Abstract
OBJECTIVE
Insulin pump therapy (continuous subcutaneous insulin infusion [CSII]) and multiple daily injections (MDIs) with insulin glargine as basal insulin and mealtime insulin lispro have not been prospectively compared in people naïve to either regimen in a multicenter study. We aimed to help close that deficiency.
RESEARCH DESIGN AND METHODS
People with type 1 diabetes on NPH-based insulin therapy were randomized to CSII or glargine-based MDI (both otherwise using lispro) and followed for 24 weeks in an equivalence design. Fifty people were correctly randomized, and 43 completed the study.
RESULTS
Total insulin requirement (mean ± SD) at end point was 36.2 ± 11.5 units/day on CSII and 42.6 ± 15.5 units/day on MDI. Mean A1C fell similarly in the two groups (CSII −0.7 ± 0.7%; MDI −0.6 ± 0.8%) with a baseline-adjusted difference of −0.1% (95% CI −0.5 to 0.3). Similarly, fasting blood glucose and other preprandial, postprandial, and nighttime self-monitored plasma glucose levels did not differ between the regimens, nor did measures of plasma glucose variability. On CSII, 1,152 hypoglycemia events were recorded by 23 of 28 participants (82%) and 1,022 in the MDI group by 27 of 29 patients (93%) (all hypoglycemia differences were nonsignificant). Treatment satisfaction score increased more with CSII; however, the change in score was similar for the groups. Costs were ∼3.9 times higher for CSII.
CONCLUSIONS
In unselected people with type 1 diabetes naïve to CSII or insulin glargine, glycemic control is no better with the more expensive CSII therapy compared with glargine-based MDI therapy.
Insulin substitution in type 1 diabetes is based on mealtime rapid-acting and basal insulin, using multiple daily injections (MDIs) or continuous subcutaneous insulin infusion (CSII) (1,2). In meta-analyses of studies, two small trials (3,4) with the long-acting insulin analog insulin glargine suggested superiority of CSII over MDI in terms of A1C lowering. Lower A1C levels and less hypoglycemia with CSII were also recently reported by Hoogma et al. (5) in a large type 1 diabetes population (272 people) comparing CSII with MDI using NPH insulin. A more recent meta-analysis (6) proposed that CSII is beneficial to “selected” people (people with recurrent and frequent severe hypoglycemia on MDI using NPH insulin) with type 1 diabetes.
Since 2000, the long-acting insulin analogs, insulin glargine and insulin detemir, have become available (2). These are progressively replacing NPH insulin as basal insulin in type 1 diabetes due to favorable pharmacokinetics and pharmacodynamics, namely, a less pronounced peak concentration and longer duration of action (7,8) resulting in lower A1C levels and less hypoglycemia (9,10). Additionally, the long-acting analogs may give more reproducible effects compared with NPH insulin (11,12).
This better glycemic control with the new analogs has reopened the question of comparisons between CSII and MDI based on long-acting analogs, rather than NPH insulin. A number of studies have attempted to compare CSII with MDI using insulin glargine, but not insulin detemir, as basal insulin (13–17). However, these studies are small, nonrandomized, or of short duration.
The aim of the present prospective, randomized, multicenter, international study was to assess the difference in glycemic control when people with type 1 diabetes using NPH insulin-based MDIs are randomized either to an MDI regimen with insulin glargine as basal insulin and mealtime insulin lispro or to continuous subcutaneous infusion of insulin lispro and managed on either regimen for 6 months.
RESEARCH DESIGN AND METHODS
Our primary objective was to investigate whether an MDI regimen using insulin glargine as basal insulin with insulin lispro at mealtimes can achieve glycemic control (A1C) equivalent to CSII (insulin lispro) in people with type 1 diabetes. Secondary objectives were to compare other measures of blood glucose control (pre- and postprandial, bedtime and 0300 h levels, and within-day glucose variability), hypoglycemia, adverse events (AEs), treatment costs, and treatment satisfaction.
This was a randomized, parallel-group, open-label, multicenter (n = 5) study performed in three European countries with a 1-week run-in period followed by a 24-week treatment period (including 4 weeks of active dose titration), with clinic visits at 0, 2, 8, 16, and 24 weeks and 2 weeks follow-up. An open-label design was necessary owing to the different insulin administration regimens. Concealed remote randomization was by an online system. Ethical approval was by local procedures, and all participants provided written informed consent. The study was performed according to Good Clinical (Research) Practice/Declaration of Helsinki.
Participants were aged 18–70 years, with a BMI ≤27.0 kg/m2 and diabetes for >1 year, A1C 6.5–9.0%, fasting plasma glucose (FPG) >7.0 mmol/l (>126 mg/dl), and fasting plasma C-peptide <0.10 nmol/l at the screening visit. Participants with a mean duration of treatment of 18.4 (CSII) and 20.7 (MDI) years, who were currently using an MDI regimen with NPH insulin, were recruited and randomized. Participants were excluded if they were prior users of CSII or insulin glargine, were unwilling or unable to use CSII or MDIs, had more than two severe hypoglycemic events in the previous 6 months, or had recent diabetic ketoacidosis or impaired hepatic/renal function.
Study treatments
Randomization was to insulin glargine (LANTUS, once daily in the evening; sanofi-aventis, Paris, France) plus mealtime insulin lispro (Humalog, three-times daily; Eli Lilly, Indianapolis, IN) or to insulin lispro administered subcutaneously using a MiniMed 508 pump (MiniMed Technologies, Northridge, CA). Insulin dose titration was to the same glucose targets on both treatments: FPG 4.4–6.6 mmol/l (80–120 mg/dl), other preprandial blood glucose 5.0–7.7 mmol/l (90–140 mg/dl), 2-h postprandial blood glucose <7.7 mmol/l (<140 mg/dl), and bedtime blood glucose 6.1–8.3 mmol/l (110–150 mg/dl).
Insulin glargine was started according to prior basal insulin dose. Titration was based on the mean FPG over at least 3 days, adjusted by +2 units if FPG was 6.6–8.8 mmol/l (120–160 mg/dl), +4 units if FPG was 8.8–11 mmol/l (160–200 mg/dl), or −2 units if FPG was <4.4 mmol/l (<80 mg/dl). Further titration of the dose was performed at investigator discretion if symptoms consistent with severe hypoglycemia occurred. The initial insulin lispro dose was investigator selected, based on prior mealtime doses, and was titrated once basal insulin dose was optimized. The insulin lispro dose was adjusted by ±1–2 units, as appropriate. Initiation of CSII was based on the pump manufacturer's recommendations, using the “normal bolus option”; a bolus wizard calculator was not used. Infusion sites were advised to be changed every 2–3 days in accordance with investigators' routine clinical practice and manufacturer's recommendations.
Study measurements
Analysis of A1C was performed at a central laboratory (Diabetes Control and Complications Trial [DCCT] Research Group aligned) at screening and weeks 8, 16, and 24. Participants were asked to perform self-monitored plasma glucose measurements four times daily (bedtime and preprandial) using a plasma-calibrated memory glucose meter (OneTouch Ultra; Lifescan, Milpitas, CA). Eight-point profiles (pre-/postprandial, bedtime, 0300 h) were requested on 3 days (including 1 weekend day) in the 2-week period prior to each study visit. All results were diary recorded. Participants were asked to complete Diabetes Treatment Satisfaction Questionnaires (DTSQs) (status DTSQ and change DTSQ) (18) at randomization and week 24.
The costs of treatment were calculated in euros (€) based on the cost of therapies, devices, and consumables. Equipment costs include the glucose meters and consumables (lancets and test strips), insulin pens and consumables (needles), and insulin pumps and consumables (batteries, and infusion sets). The pump acquisition cost was amortized over 5 years. The costs of insulin were calculated by two methods: insulin dispensed and insulin used. Insulin dispensed was based on the entire cost of treatment, including wastage according to dispensing records (based on pharmacy and investigator dispensing records); insulin used was based on insulin doses recorded in treatment diaries by participants.
Hypoglycemia was recorded in diaries and extracted at study visits. Nonsevere hypoglycemia was defined as symptoms consistent with hypoglycemia not requiring the assistance of another person and confirmed by plasma glucose <4.0 mmol/l (<72 mg/dl); severe hypoglycemia was defined as similar symptoms but requiring management assistance and either plasma glucose <2.0 mmol/l (<36 mg/dl) or prompt recovery after oral or intravenous carbohydrate or glucagon; nocturnal hypoglycemia was defined as between bedtime and rising. Other AEs were collected by direct questioning at study visits. Unprogrammed changes of CSII or injection equipment were also captured.
Statistical analyses
Sixty randomized individuals were estimated to give 80% power, after 15% dropout, to demonstrate equivalence at ±0.6% A1C at a mean A1C of 7.0% and an SD of 0.7%. ANCOVA, with center and group as the fixed variables and baseline values as covariates, was used for continuous variables. The Cochran-Mantel-Haenszel test, stratified by center, was used for discrete end points. Two-sided 95% CIs for treatment differences were calculated without adjustment for multiple end points. Safety measures and adverse effects were compared between treatment groups using the Wilcoxon test. Data were summarized as means ± SD or means (95% CI). The mean amplitude of glycemic excursion was calculated from the eight-point plasma glucose profiles. All results are presented for the per-protocol population as is appropriate for an equivalence study to minimize bias. This population included all randomized participants treated with at least one dose of study insulin and without a major protocol deviation that would interfere with treatment efficacy. An intent-to-treat (ITT) analysis (all randomized patients) was also performed as a secondary analysis. Safety and hypoglycemia were reported for all participants who took at least one dose of study insulin and provided follow-up data (safety population).
RESULTS
Study population
Of 67 people screened, 58 met inclusion criteria and were randomized: 28 to CSII and 30 to MDI. One participant was randomized in error and was not treated. Of 57 patients treated, 7 were protocol violators, 1 had a baseline A1C >9.0%, 1 had previously used insulin glargine/CSII, 1 had no C-peptide status, and 4 had used corticosteroids/glucocorticoids. Therefore, the population analyzed (per-protocol population, n = 50) included 24 patients in the CSII group and 26 in the MDI group (Table 1); the ITT and safety analysis comprised 57 patients (screening failures excluded). Seven participants dropped out before completion—three withdrawing consent, one due to an AE, one due to pregnancy, and two due to protocol noncompliance. Baseline characteristics were similar for both treatment groups (Table 1).
Table 1.
Baseline characteristics of the people with type 1 diabetes studied (per-protocol population)
| CSII (n = 24)* | MDI (n = 26)† | |
|---|---|---|
| n (men/women) | 13/11 | 14/12 |
| Age (years) | 37.6 ± 12.3 | 42.4 ± 9.9 |
| Body weight (kg) | 70.1 ± 11.6 | 70.8 ± 10.5 |
| BMI (kg/m2) | 23.8 ± 2.7 | 24.3 ± 1.9 |
| Duration of diabetes (years) | 18.5 ± 8.4 | 20.9 ± 10.6 |
| Age at diagnosis (years) | 19.1 ± 10.8 | 21.5 ± 11.5 |
| Duration of treatment (years) | 18.4 ± 8.5 | 20.7 ± 10.5 |
| Prior insulin dose (units/day)‡ | ||
| Basal | 20.2 ± 12.7 | 20.0 ± 7.5 |
| Total | 51.0 ± 15.7 | 51.2 ± 16.8 |
| A1C (%) | 7.7 ± 0.7 | 7.8 ± 0.6 |
Data are means ± SD. The per-protocol population consisted of patients who were correctly randomized and receiving study insulin.
*Insulin lispro;
†insulin glargine plus prandial insulin lispro;
‡prerandomization dose.
Insulin dose
In the CSII group after randomization, basal and prandial doses were 17.2 ± 8.5 and 17.4 ± 6.7 units/day, respectively (total: 34.6 ± 13.0 units/day), and at 24 weeks 18.2 ± 8.0 and 18.0 ± 6.1 units/day, respectively (total: 36.2 ± 11.5 units/day). In the MDI group after randomization, basal and prandial doses were 19.6 ± 6.3 and 25.7 ± 12.0 units/day, respectively (total: 45.3 ± 15.0 units/day), and at 24 weeks 22.5 ± 7.0 and 20.1 ± 9.8 units/day, respectively (total: 42.6 ± 15.5 units/day). Similar changes in insulin dose were observed for the ITT population.
Blood glucose control
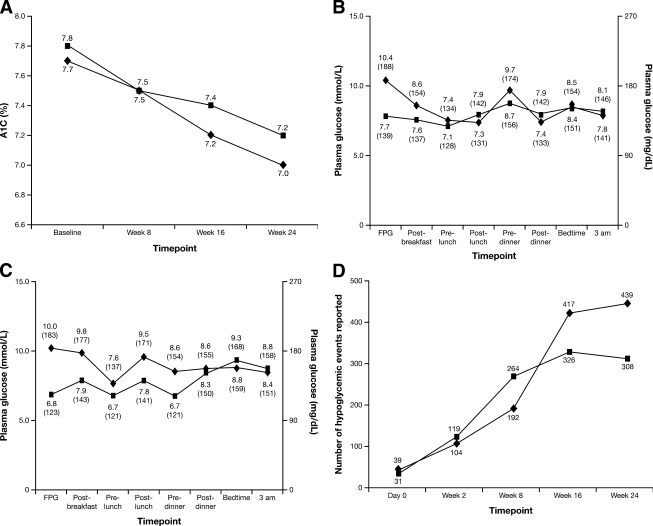
A similar decrease in A1C from baseline to end point was observed in the two groups (Fig. 1A): −0.7 ± 0.7% on CSII and −0.6 ± 0.8% on MDI, with a baseline-adjusted difference of −0.1% (95% CI −0.5 to 0.3). Mean blood glucose decreased in both treatment groups (Table 2) (Fig. 1), with a difference of 0.03 mmol/l (−0.8 to 0.8) (0.6 mg/dl [−13.5 to 14.7]). The primary efficacy results for the ITT population were similar.
Figure 1.
A: Data are the change in A1C (%) from baseline over the course of the study in the per-protocol population with type 1 diabetes managed with CSII with insulin lispro (insulin pump therapy) or insulin glargine plus insulin lispro MDI therapy. ♦, CSII; ■, MDI. B: Data are the mean eight-point blood glucose profiles at baseline and end point over the course of the study for people in the per-protocol population randomized to insulin glargine plus insulin lispro MDI therapy. ♦, baseline; ■, end point. C: Data are the mean eight-point blood glucose profiles at baseline and end point over the course of the study for people in the per-protocol population randomized to CSII with insulin lispro (insulin pump therapy). ♦, baseline; ■, end point. D: Data are the number of hypoglycemic events by visit for the two insulin regimens (safety population). ♦, CSII; ■, MDI.
Table 2.
Summary of blood glucose control results (per-protocol population)
| Parameter | CSII | MDI | Difference at end point (95% CI) |
|---|---|---|---|
| A1C (%) | |||
| Baseline | 7.7 ± 0.7 | 7.8 ± 0.6 | |
| End point | 7.0 ± 0.8 | 7.2 ± 0.7 | −0.1 (−0.5 to 0.3) |
| SMBG [mmol/l (mg/dl)] | |||
| Fasting | |||
| Baseline | 10.1 ± 3.3 (183 ± 60) | 10.4 ± 3.2 (188 ± 57) | |
| End point | 6.8 ± 1.7 (123 ± 30) | 7.7 ± 2.4 (139 ± 43) | −0.7 (−1.8 to 0.5) |
| Preprandial | |||
| Baseline | 7.8 ± 3.6 (140 ± 64) | 8.0 ± 3.0 (145 ± 53) | |
| End point | 6.7 ± 2.4 (120 ± 43) | 7.7 ± 2.4 (138 ± 42) | −0.9 (−2.3 to 0.4) |
| Postprandial | |||
| Baseline | 8.4 ± 3.6 (151 ± 66) | 6.9 ± 2.6 (125 ± 47) | |
| End point | 8.2 ± 2.3 (147 ± 42) | 7.6 ± 2.1 (136 ± 39) | 0.3 (−1.1 to 1.7) |
| 0300 h blood glucose [mmol/l (mg/dl)] | |||
| Baseline | 8.4 ± 4.8 (151 ± 87) | 7.8 ± 5.0 (141 ± 90) | |
| End point | 8.8 ± 2.3 (158 ± 42) | 8.1 ± 4.3 (146 ± 78) | 3.0 (−0.4 to 6.5) |
| Blood glucose [mmol/l (mg/dl)] | |||
| Baseline | 9.1 ± 2.3 (164 ± 41) | 8.9 ± 1.6 (160 ± 30) | |
| End point | 8.1 ± 1.8 (146 ± 32) | 8.0 ± 1.1 (144 ± 20) | 0.03 (−0.7 to 0.8) |
| MAGE [mmol/l (mg/dl)] | |||
| Baseline | 8.0 ± 2.4 (144 ± 43) | 7.6 ± 1.7 (137 ± 31) | |
| End point | 6.4 ± 2.2 (115 ± 40) | 6.4 ± 2.1 (115 ± 38) | −0.4 (−1.8 to 1.0) |
Data are means ± SD unless otherwise indicated. The per-protocol population consisted of patients who were correctly randomized and receiving study insulin. MAGE, mean amplitude of glycemic excursion; SMBG, self-monitored blood glucose.
Fasting blood glucose levels improved in both groups (Table 2), with a difference of −0.7 mmol/l (95% CI −1.8 to 0.5) (−12.3 mg/dl [–32.9 to 8.2]). Coefficient of variation (CV) of fasting blood glucose was unchanged (CSII from 41 ± 12 to 42 ± 13%; MDI from 43 ± 18 to 45 ± 12%) and did not differ between groups (difference: −2.4% [−9.5 to 4.7]). Preprandial blood glucose levels decreased in both groups (Table 2), with a treatment difference of −0.9 mmol/l (−2.3 to 0.4) (−17.1 mg/dl [–42.1 to 8.0]). Postprandial blood glucose levels decreased in the CSII group and increased in the MDI group (Table 2) but with no statistically significant differences between groups (difference: 0.3 mmol/l [−1.1 to 1.7]) (5.5 mg/dl [–18.9 to 29.9]). At 0300 h, plasma glucose increased with both CSII and MDI treatment (Table 2), with a treatment difference of 3.0 mmol/l (−0.4 to 6.5) (54.8 mg/dl [–7.2 to 116.7]).
Baseline and end point eight-point blood glucose profiles are shown in Fig. 1B and C. The CV of the eight-point blood glucose profile decreased from 53 ± 10 to 46 ± 8% (CSII) and from 52 ± 12 to 47 ± 11% (MDI), a treatment difference of −1.4% (95% CI −6.6 to 3.7). The mean amplitude of glycemic excursion decreased similarly in both groups (Table 2), with a treatment difference of −0.4 mmol/l (−1.8 to 1.0) (−7.1 mg/dl [–31.9 to 17.8]). The ITT population was comparable with the per-protocol results for all secondary end points of blood glucose control.
Hypoglycemia
On CSII, 1,152 hypoglycemic events were recorded by 23 of 28 participants (82%), and in the MDI group 1,022 hypoglycemic events were recorded by 27 of 29 participants (93%). The incidence of hypoglycemia was similar with CSII and MDI for overall (41 ± 43 vs. 35 ± 35 events/patient; P = 0.93), nonsevere (35 ± 37 vs. 31 ± 32 events/patient; P = 0.97), nocturnal (3 ± 5 vs. 5 ± 7 events/patient; P = 0.34), symptomatic (13 ± 12 vs. 14 ± 15 events/patient; P = 0.84), and asymptomatic (1.2 ± 2.0 vs. 1.4 ± 2.3 events/patient; P = 0.95) hypoglycemia. Two participants in both groups experienced one severe hypoglycemic event. Number of events by visit is shown in Fig. 1D.
Treatment satisfaction
The DTSQ treatment satisfaction score (±SD) increased from 22.8 ± 8.1 at baseline to 31.5 ± 4.9 at 24 weeks in the CSII group and from 24.0 ± 6.3 to 28.8 ± 5.4 in the MDI group (treatment difference: 3.1 [95% CI 0.1–6.1]; P = 0.042). Differences between treatment groups regarding perception of hyperglycemia and hypoglycemia were not statistically significant. At 24 weeks, change DTSQ values of 13.3 ± 5.3 (CSII) and 12.9 ± 4.9 (MDI) were observed (difference: 0.4 [−2.4 to 3.3]), with no statistical difference between groups in perception of hyperglycemia and hypoglycemia. Results of the ITT analyses were comparable.
Costs
At the time of study conduct, the unit cost of insulin for CSII was €0.021 and for prandial and basal insulin on MDI was €0.024 and €0.025, respectively. The average cost of insulin dispensed per subject was €295 for CSII (range 131–657) and €293 for MDI (€127 prandial and €166 basal) (range 211–755). The average cost of insulin used per participant in the study based on diaries was €140 for CSII (range 76–283) and €212 for MDI (€96 prandial and €116 basal) (range 135–444).
The average cost per treatment during the study, including all items of equipment and dispensed insulin, was ∼3.9 times higher for insulin lispro–based CSII versus MDI (€3,020 vs. €778, respectively), including the cost of the pump and cannula insertion kits (see online appendix Table 1 [available at http://dx.doi.org/10.2337/dc08-1874]).
Safety profile
A total of 115 AEs were reported, 1 of which was not treatment emergent. Therefore, a total of 18 patients experienced 59 treatment-emergent AEs in the CSII group, and 22 patients experienced 56 treatment-emergent AEs in the MDI group. One patient in the MDI group withdrew due to an AE (skin rash) and one due to pregnancy. Two individuals on CSII each reported one serious AE (both of severe hypoglycemia).
Three participants from three centers had pumps replaced once, as soon as failure was suspected. However, no mechanical failure was detected by the manufacturer in these cases. Twenty instances of giving set occlusion were reported for nine participants from five centers. Seven patients had one occlusion reported; one of these later experienced two additional events and had six changes of the giving set as a preventative measure due to an episode of infection at the infusion site (not recorded as occlusion events); one participant had two events, and one reported nine events.
CONCLUSIONS
This study suggests that the optimization of basal NPH insulin replacement with insulin lispro by CSII or an MDI-based regimen of once-daily insulin glargine plus insulin lispro results in similar improvement of glycemic control in people with type 1 diabetes who are naïve to either treatment regimen. Both regimens achieved similar improvements in A1C, self-monitored plasma glucose, and hypoglycemia.
The best estimate of difference between the regimens is −0.1% A1C, but study power implies that the actual difference could be between a CSII advantage of 0.5% and an MDI advantage of 0.3%. The present study is limited by the number of participants and a duration of 6 months but has the advantage of being the first prospective, multicenter study demonstrating noninferiority of the insulin glargine–based regimen versus CSII. Another possible limitation of the present study is that the MiniMed 508 pump, rather than the recent Paradigm 522 or 722 pump with the bolus wizard, was used. The latter pumps might be helpful to estimate bolus doses by calculating the insulin-to-carbohydrate ratio, insulin sensitivity factor, target blood glucose, and insulin on board (putative length of action of insulin bolus). However, randomized, controlled trials are required in the adult population to prove these benefits. In addition, an expert system might prove useful in MDI as well.
The mean end-of-study A1C levels for both regimens were close to American Diabetes Association targets and were similar to those in the intensive arm of the DCCT trial (1,19). Although the baseline levels were higher on NPH insulin regimens, the present study did not examine the use of NPH insulin and the fall from baseline may have been a study effect, though consistent with previous comparisons of CSII and insulin glargine–based MDI with NPH insulin–based MDI (16,17). However, NPH insulin regimens can be optimized by multiple NPH insulin injections (20).
As expected, intensification of blood glucose control and careful recording resulted in increased reported rates of nonsevere hypoglycemia with no difference between regimens. Severe hypoglycemia was rare, but the inclusion criteria excluded people prone to this problem. Others have noted that the DCCT-derived conclusion of an increase in severe hypoglycemia is not necessarily reproduced when intensive insulin therapy is based on insulin analogs (9,10).
Although blood glucose control with CSII and insulin glargine–based MDI was similar by other measures, plasma glucose predinner was higher with the latter regimen. This elevation in blood glucose in the late afternoon hours on an insulin glargine–based MDI regimen has been noted previously (21). In some people with type 1 diabetes, the duration of action of insulin glargine appears to be <24 h. However, continued food absorption beyond the duration of action of the lunchtime insulin may be another cause of predinner hyperglycemia, as the phenomenon is attenuated when lunch is skipped (21). With CSII it is possible that this was counteracted by higher basal rates in the afternoon, but these data were not collected. With insulin glargine, an additional bolus of rapid-acting analog 2–3 h after lunch can be given (21); this was not part of the protocol of the present study. Interestingly, the theoretical advantage of multiple basal rates with CSII did not translate into lower basal plasma glucose levels at other times of day (Fig. 1C). Furthermore, this study confirms that the magnitude of any “dawn phenomenon” is modest when basal insulin is optimally replaced with either CSII or an insulin glargine regimen (22).
In the present study, glucose variability with CSII and insulin glargine–based MDI was not different. A previous study (23) that compared people on long-term CSII with those switched to insulin glargine–based MDI found a small advantage of CSII in glucose variability, despite no difference in overall glycemic control. However, the importance of variability of blood glucose when A1C, hypoglycemia, and plasma glucose profile are identical is doubtful. Other data support the importance of glucose variability for vascular complications in diabetes, but clinical studies have yet to confirm this (24).
That the costs of CSII are higher than those of MDI is perhaps obvious given the costs of pumps and infusion sets. There will also be a small saving in any market where insulin glargine is more expensive than insulin lispro. In economic terms, with similar efficacy but higher costs, CSII is “dominated” by MDI. The National Institute for Health and Clinical Excellence (U.K.) calculated a higher cost in the order of €1,392 to €1,772 per year, compatible with our results (25).
The present study adds to previous observations (14,15,17) of the noninferiority of insulin glargine–based MDI versus CSII in different study designs. A similar conclusion to ours was recently reported (23) in a study in which people with type 1 diabetes were transferred from long-term CSII initiated primarily in the NPH insulin era to insulin glargine–based MDI. Thus, it is likely that the majority of people with type 1 diabetes who started CSII in the NPH insulin era did so due to the poor performance of basal NPH insulin. Similarly, it is likely that the majority of these people might today switch to MDI based on insulin glargine with no deterioration of blood glucose control (23).
While the conclusions of the present study establish noninferiority of insulin glargine–based MDI against CSII in unselected (except for study purposes) people with type 1 diabetes, they cannot be applied to “selected” people with type 1 diabetes who may have special indications to CSII, such as long duration of disease with low insulin requirements or hypoglycemia unawareness with frequent, severe hypoglycemia on long-acting insulin analog–based MDI (6). Additional studies are required in these groups of selected people with type 1 diabetes to establish the possible equivalence or otherwise of insulin glargine–based MDI against CSII with regard to glycemic control and the incidence and awareness of hypoglycemia.
Supplementary Material
Acknowledgments
Various authors, on behalf of themselves or the institutions in which they are involved, have received funds from sanofi-aventis for research, lecturing/educational, advisory, or health development activities. G.B.B. has received honoraria for scientific consulting and lecturing and financial research support from sanofi-aventis, Eli Lilly, Novo Nordisk, Medtronic, and Roche Diagnostics. P.D.H. does not take fees personally but has research/educational/project support from all major insulin manufacturers, including sanofi-aventis. R.T. was supported through Newcastle University by sanofi-aventis during the course of this study. The study was devised by G.B.B. and P.D.H. and supported by sanofi-aventis, the manufacturers of insulin glargine. Editorial support was provided by the global publications group of sanofi-aventis. No other potential conflicts of interest relevant to this article were reported.
We thank the people with type 1 diabetes who gave their time to this study.
APPENDIX
List of investigators: France: S.G. and J.L.S.; Italy: G.B.B., Fabio Capani, E.T. and E.V.; U.K.: P.D.H., D.K. and R.T.
Footnotes
Clinical trial reg. no. NCT00540709, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 2. Owens DR, Zinman B, Bolli GB: Insulins today and beyond. Lancet 2001; 358: 739– 746 [DOI] [PubMed] [Google Scholar]
- 3. Pickup J, Mattock M, Kerry S: Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2002; 324: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, Siebenhofer A: Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008; 51: 941– 951 [DOI] [PubMed] [Google Scholar]
- 5. Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, Wiefels KJ, de la Calle H, Schweitzer DH, Pfohl M, Torlone E, Krinelke LG, Bolli GB: Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med 2006; 23: 141– 147 [DOI] [PubMed] [Google Scholar]
- 6. Pickup JC, Sutton AJ: Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008; 25: 765– 774 [DOI] [PubMed] [Google Scholar]
- 7. Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, Cordoni C, Costa E, Brunetti P, Bolli GB: Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000; 49: 2142– 2148 [DOI] [PubMed] [Google Scholar]
- 8. Porcellati F, Rossetti P, Busciantella NR, Marzotti S, Lucidi P, Luzio S, Owens DR, Bolli GB, Fanelli CG: Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes mellitus: a double-blind, randomized, cross-over study. Diabetes Care 2007; 30: 2447– 2452 [DOI] [PubMed] [Google Scholar]
- 9. Ashwell SG, Amiel SA, Bilous RW, Dashora U, Heller SR, Hepburn DA, Shutler SD, Stephens JW, Home PD: Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with type 1 diabetes. Diabet Med 2006; 23: 285– 292 [DOI] [PubMed] [Google Scholar]
- 10. Porcellati F, Rossetti P, Pampanelli S, Fanelli CG, Torlone E, Scionti L, Perriello G, Bolli GB: Better long-term glycaemic control with the basal insulin glargine as compared with NPH in patients with type 1 diabetes mellitus given meal-time lispro insulin. Diabet Med 2004; 21: 1213– 1220 [DOI] [PubMed] [Google Scholar]
- 11. Gerich J, Becker RH, Zhu R, Bolli GB: Fluctuation of serum basal insulin levels following single and multiple dosing of insulin glargine. Diabetes Technol Ther 2006; 8: 237– 243 [DOI] [PubMed] [Google Scholar]
- 12. Heise T, Nosek L, Ronn BB, Endahl L, Heinemann L, Kapitza C, Draeger E: Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004; 53: 1614– 1620 [DOI] [PubMed] [Google Scholar]
- 13. Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV: A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004; 27: 1554– 1558 [DOI] [PubMed] [Google Scholar]
- 14. Garg SK, Walker AJ, Hoff HK, D'Souza AO, Gottlieb PA, Chase HP: Glycemic parameters with multiple daily injections using insulin glargine versus insulin pump. Diabetes Technol Ther 2004; 6: 9– 15 [DOI] [PubMed] [Google Scholar]
- 15. Harmel AP, Mathur R: Similar A1C outcomes in type 1 diabetic patients undergoing intensive diabetes management with preprandial rapid-acting insulin and either CSII or glargine. Diabetes Care 2004; 27: 272– 273 [DOI] [PubMed] [Google Scholar]
- 16. Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, Santiago OM, Kolaczynski JW: Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28: 533– 538 [DOI] [PubMed] [Google Scholar]
- 17. Lepore G, Dodesini AR, Nosari I, Trevisan R: Both continuous subcutaneous insulin infusion and a multiple daily insulin injection regimen with glargine as basal insulin are equally better than traditional multiple daily insulin injection treatment. Diabetes Care 2003; 26: 1321– 1322 [DOI] [PubMed] [Google Scholar]
- 18. Bradley C: The diabetes treatment satisfaction questionnaire: DTSQ. In Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Bradley C. Ed. Chur, Switzerland, Harwood Academic Publishers, 1994 [Google Scholar]
- 19. American Diabetes Association. Standards of medical care in diabetes—2007 (Position Statement). Diabetes Care 2007; 30( Suppl. 1): S4– S42 [PubMed] [Google Scholar]
- 20. Lalli C, Ciofetta M, Del Sindaco P, Torlone E, Pampanelli S, Compagnucci P, Cartechini MG, Bartocci L, Brunetti P, Bolli GB: Long-term intensive treatment of type 1 diabetes with the short-acting insulin analog lispro in variable combination with NPH insulin at mealtime. Diabetes Care 1999; 22: 468– 477 [DOI] [PubMed] [Google Scholar]
- 21. Rossetti P, Porcellati F, Fanelli C, Bolli G: Mechanisms and treatment of the afternoon phenomenon in patients with type 1 diabetes mellitus using glargine as basal insulin (Abstract). Diabetes 2005; 54 ( Suppl. 1): A68 [Google Scholar]
- 22. Bending JJ, Pickup JC, Collins AC, Keen H: Rarity of a marked “dawn phenomenon” in diabetic subjects treated by continuous subcutaneous insulin infusion. Diabetes Care 1985; 8: 28– 33 [DOI] [PubMed] [Google Scholar]
- 23. Bruttomesso D, Crazzolara D, Maran A, Costa S, Dal Pos M, Girelli A, Lepore G, Aragona M, Iori E, Valentini U, Del Prato S, Tiengo A, Buhr A, Trevisan R, Baritussio A: In type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet Med 2008; 25: 326– 332 [DOI] [PubMed] [Google Scholar]
- 24. Kilpatrick ES, Rigby AS, Atkin SL: The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006; 29: 1486– 1490 [DOI] [PubMed] [Google Scholar]
- 25. National Institute for Health and Clinical Excellence. Diabetes (type 1)-insulin pump therapy: the clinical effectiveness and cost effectiveness of insulin pump therapy, 2003. Available at http://www.nice.org.uk/guidance/index.jsp?action=byID&r= true&o=11492. Accessed 23 June 2008
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.