Abstract
OBJECTIVE
Adiponectin has been postulated to affect lipid and insulin signal transduction pathways. We evaluated the relationships of plasma adiponectin with lipoprotein mean particle size and subclass concentrations, independent of obesity and insulin sensitivity.
RESEARCH DESIGN AND METHODS
A cross-sectional analysis of 884 young Israeli adults who participated in the population-based Jerusalem Lipid Research Clinic (LRC) study was conducted. Lipoprotein particle size was assessed using proton nuclear magnetic resonance.
RESULTS
In multivariable linear regression models that included sex, BMI, waist circumference, homeostasis model assessment of insulin resistance, and leptin, adiponectin was associated with mean LDL size (standardized regression coefficient B = 0.20; P < 0.001), VLDL size (B = −0.12; P < 0.001), and HDL size (B = 0.06; P = 0.013). Adiponectin was inversely related to large VLDL (P < 0.001) but positively to small VLDL (P = 0.02), inversely related to small LDL (P < 0.006) but positively to large LDL (P < 0.001), and positively related to large HDL (P < 0.001) subclass concentrations.
CONCLUSIONS
Adiponectin is favorably associated with lipoprotein particle size and subclass distribution independent of adiposity and insulin sensitivity.
Adiponectin is a fat-derived adipocytokine (1,2) that is strongly associated with insulin sensitivity and favorable cardiovascular outcomes. Given that insulin resistance is associated with reduced adiponectin levels (3), we hypothesized that adiponectin may have a direct role in hepatic lipoprotein metabolism. Thus, the aim of this study was to evaluate the relationships of adiponectin with lipoprotein particle size, independent of the degree of obesity and insulin sensitivity, in a population-based sample of healthy young adults.
RESEARCH DESIGN AND METHODS
The Jerusalem Lipid Research Clinic (LRC) prevalence study has previously been described (4). In a follow-up study of a sex-stratified random sample, we examined 570 men (73% response) and 314 women (68%) aged 28–32 years, as previously reported (5).
Lipoprotein particle size analysis used a 400-MHz proton nuclear magnetic resonance analyzer (LipoScience, Raleigh, NC) (6). Adiponectin was analyzed using an in-house immunofluorometric assay (TRIFMA) as previously described (7). Plasma leptin and insulin concentrations were determined by radioimmunometric methods (Linco, St. Charles, MO). Sex-specific Pearson correlations, partial correlations, and multivariable linear regression modeling were used. Regression results are expressed as standardized coefficient B. Collinearity tolerances were acceptable.
RESULTS
The study population included 884 young adults (570 men and 314 women; mean ± SD age 30.1 ± 0.8 years). Men had greater BMI than women (25.1 ± 3.6 vs. 23.8 ± 3.9 kg/m2; P < 0.001), whereas women had greater adiponectin (8.9 ± 3.4 vs. 6.4 ± 2.6 mg/l; P < 0.001) and leptin concentrations (13.2 ± 8.3 vs. 5.1 ± 3.5 μg/l; P < 0.001).
Adiponectin was associated with lipoprotein mean particle size in both sexes. Its strongest relation in men was an inverse association with VLDL size (r = −0.39; P < 0.001) that persisted after adjustment for BMI, waist circumference, and homeostasis model assessment of insulin resistance (HOMA-IR) (r = −0.28; P < 0.001). This relation was weaker in women (r = −0.21; P < 0.001) and was attenuated by adjustment (r = −0.12; P < 0.05). A positive association of adiponectin with HDL and LDL particle size evident in both sexes (r = ∼0.30–0.35) remained significant after adjustment.
VLDL, LDL, and HDL particle sizes were introduced separately as dependent variables in backward stepwise multivariable linear regression models that included sex, BMI, waist circumference, HOMA-IR, the relevant plasma lipid or lipoprotein (triglycerides, HDL cholesterol, or LDL cholesterol), leptin, and adiponectin. Adiponectin was significantly inversely associated with VLDL size (B = −0.12; P < 0.001), whereas waist circumference (B = 0.12; P < 0.001), HOMA-IR (B = 0.09; P = 0.002), and triglycerides (B = 0.58; P < 0.001) were positively associated (model R2 = 0.57). Adiponectin was associated with larger LDL particle size (B = 0.20; P < 0.001), whereas waist circumference (B = −0.31; P < 0.001) and sex (B = −0.20; P < 0.001) were associated with smaller particle size (model R2 = 0.32). Adiponectin (B = 0.06; P = 0.013) and leptin (B = −0.10; P = 0.001) showed opposite associations with HDL particle size. HDL cholesterol was strongly associated with larger HDL particle size (B = 0.58; P < 0.001), whereas waist circumference (B = −0.14; P < 0.001), HOMA-IR (B = −0.04; P = 0.06), and male sex (B = −0.25; P < 0.001) were associated with smaller HDL particles (model R2 = 0.69).
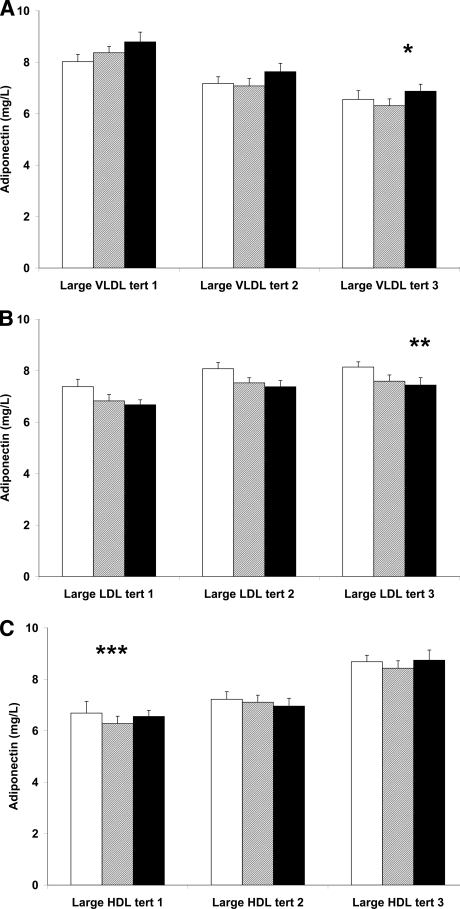
We stratified the study sample into tertiles of lipoprotein subclasses and displayed the concentration of adiponectin in each tertile of a lipoprotein subclass (i.e., large, intermediate, and small) while adjusting for sex, waist circumference, BMI, and HOMA-IR. As shown in Fig. 1A, the adiponectin concentrations differed substantially between tertiles of large VLDLs and were inversely associated with increasing concentrations of large VLDL particles (B = −0.19; P for trend < 0.001). In contrast, adiponectin was highest in the upper small VLDL tertile within each large VLDL tertile category (P for trend = 0.02). Adiponectin was highest in the upper large LDL tertile (P for trend < 0.001) (Fig. 1B). In contrast, within each large LDL tertile, adiponectin decreased consistently with increasing concentrations of small LDL particles (P for trend = 0.009). Adiponectin was positively associated with concentrations of large HDLs (P for trend < 0.001) (Fig. 1C) but was not related to small HDL particle concentrations. Thus, adiponectin concentration showed significant contrasting associations with small and large VLDL and LDL subclasses but was associated with only large HDL and not small HDL particles.
Figure 1.
Adjusted plasma adiponectin concentration as a function of lipoprotein subclass tertiles adjusted for sex, waist circumference, BMI, and HOMA-IR. A: The cohort divided into tertiles of large VLDL concentrations is shown. Each of these tertiles is subdivided into tertiles of small VLDL concentrations. □, the lowest tertile (tert); ▩, the middle tertile; and ■, the upper tertile of small VLDL. B: The cohort divided into tertiles of large LDL concentrations is shown; each of these tertiles is subdivided into tertiles of small LDL concentrations. □, the lowest tertile; ▩, the middle tertile; and ■, the upper tertile of small LDL. C: The cohort divided into tertiles of large HDL concentrations is shown; each of these tertiles is subdivided into tertiles of small HDL concentrations. □, the lowest tertile; ▩, the middle tertile; and ■, the upper tertile of small HDL. *P for trend < 0.001 and 0.02 for large VLDL and small VLDL median tertile values, respectively. **P for trend < 0.001 and 0.009 for large LDL and small LDL median tertile values, respectively. ***P for trend < 0.001 for large HDL median tertile values.
We modeled adiponectin and leptin separately as dependent variables in a backward stepwise procedure that included all lipoprotein subclasses, anthropometric measures (BMI and waist circumference), and HOMA-IR. In men, adiponectin was independently inversely associated with concentrations of large VLDLs and positively associated with small VLDLs and large and intermediate HDLs. Leptin was positively associated only with small VLDLs and adiponectin after adjustment for anthropometric measures and HOMA-IR. In women, adiponectin showed an independent positive association only with large HDLs, whereas leptin was inversely associated with large VLDLs and positively with small and intermediate HDLs.
CONCLUSIONS
This analysis, in ostensibly healthy young adults, demonstrates a substantial association between plasma adiponectin and lipoprotein particle size. Adiponectin was associated with mean particle size of the three lipoprotein moieties after adjustment for measures of obesity, insulin sensitivity, leptin, and chemically measured relevant lipoprotein (triglycerides and HDL or LDL cholesterol). In addition, adiponectin was significantly linked to LDL and VLDL subclass distribution, with lower adiponectin concentrations associated with lower concentrations of large LDLs and higher concentrations of small LDLs. Such associations have previously been described in the context of insulin resistance and obesity (8); however, our findings shed further light on these relations because we show that adiponectin levels are inversely associated with the concentrations of small LDL and large VLDL particles independent of insulin sensitivity, obesity, and body fat distribution.
The presence of elevated levels of small, dense LDL particles seems to be associated with increased levels of large VLDL particles and is closely associated with insulin resistance (9). Our results show that those subjects with greater concentrations of large VLDL particles had reduced adiponectin levels, whereas the opposite was found for small VLDL particles. Thus, the clustering of elevated triglycerides (as a result of increased large VLDL production) and small, dense LDL particles in the context of insulin resistance and obesity may be mediated by adiponectin that is typically reduced in this clinical setting (10,11), although the cross-sectional nature of this study cannot resolve directionality. Thus, the seemingly protective role of adiponectin against cardiovascular diseases and diabetes may not necessarily be mediated through its association with insulin sensitivity or obesity but, rather, could reflect an independent effect on lipoprotein metabolism.
Acknowledgments
This study was financially supported by the U.S.-Israel Binational Science Foundation, the Israel Science Foundation, the Steven Morse Diabetes Research Fund, the Danish Research Council for Health and Disease, the Danish Diabetes Association, and the Clinical Institute, Aarhus University.
J.D.O. is employed by, holds stock in, and serves on the board of directors of LipoScience, a diagnostic company that performed the lipoprotein particle analyses described in this article. No other potential conflicts of interest relevant to this article were reported.
We thank Karen Mathiassen and Hanne Petersen for their excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996; 271: 10697– 10703 [DOI] [PubMed] [Google Scholar]
- 2. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005; 26: 439– 451 [DOI] [PubMed] [Google Scholar]
- 3. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79– 83 [DOI] [PubMed] [Google Scholar]
- 4. Slater PE, Friedlander Y, Baras M, Harlap S, Halfon ST, Kaufmann NA, Eisenberg S, Davies AM, Stein Y. The Jerusalem Lipid Research Clinic: sampling, response and selected methodological issues. Isr J Med Sci 1982; 18: 1106– 1112 [PubMed] [Google Scholar]
- 5. Kark JD, Sinnreich R, Leitersdorf E, Friedlander Y, Shpitzen S, Luc G. Taq1B CETP polymorphism, plasma CETP, lipoproteins, apolipoproteins and sex differences in a Jewish population sample characterized by low HDL-cholesterol. Atherosclerosis 2000; 151: 509– 518 [DOI] [PubMed] [Google Scholar]
- 6. Otovs JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. In Handbook of Lipoprotein Testing. Rifai N, Wainick R, Cominazak M. Eds. Washington, DC, AACC Press, 2000, p. 609– 623 [Google Scholar]
- 7. Saraheimo M, Forsblom C, Fagerudd J, Teppo A-M, Petterson-Fernholm K, Frystyk J, Flyvbjerg A, Groop P-H, the FinnDiane Study Group. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care 2005; 28: 1410– 1414 [DOI] [PubMed] [Google Scholar]
- 8. Goff DC, Jr, D'Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism 2005; 54: 264– 270 [DOI] [PubMed] [Google Scholar]
- 9. Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003; 52: 453– 462 [DOI] [PubMed] [Google Scholar]
- 10. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004; 350: 2362– 2374 [DOI] [PubMed] [Google Scholar]
- 11. Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, Tamborlane WV, Dziura J, Shulman GI, Caprio S. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 2005; 90: 3731– 3737 [DOI] [PubMed] [Google Scholar]