Abstract
OBJECTIVE
To determine whether serum uric acid predicts incident type 2 diabetes by glucose tolerance status in older community-dwelling adults.
RESEARCH DESIGN AND METHODS
Participants without diabetes at baseline were evaluated for incident type 2 diabetes 13 years later. Baseline glucose tolerance status was defined as normoglycemia, impaired fasting glucose, and impaired postchallenge glucose tolerance.
RESULTS
A total of 566 participants were included (mean age 63.3 ± 8.6 years; 41% men). Regression models adjusted for age, sex, BMI, diuretic use, and estimated glomerular filtration rate showed that for each 1 mg/dl increment in uric acid levels, incident type 2 diabetes risk increased by ∼60%. When analyses were stratified by glucose status, uric acid levels independently predicted incident type 2 diabetes among participants who had impaired fasting glucose (odds ratio 1.75, 95% CI 1.1–2.9, P = 0.02).
CONCLUSIONS
Uric acid may be a useful predictor of type 2 diabetes in older adults with impaired fasting glucose.
Increased levels of serum uric acid have been associated with insulin resistance (1) and with established type 2 diabetes (2). Previous studies demonstrated that uric acid is an independent predictor of incident type 2 diabetes in general populations (3,4), but whether uric acid predicts incident type 2 diabetes in individuals who have abnormal glucose tolerance is unknown. We examined whether baseline uric acid levels predict incident type 2 diabetes by glucose tolerance status in older adults.
RESEARCH DESIGN AND METHODS
Participants were older adults who had an oral glucose tolerance test and uric acid measured between 1984 and 1987. The 566 participants without baseline diabetes by history and oral glucose tolerance test were evaluated for incident diabetes an average of 13 years later (±0.85 years; maximum 22 years). Baseline glucose tolerance status was defined by American Diabetes Association criteria as 1) normoglycemia (n = 276): fasting plasma glucose <100 mg/dl and 2-h postchallenge glucose <140 mg/dl; 2) impaired fasting glucose (IFG; n = 152): fasting plasma glucose ≥100 mg/dl and <126 mg/dl; and 3) impaired glucose tolerance (IGT; n = 167): 2-h postchallenge glucose ≥140 mg/dl and <200 mg/dl.
Participants provided written informed consent. The Human Research Protection Program at the University of California, San Diego, approved the study protocol.
Laboratory and anthropometric data were collected as described (5). Incident type 2 diabetes was defined as follows: fasting blood glucose ≥126 mg/dl and/or postchallenge glucose ≥200 mg/dl and/or physician-prescribed medication for type 2 diabetes. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula: 186 × [plasma creatinine (mg/dl)−1.154 × age (years)−0.203 × (1.212 if black) × (0.742 if female)] (6).
Analyses were performed using SPSS, version 13.1 (SPSS, Chicago, IL). The association between uric acid and incident type 2 diabetes was examined using multivariate regression models after adjustment for potential confounding variables (age, sex, BMI, diuretic use, and estimated glomerular filtration rate). Receiver-operating characteristic curves were constructed to calculate sensitivity and specificity of uric acid in identifying incident type 2 diabetes by glucose tolerance status. P values <0.05 were considered significant.
RESULTS
Mean age at baseline was 63.3 ± 8.6 years; 41% were men. During follow-up, there were 55 (9.7%) new cases of type 2 diabetes (8, 25, and 22 among normoglycemia, IFG, and IGT groups, respectively). At baseline, participants who developed type 2 diabetes during the follow-up had higher BMI (27 ± 3.2 vs. 24.6 ± 3.2 kg/m2, P < 0.001), blood pressure levels (systolic: 136.8 ± 16.1 vs. 129.4 ± 17.7 mmHg, P = 0.003; diastolic: 80 ± 8.5 vs. 75.7 ± 8.5 mmHg, P < 0.001), total cholesterol/HDL ratio (4.3 ± 1.2 vs. 3.7 ± 1.1, P < 0.001), and uric acid levels (6.8 ± 1.3 vs. 5.6 ± 1.2 mg/dl, P < 0.001) compared with those who did not develop diabetes.
In regression models adjusted for age, sex, BMI, diuretic use, and estimated glomerular filtration rate, the risk for incident type 2 diabetes in the cohort overall increased by ∼60% for each 1 mg/dl increment in uric acid levels (odds ratio [OR] 1.65, 95% CI 1.25–2.18, P < 0.001). Uric acid still predicted incident type 2 diabetes (OR 1.63, 95% CI 1.21–2.19, P = 0.001) after including IFG and/or IGT in the model (OR 4.7, 95% CI 2–11, P < 0.001). In the same adjusted analyses stratified by glucose status, uric acid levels independently predicted incident type 2 diabetes among the 152 subjects who had IFG (OR 1.75, 95% CI 1.1–2.9, P = 0.02) but not among those with normoglycemia (OR 2.1, 95% CI 0.93–4.84, P = 0.07) or IGT (OR 1.42, 95% CI 0.9–2.3, P = 0.15). Further adjustment for regular physical exercise, family history of type 2 diabetes, and triglyceride levels did not materially change the results.
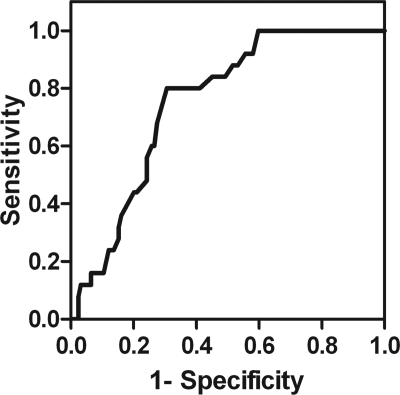
Among participants with IFG, a uric acid level >6.35 mg/dl had 80% sensitivity and 70% specificity to identify individuals who later developed type 2 diabetes (area under receiver-operating characteristic curve 0.751) (Fig. 1). The negative predictive value of a uric acid level <5.35 mg/dl was 100%; this value was calculated using its receiver-operating characteristic curve sensitivity (100%) and specificity (40%) based on the prevalence of diabetes in the U.S population (7). In our sample, 30% of individuals who had IFG also had uric acid levels <5.35 mg/dl.
Figure 1.
Serum uric acid receiver-operating characteristic curve of individuals with impaired fasting glucose for type 2 diabetes incidence.
CONCLUSIONS
In this population-based study, uric acid levels independently predicted incident type 2 diabetes (after adjustment for variables known to be associated with type 2 diabetes) only among individuals with IFG.
The association of uric acid levels and type 2 diabetes incidence has been reported in two other populations: in a 6-year follow-up study of 2,310 Japanese male adults, OR the odds ratio for type 2 diabetes incidence of the highest uric acid quintile compared to lower quintiles was 1.63 (95% CI 1.2–2.2, P < 0.001) after adjustments for age, sex, BMI, and other covariates (4). The highest quartile of uric acid was also associated with incident type 2 diabetes in the Rotterdam study (4,536 adults followed for 10 years) (3). Although these studies demonstrated an association of uric acid with type 2 diabetes incidence, neither addressed the association of uric acid with incident type 2 diabetes by glucose tolerance status.
Uric acid levels had high specificity for individuals with IFG. The current recommendation is to perform an oral glucose tolerance test in individuals with IFG to better define the risk of type 2 diabetes (8); but testing glucose tolerance is unpleasant and expensive. In this small cohort, a simple measurement of uric acid <5.35 mg/dl demonstrated a 100% negative predictive value of incident type 2 diabetes over a maximum 22-year follow-up. If confirmed, this simple inexpensive test might help to identify older adults with IFG who are at little risk for developing type 2 diabetes and would not need an oral glucose tolerance test. This observation would be important, since in the U.S., the elderly have the greatest current burden and expected increase in prevalence of type 2 diabetes (9,10).
This study has limitations. The Rancho Bernardo cohort is a homogeneous population (largely Caucasian and middle class), so results may not be generalizable to other populations. The average age of participants was 68 years at baseline; the role of uric acid in predicting incident type 2 diabetes among younger adults needs further study. Because of the small number of new type 2 diabetes cases, this study had limited power to exclude complete effectiveness of uric acid prediction among IGT and normoglycemia groups. This does not obscure the main result, showing that uric acid adds to the prediction of type 2 diabetes among adults with IFG.
In conclusion, adding uric acid to fasting blood glucose may help identify older adults with IFG who are at low risk of diabetes and who do not need a glucose tolerance test.
Acknowledgments
The Rancho Bernardo Study was funded by the National Institutes of Health/National Institute on Aging Grant AG07181 and Grant AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK31801. C.K.K. was a recipient of a grant from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) Brazil (Programa de Doutorada no Pais com Estagio no Exterior [PDEE] sandwich).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Modan M, Halkin H, Karasik A, Lusky A. Elevated serum uric acid: a facet of hyperinsulinaemia. Diabetologia 1987; 30: 713– 718 [DOI] [PubMed] [Google Scholar]
- 2. Wun YT, Chan CS, Lui CS. Hyperuricaemia in type 2 diabetes mellitus. Diabetes Nutr Metab 1999; 12: 286– 291 [PubMed] [Google Scholar]
- 3. Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008; 31: 361– 362 [DOI] [PubMed] [Google Scholar]
- 4. Chien KL, Chen MF, Hsu HC, Chang WT, Su TC, Lee YT, Hu FB. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem 2008; 54: 310– 316 [DOI] [PubMed] [Google Scholar]
- 5. Kramer CK, von Muhlen D, Gross JL, Laughlin GA, Barrett-Connor E. Blood pressure and fasting plasma glucose rather than metabolic syndrome predict coronary artery calcium progression: the Rancho Bernardo Study. Diabetes Care 2009; 32: 141– 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461– 470 [DOI] [PubMed] [Google Scholar]
- 7. Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 2006; 29: 1263– 1268 [DOI] [PubMed] [Google Scholar]
- 8. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32 ( Suppl. 1): S13– S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001; 24: 1936– 1940 [DOI] [PubMed] [Google Scholar]
- 10. Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the U.S.: 1990–1998. Diabetes Care 2000; 23: 1278– 1283 [DOI] [PubMed] [Google Scholar]