in the placebo group (0.6 ± 0.3 kg). The weight loss in the 1.8 mg liraglutide/day group was significantly greater than the 1.2 mg liraglutide/day group (P = 0.011).
The 1.2 and 1.8 mg liraglutide/day groups had significant reductions in mean SBP compared with the placebo group (Table 2) (Fig. 1E) (placebo-corrected difference: 1.2 mg liraglutide/day: −5.6 mmHg, P < 0.0001; 1.8 mg liraglutide/day: −4.5mmHg, P = 0.0009). There were no significant differences between treatment groups in diastolic blood pressure (DBP). Minor, but statistically significant, increases in pulse rate were observed in the liraglutide-treated groups versus placebo (2 and 3 bpm for 1.2 mg (P = 0.0071) and 1.8 mg liraglutide (P = 0.0001), respectively) with a decrease of 0.5 bpm for placebo. Changes in lipids from baseline are presented in Table 2 showing that free fatty acid values decreased with liraglutide treatment as compared with an increase with placebo, and LDL cholesterol and triglycerides decreased significantly more in the 1.2 mg liraglutide group than in the placebo group.
Table 2.
Other end points of interest/metabolic intermediates change from baseline to end of study
| Liraglutide 1.2 mg | Liraglutide 1.8 mg | Placebo | |
|---|---|---|---|
| Blood pressure (mmHg) | |||
| SBP | −6.7 ± 1.1* | −5.6 ± 1.1* | −1.1 ± 1.2 |
| DBP | −2.3 ± 0.7 | −1.9 ± 0.7 | −0.8 ± 0.7 |
| β-Cell function | |||
| Insulin (pmol/l) | 6.0 ± 5.8 | 5.6 ± 5.5 | 6.8 ± 6.0 |
| C-peptide (pmol/l) | 131 ± 32* | 144 ± 31* | 51 ± 34 |
| Proinsulin-to-insulin ratio | −0.029 ± 0.026* | −0.085 ± 0.26* | 0.036 ± 0.029 |
| β-Cell function (%) (HOMA-B) | 27 ± 4.4* | 27 ± 4.2* | 6 ± 4.5 |
| Insulin resistance (HOMA-IR) | −0.6 ± 0.3 | −0.7 ± 0.3 | −0.3 ± 0.3 |
| Proinsulin–to–C-peptide ratio | −0.007 ± 0.001* | −0.008 ± 0.001* | −0.002 ± 0.001 |
| Fasting glucagon (pg/ml) | −5.9 ± 2.9 | −6.7 ± 2.8 | −0.4 ± 3.0 |
| Lipids | |||
| Total cholesterol (mmol/l) | −0.21 ± 0.9 | −0.20 ± 0.09 | −0.02 ± 0.10 |
| LDL cholesterol (mmol/l) | −0.28 ± 0.07* | −0.23 ± 0.07 | −0.10 ± 0.07 |
| VLDL cholesterol (mmol/l) | 0.12 ± 0.03 | 0.10 ± 0.03 | 0.11 ± 0.03 |
| HDL cholesterol (mmol/l) | −0.03 ± 0.02 | −0.04 ± 0.02 | −0.03 ± 0.02 |
| Triglycerides (mmol/l) | −0.38 ± 0.10* | −0.32 ± 0.10 | −0.13 ± 0.11 |
| Free fatty acids (mmol/l) | −0.03 ± 0.02* | −0.05 ± 0.02* | 0.02 ± 0.02 |
Data are means ± SE, unless otherwise noted.
*P < 0.05 vs. placebo.
The decreases in the proinsulin-to-insulin ratio from baseline (baseline of 0.4 across all groups) for the liraglutide groups were significant (P < 0.05) compared with the placebo group, which increased from baseline (Table 2). The increase in C-peptide was significantly greater in the liraglutide groups (131 and 144 pmol/l for 1.2 and 1.8 mg liraglutide, respectively) compared with an increase of 51 pmol/l for placebo (P < 0.05 for comparison of both liraglutide groups to placebo). Both liraglutide treatment groups had significant improvements (increase of 27 absolute percentage points) in HOMA-B for both groups from baseline values of 34 to 37%, respectively, compared with an improvement in the placebo group of 6 absolute percentage points from a baseline of 40% (P < 0.0001 for both groups vs. placebo). Insulin resistance (measured by HOMA-IR) was reduced in all three treatment groups but was not significantly different between groups. No significant differences in the change-from-baseline of HOMA-IR and fasting insulin and glucagon values were observed between either of the liraglutide groups versus the placebo group (Table 2).
Safety
Gastrointestinal disorders (including nausea, vomiting, and diarrhea) were the most frequently reported adverse events in the liraglutide groups and were reported by 45, 56, and 19% of the subjects in the 1.2 and 1.8 mg liraglutide and placebo groups, respectively. One episode or more of nausea was experienced by 29 and 40% in the 1.2 and 1.8 mg liraglutide groups, respectively, and vomiting was experienced by 7 and 17%, respectively. The majority of nausea was transient, as it occurred in the first 4 weeks of liraglutide treatment (216 events in weeks 1–4 vs. 65 events in weeks 4–26) (Fig. 1F). During the first 8 weeks of treatment, 71–84% of subjects in the liraglutide groups and 98% of subjects in the placebo group reported ≤7 days of nausea. Peripheral edema was reported by 5.1, 1.7, and 8.0% in the 1.2 mg liraglutide, 1.8 mg liraglutide, and placebo groups, respectively.
The percentages of subjects withdrawn because of adverse events were greater in the liraglutide groups than in the placebo group (Table 1). Nausea, vomiting, and/or diarrhea were the gastrointestinal events that lead to the withdrawal of five subjects treated with 1.2 mg liraglutide and 19 subjects treated with 1.8 mg liraglutide (Table 1). Most gastrointestinal adverse events resulting in withdrawal occurred during the first month of therapy. There were no episodes of pancreatitis, and no deaths occurred. Serious adverse events were infrequent (8 subjects [8 total events] for 1.2 mg liraglutide, 7 subjects [10 events] for 1.8 mg liraglutide, and 12 subjects [13 events] for placebo).
Minor hypoglycemia occurred at low incidence (9.0, 7.9, and 5.1% of subjects) resulting in a low rate of reported minor hypoglycemia (0.4. 0.6, and 0.2 events per year) for the 1.2 mg liraglutide, 1.8 mg liraglutide, and placebo groups, respectively. The rate of minor hypoglycemia for the 1.8 mg liraglutide group was significantly higher than placebo (P = 0.004). No major hypoglycemic event was reported.
No clinically relevant between-treatment differences were observed in physical examination findings, laboratory analyses (hematology and biochemistry analyses), electrocardiogram, or ophthalmoscopy. There was no significant treatment effect with 1.8 mg liraglutide versus placebo on calcitonin. Geometric mean– estimated repeated-measurement analysis showed calcitonin levels of 0.89, 0.83, and 0.75 ng/l for 1.2 mg liraglutide, 1.8 mg liraglutide, and placebo, respectively, at the end of the study (all values within the normal range). There was a significant increase for the 1.2 mg liraglutide group versus placebo group (P = 0.022) but no significant difference with the 1.8 mg liraglutide group. No difference in cardiovascular adverse events was reported between the liraglutide groups and placebo (five events [five subjects] with liraglutide 1.2, three events [three subjects] with liraglutide 1.8, and four events [four subjects] with placebo). There were 4.1 and 6.7% of subjects treated with 1.2 and 1.8 mg liraglutide and positive for liraglutide antibodies at the end of the study (versus none with placebo). Subjects with antibodies did not have an attenuated A1C response.
CONCLUSIONS
Liraglutide therapy in combination with metformin and TZD provided significant decreases in A1C, FPG, and PPG with weight loss; decreases in SBP; and a low rate of minor hypoglycemia. In addition, there were indications of improvement in β-cell function with liraglutide treatment compared with placebo. While improvement in β-cell function may have been a consequence of improved glucose control, it could well be a direct effect of liraglutide, which is known to stimulate glucose-dependent endogenous insulin secretion. Gastrointestinal adverse events were reported more frequently with liraglutide treatment, with most of the events occurring early in treatment. The glucose-lowering effects of the two doses of liraglutide were similar, although there were significantly more gastrointestinal adverse events with the higher dose. However, it is likely that there is significant individual variation in the development of nausea and glycemic effectiveness.
The underlying pathophysiology of type 2 diabetes is complex and involves three main factors: a relative decrease in β-cell insulin secretory function; increased glucose production by the liver, which is at least partially mediated by inappropriately increased glucagon levels; and decreased glucose uptake by muscle. The triple therapy of metformin, TZD, and GLP-1 receptor agonists has the potential of addressing all three underlying abnormalities and results in improved glycemic control, potential weight loss, and improvements in β-cell function with minimal risk of hypoglycemia. The use of this triple therapy has demonstrated the largest decreases in A1C and SBP values in the LEAD program. It should be noted that ∼50% of the subjects initiated TZD treatment during the run-in period, and doses of metformin and TZD were maximized during this period, which may account for the improvements observed in the placebo arm of this study.
Exenatide, a commercially available GLP-1 receptor agonist, is a synthetic version of exendin-4. Unlike liraglutide, which is dosed once daily independently of meals, exenatide is dosed twice daily within 60 min of breakfast and dinner (14). The findings in this study support the findings of a previous study (15) in which 233 subjects inadequately controlled with a stable dose of TZD (rosiglitazone ≥4 mg/day or pioglitazone ≥30 mg/day) with or without metformin treatment (79% were treated with metformin) were randomized to add exenatide treatment (n = 121) or placebo (n = 112) for 16 weeks of treatment. At the end of the study, A1C values decreased by 0.89%, compared with a slight increase of 0.09% in the placebo group (P < 0.001). Body weight decreased by 1.75 kg (vs. 0.24 kg in the placebo group; P < 0.001), and other measurements of glycemic control (FPG, mean SMPG, and mean postprandial SMPG values) all showed significant improvement with exenatide treatment. These studies support the effectiveness of this type of diabetes regimen, particularly as it is associated with modest weight loss and low risk of hypoglycemia.
The very significant change in SBP observed in this study appears to be of larger magnitude than that observed in the other LEAD studies. TZD treatment is associated with a modest reduction in blood pressure but is also associated with fluid retention. There may be an interaction between the cardiovascular effects of liraglutide and TZD. Further study would be of obvious interest, particularly if there was potential for long-term cardiovascular benefit.
The specific mechanism(s) of SBP reduction and the slight increases in pulse with liraglutide remain to be further studied. Based on data with native GLP-1, it could be speculated that the effect on SBP relates to reduced renal sodium reabsorption (16,17). Native GLP-1 has been shown to improve endothelial function in patients with type 2 diabetes and coronary heart disease (18) and in vitro endothelial cell models (19). The latter has also been shown for liraglutide (20). Potentially, the slight increases in pulse observed with liraglutide may be compensatory for the decreases in SBP.
In summary, this study demonstrated that the triple combination of liraglutide, metformin, and TZD is an effective and safe treatment for patients with type 2 diabetes. This combination significantly improved glycemic control and other efficacy parameters, in addition to resulting in significant weight loss and improved blood pressure.
Supplementary Material
Acknowledgments
B.Z. has attended advisory boards for Amylin, Eli Lilly, GSK, Merck, sanofi-aventis, and Servier and has received research support from GlaxoSmithKline, Merck, Novartis, and Novo Nordisk. J.B.B. has conducted clinical trials and provided consultation for Novo Nordisk, Amylin Pharmaceuticals, and Eli Lilly under contract with the University of North Carolina; these relationships do not provide him with direct financial benefit. A.L. has received research grants from 7TM Pharma, Abbott Laboratories, ActivX Biosciences, Amylin Pharmaceuticals, AstraZeneca, Aura Laboratories, Aventis Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Calpis, Conjuchem, Daiichi Sankyo, DepoMed, Eli Lilly & Company, Elixir Pharmaceuticals, Essentialis, Forest Laboratories/Forest Research Institute, Generex Pharmaceuticals, GlaxoSmithKline, Hollis-Eden, Hoffmann-La Roche, Incyte, InteKrin Therapeutics, Johnson & Johnson, King Pharmaceuticals, Kowa Research Institute, Lilly ICOS, Merck & Company, Metabasis, Metabolic Solutions, Microbia, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Pharmacopeia, Regeneron Pharmaceuticals, Reliant Pharmaceuticals, Sankyo Pharma Development/Sankyo USA, sanofi-aventis, Schering-Plough, Sepracor, Takeda Pharmaceuticals America, TAP Holdings, Transition Therapeutics, Tularik, VIVUS, and Wyeth-Ayerst. P.R. has attended advisory boards for Novo Nordisk. P.H. and M.Z. are both employees of Novo Nordisk. L.B. has acted as an investigator for Amylin Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim Pharmaceutical, Bristol-Myers Squibb, Eli Lilly and Company, MannKind Corporation, Merck & Company, Novo Nordisk, Novartis, Pfizer, and sanofi-aventis; has been a speaker for Abbott, Amylin Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly & Company, GlaxoSmithKline, LifeScan, Merck & Company, Novartis, Novo Nordisk, Pfizer, and sanofi-aventis; and has acted as consultant for Boehringer-Ingelheim Pharmaceutical and Hazlozyme. No other potential conflicts of interest relevant to this article were reported.
The authors acknowledge the assistance of the LEAD-4 Met+TZD Study Group, their staff, clinical trial personnel, and the subjects for participating in the study. The LEAD-4 Study Investigators are listed in online appendix 1. We also thank C.T. Chang for statistical assistance and Angela M. Campbell and Jennifer M. Faleska for writing assistance (all from Novo Nordisk).
Footnotes
Clinical trial reg. no. NCT00333151, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–43 [DOI] [PubMed] [Google Scholar]
- 2. UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53 [PubMed] [Google Scholar]
- 3. Green J, Feinglos M: Update on type 2 diabetes mellitus: understanding changes in the diabetes treatment paradigm. Int J Clin Pract 2007;61(Suppl. 154):3–11 [DOI] [PubMed] [Google Scholar]
- 4. Holst JJ: The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 5. Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H: Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000;43:1664–1669 [DOI] [PubMed] [Google Scholar]
- 6. Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M: The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 2002;45:195–202 [DOI] [PubMed] [Google Scholar]
- 7. Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B: the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2008;373:473–481 [DOI] [PubMed] [Google Scholar]
- 8. Marre M, Shaw J, Brändle M, Bebakar WMW, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S: the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2008;26:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR: the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell-Jones D, Vaag A, Schmitz O, Sethi B, Lalic NM, Antic S, Zdravkovic M, Ravn GM, Simo R: Significantly better glycaemic control and weight reduction with liraglutide, a once-daily human GLP-1 analog, compared with insulin glargine: all as add-on to metformin and a sulphonylurea in type 2 diabetes (Abstract). Diabetes 2008;57(Suppl. 1):A159 [Google Scholar]
- 11. Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, During M, Zdravkovic M, Strauss BJ: Liraglutide, a once-daily human GLP-1 analog, reduces fat percentage, visceral and subcutaneous adipose tissue and hepatic steatosis compared with glimepiride when added to metformin in subjects with type 2 diabetes (Abstract). Diabetes 2008;57(Suppl. 1):A32 [Google Scholar]
- 12. World Medical Association. Declaration of Helsinki: recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 13. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 14. Byetta (exenatide) [package insert]. Available from http://pi.lilly.com/us/byetta-pi.pdf. Accessed 27 October 2008
- 15. Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG: The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes. Ann Intern Med 2007;146:477–485 [DOI] [PubMed] [Google Scholar]
- 16. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C: Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004;89:3055–3061 [DOI] [PubMed] [Google Scholar]
- 17. Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C: Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 2006;73:142–150 [DOI] [PubMed] [Google Scholar]
- 18. Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A: Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:1209–1215 [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Hu Y, Simpson RW, Dear AE: Glucagon-like peptide-1 attenuates tumour necrosis factor-alpha-mediated induction of plasmogen activator inhibitor-1 expression. J Endocrinol 2008;196:57–65 [DOI] [PubMed] [Google Scholar]
- 20. Liu H, Dear A, Knudsen L, Simpson R: A long-acting GLP-1 analogue attenuates induction of PAI-1 and vascular adhesion molecules. J Endocrinol 2009;201:59–66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.