Abstract
OBJECTIVE
To compare whether depressive symptoms are more strongly related to subsequent or prior glycemic control in type 2 diabetes and to test whether patient characteristics modify these longitudinal associations.
RESEARCH DESIGN AND METHODS
On two occasions separated by 6 months, depressive symptoms and glycemic control were assessed in 253 adults with type 2 diabetes. Regression analyses examined depressive symptoms as both a predictor and outcome of glycemic control and tested whether medication regimen (e.g., insulin versus oral drugs) was an effect modifier before and after adjusting for baseline levels of the outcome being predicted.
RESULTS
Depressive symptom severity predicted poor glycemic control 6 months later (P = 0.018) but not after baseline glycemic control was taken into account (P = 0.361). Although baseline glycemic control did not generally predict depressive symptoms 6 months later (P = 0.558), it significantly interacted with regimen (P = 0.008). Specifically, glycemic control predicted depressive symptoms among patients prescribed insulin (β = 0.31, P = 0.002) but not among those prescribed oral medication alone (β = −0.10, P = 0.210). Classifying depression dichotomously produced similar but weaker findings.
CONCLUSIONS
Depressive symptoms do not necessarily lead to worsened glycemic control. In contrast, insulin-treated patients in poor glycemic control are at moderate risk for worsening of depressive symptoms. These patients should be carefully monitored to determine whether depression treatment should be initiated or intensified.
Like type 2 diabetes, depression is a major public health concern, affecting ∼20 million Americans yearly (1). Approximately 30% of individuals with diabetes exhibit elevated depressive symptoms, and about one-third of these individuals meet psychiatric criteria for depression (2).
Although epidemiological data (3,4) suggest that depression may increase the risk for diabetes onset, the nature and directionality of the association between depressive symptoms and subsequent diabetes course remain unclear. This is because cross-sectional studies generally indicate modest contemporaneous associations between depression and both glycemic control and complication rate but cannot speak to directionality. Some depression trials (5) suggest that mood improvement is associated with glycemic improvement, whereas other trials (6–8) do not support this conclusion. Most diabetes treatment trials overlook depression assessment or include insufficient numbers of depressed patients to permit subgroup analyses. With these exceptions, longitudinal studies relating type 2 diabetes course to temporal variation in depressive symptoms are rare. Several underlying mechanisms seem plausible. On one hand, poor diabetes control is probably stressful enough to induce or worsen depressive symptoms. In the other temporal direction, depression may worsen glycemic control through physiological channels such as catecholaminic and noradrenergic mechanisms and/or behaviorally by disrupting patients' medical adherence and self-care routines (9).
While naturalistic studies obviously cannot prove causality, certain longitudinal designs can test for “temporal priority,” in which one variable predicts future values of a second variable, which in turn does not predict future values of the first. The objective of the current study was to clarify the temporal priority of the possible longitudinal associations between type 2 diabetes control and depression by testing whether depressive symptoms are more strongly related to subsequent than to prior glycemic control.
A second issue in the literature is that depression, whether conceptualized as a syndromal entity or symptom continuum, has at best explained only a modest portion (<5%) of variance in diabetes outcomes (3). However, certain patient characteristics might modify this effect. In particular, recent preliminary studies (10,11) suggest that depression has a more potent role among patients who are prescribed insulin. For example, perhaps the need for insulin represents a psychological threat related to injection performance anxiety, needle phobia, fear of hypoglycemia, concerns about how others will react to injections, and worries about diabetes severity and progression (12). Additionally, insulin regimen tasks (e.g., glucose monitoring, dosage adjustment) may be easily disrupted by depressive symptoms such as apathy and impaired task initiation. Therefore, the current study also aimed to build upon existing work by testing whether insulin use strengthens any longitudinal associations between depression and glycemic control.
RESEARCH DESIGN AND METHODS
Potential participants were identified using the administrative and clinical databases of a large Midwestern urban health care system. Eligible patients had type 2 diabetes as indicated by at least one of the following: 1) at least one hospitalization with a diabetes-related ICD-9 code (250.x, 357.2, 362.0, or 366.41), 2) at least two outpatient visits with a diabetes-related ICD-9 code, or 3) at least one prescription for a glucose control medication or monitoring supplies, being between 18 and 80 years of age, and being able to complete self-report instruments. Type 1 diabetes was further ruled out through medical record review and telephone screening by research staff.
Following an institutional review board–approved protocol, eligible patients were mailed a study invitation letter followed by a recruitment telephone call from research staff for further screening and enrollment scheduling. After informed consent, participants attended research appointments at baseline and 6 months later for assessment of depressive symptoms, glycemic control, and other variables.
The presence of probable depressive disorder was assessed with the Patient Health Questionnaire-9 (PHQ-9), upon which respondents indicated “how often, over the last two weeks, were you bothered by any of the following problems?” for each of nine depressive symptoms included in the DSM-IV criteria for depressive episodes paired with a four-point scale ranging from “not at all” to “nearly every day.” The PHQ-9 is 88% sensitive and 88% specific for interview-detected major depression in medical patients (13). Participants were classified as either depressed or not using the established cutoff of PHQ-9 total ≥10. Glycemic control (A1C) was measured with the DCA 2000 (GMI, Ramsey, MN), which analyzes capillary blood samples through a monoclonal antibody method. Comorbid medical illness was assessed by abstracting electronic medical records using a checklist of 13 common medical illnesses used in prior primary care studies (14,15), and the presence of diabetes complications was measured using a standard self-report checklist of visual, cardiovascular, kidney, genitourinary, and other common diabetes complications taken from the validated Diabetes Care Profile (16). Participants classified themselves using U.S. Census racial/ethnic categories. Socioeconomic status (SES) was assessed using the U.S. Census Bureau Index of Socioeconomic Status (17) adjusted for current regional Consumer Price Index.
Data analysis
Data were analyzed using STATA 10.1 software (StataCorp, College Station, TX). Descriptive analyses were conducted to characterize the sample and examine the data for violation of statistical assumptions. Nonnormal distributions were rank converted for analysis. Relationships between glycemic control and depressive symptoms were analyzed using ordinary least-squares regression for the prediction of 6-month depressive symptom severity and glycemic control and logistic regression for the prediction of 6-month elevation in depressive symptoms. Main effects were evaluated prior to including interaction terms, which were computed as the product of regimen (0 = oral hypoglycemic alone, 1 = on insulin) and the other baseline predictor of interest (i.e., either depression or glycemia). Comparisons were made between coefficients estimated before and after adjusting models for baseline values of the 6-month outcome being predicted.
RESULTS
Enrollment
Of 828 eligible patients solicited, 271 (33%) consented to the study and 13% dropped out before the month-6 follow-up. Attrition was significantly more likely among those who were aged <60 years (P = 0.032) or African American (P = 0.024) but not significantly related to socioeconomic status, illness duration, diabetes complications, medical comorbidity, or any primary study variable (glycemic control, depressive symptoms, and regimen type). The final sample (n = 253) was demographically and medically heterogenous (Table 1). That is, half were female subjects, 54% were African American, age ranged from 27 to 88 years, 40% were prescribed insulin (almost always in addition to an oral hypoglycemic agent), diabetes duration ranged from 1 to 60 years, and 21% had at least two significant comorbid medical conditions.
Table 1.
Demographic and clinical characteristics by regimen
| Pooled sample (n = 253) | On oral hypoglycemics alone (n = 153) | On insulin (n = 100) | P | |
|---|---|---|---|---|
| Age | 57.3 ± 8.3 | 57.8 | 56.6 | 0.246 |
| Female | 50 | 51.3 | 46.1 | 0.415 |
| African American | 55 | 49.7 | 64.1 | 0.023 |
| Socioeconomic status index* | 65.0 ± 17.5 | 64.1 ± 18.0 | 66.3 ± 16.7 | 0.349 |
| Diabetes duration (years) | 10.9 ± 8.2 | 9.0 ± 7.0 | 13.8 ± 9.1 | <0.001 |
| Diabetes complications† | 0.7 ± 0.8 | 0.6 ± 0.8 | 0.8 ± 0.9 | 0.157 |
| Two or more comorbid medical conditions‡ | 20 | 19.1 | 23.8 | 0.371 |
| A1C at baseline (%) | 7.57 ± 1.61 | 7.37 ± 1.54 | 7.89 ± 1.67 | 0.015 |
| Depressive symptom severity (baseline PHQ-9 total) | 5.5 ± 4.7 | 5.0 ± 4.1 | 6.2 ± 5.4 | 0.060 |
Data are means ± SD or percent.
*U.S. Census Bureau Index of Socioeconomic Status adjusted for inflation and regional Consumer Price Index.
†Coded as 0, 1, or ≥2.
‡Coded from count of the following illnesses: asthma, chronic obstructive lung disease, congestive heart failure, osteoarthritis, rheumatoid arthritis, arthritis associated with lupus (SLE) or scleroderma, peripheral vascular disease, cirrhosis, chronic hepatitis, coronary artery disease, thyroid disease, Addison's disease, and Cushing's syndrome.
Prediction of month-6 glycemic control by baseline depression
First, month-6 glycemic control was regressed upon baseline depressive symptoms, diabetes regimen type, and the depression × regimen interaction term. Results (Table 2) indicated that depressive symptoms were prospectively associated with poor glycemic control (P = 0.018). However, this effect was no longer significant when the model was adjusted for baseline glycemic control (P = 0.361). The interaction effect did not reach statistical significance in either the unadjusted model (0.795) or the adjusted model (P = 0.251).
Table 2.
Results of regression analyses of depressive symptoms and glycemic control
| Outcome variable | Predictor variables | Adjusted for confounders only* |
Adjusted for confounders and baseline of outcome being predicted |
||
|---|---|---|---|---|---|
| β† | P | β† | P | ||
| Month 6 glycemic control | Baseline glycemic control | — | — | 0.78 | <0.001 |
| Baseline depressive symptoms | 0.15 | 0.018 | 0.04 | 0.361 | |
| Regimen | 0.10 | 0.121 | −0.02 | 0.559 | |
| Depressive symptoms × regimen | −0.02 | 0.795 | −0.10 | 0.251 | |
| Month 6 depressive symptoms | Baseline depressive symptoms | — | — | 0.69 | <0.001 |
| Baseline glycemic control | 0.07 | 0.258 | −0.03 | 0.558 | |
| Regimen | 0.01 | 0.990 | −0.04 | 0.386 | |
| Glycemic control × regimen | 0.42 | 0.006 | 0.27 | 0.020 | |
*Adjusted for race/ethnicity and diabetes duration.
†Standardized regression coefficient, with main effects estimated simultaneously and prior to including interaction term in model.
Prediction of month-6 depressive symptoms by baseline glycemic control
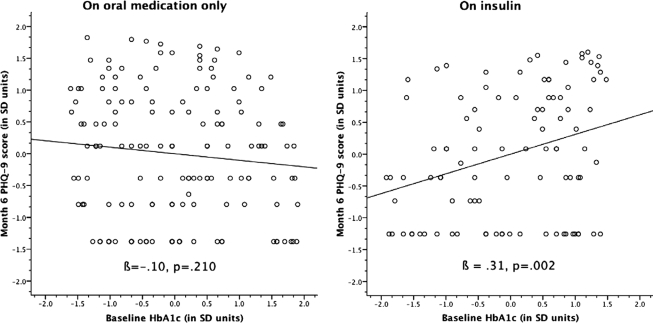
Next, we regressed month-6 depressive symptoms upon baseline glycemia, regimen, and the regimen × glycemia interaction. Glycemia did not predict subsequent depressive symptoms (P = 0.558). However, there was a significant regimen × glycemia interaction effect (P = 0.006) that persisted (P = 0.020) when the model was adjusted for baseline depressive symptoms. Regimen-stratified follow-up regression analysis and examination of regression plots (Fig. 1) indicated that hyperglycemia was positively associated with subsequent depressive symptoms among patients who were prescribed insulin (β = 0.31, P = 0.002, indicating “moderate” effect magnitude) but not among those on oral hypoglycemic medication alone (β = −0.10, P = 0.210).
Figure 1.
Standardized scatterplots of baseline glycemic control and month 6 depressive symptoms by regimen.
Examination of depression as a binary indicator
Using a PHQ-9 cutoff of 10 to classify participants as having probable depression or not at baseline and month 6, we reanalyzed the data with depressive symptoms categorized as present versus absent. Although the general pattern of findings was similar, the effect magnitudes were somewhat less pronounced and not statistically significant in all cases. As above, baseline depression predicted month-6 glycemic control in unadjusted (β = 0.14, P = 0.026) but not adjusted analyses (β = 0.01, P = 0.903). Also as above, there was a significant glycemia × regimen interaction effect on month-6 depression (odds ratio 1.01 [95% CI 1.01–1.03], P = 0.042). However, unlike above, the interaction effect did not remain significant (1.00 [0.99–1.02], P = 0.616) when the model was adjusted for the baseline presence of depression.
Consideration of other potential confounders
As can be seen in Table 1, insulin use was associated with diabetes duration and with being African American. By adjusting for regimen type, we assumed that we also indirectly adjusted for this potential confound. Indeed, regression models were adjusted for diabetes duration and minority status; the pattern and significance of findings was similar to unadjusted models. Because another study (18) suggests that the association between depression and A1C may vary by sex, we tested both prospective interactions between sex and depressive symptoms. However, sex did not interact with baseline glycemic control in the prediction of month-6 depressive symptoms (P = 0.946), nor did sex interact with baseline depressive symptoms in the prediction of month-6 glycemic control (P = 0.727).
CONCLUSIONS
Results indicated that among type 2 diabetic patients prescribed insulin, poor glycemic control predicts worsened depressive symptoms 6 months later, even after adjusting for concurrent depressive symptoms. This does not occur among patients who are on oral medication alone, suggesting that either insulin use itself (or more likely some property of insulin-treated diabetes) may exacerbate the impact of glycemic control upon mood symptoms. In sum, depressive symptoms seem to be more linked with hyperglycemia among insulin-treated patients.
Although depressive symptoms in turn predict future glycemic control, this appears to be overshadowed by the effects of current glycemic control, indicating that depression level does not have a prospective glycemic effect beyond that explained by the stability of glycemia itself. In other words, if depression has a prospective and durable effect upon glycemic control, this will be extraordinarily difficult to observe within the context of chronic hyperglycemia.
The directional relationship between glycemia and change in depressive symptoms was more pronounced among patients prescribed insulin than among those on oral antihyperglycemic medicines alone. This builds upon existing preliminary data indicating that glycemic control (19) and quality of life (20) are only associated with depression among insulin users. Moreover, we have elsewhere argued that as blood glucose becomes more fluctuating and elevated in both type 1 and type 2 diabetes, it becomes more strongly related to psychological factors in general (11,21). This pattern could be due to regimen complexity and burden in terms of multiple daily injections, regimen-related psychological distress, and other psychological impacts of requiring insulin (9,12). Patients may ascribe significant negative meaning to the concept of requiring injections, which might increase the influence of depressed mood upon outcomes, particularly quality of life. Finally, exogenous insulin may have some directly depressogenic effect on brain function, although this has not been previously reported.
Alternatively, the apparent “regimen specificity” of the observed temporal effects may simply be reflective of diabetes severity, insofar as insulin therapy often marks advanced diabetes. Perhaps psychiatric vulnerability increases as diabetes advances, a hypothesis that would require long-term observation to verify. Our analysis of potential confounds, however, helps rule out the possibilities that the findings are explained by illness duration, diabetes complications, or other medical conditions that might be expected among patients whose diabetes is severe enough to require insulin treatment. Although a prior report (18) suggests that sex may modify the association between depression and A1C, we were unable to replicate this effect.
One of the most important study limitations is that there are multiple plausible explanations for this result, many of which cannot be addressed within this study. First, both chronic hyperglycemia and subsequent depressive symptoms could be caused by a third factor that they happen to share. Examples are low levels of physical activity, functional restrictions due to diabetes or another medical condition, medical nonadherence, inadequate treatment secondary to poor appointment attendance, and the exacerbation of another medical condition. Underlying biological mechanisms have also been suggested, such that both depression and severe diabetes may be associated with neurohormonal abnormalities such as elevated cortisol and catecholamines (22). Additional study limitations should be considered. The findings should be generalized with caution because, although the sample was demographically diverse, enrollment occurred within a single health system and only one-third of solicitants actually participated, which may have biased toward the selection of more motivated individuals. The measure and its cutoffs used to classify probable depression were originally validated against psychiatric interviews; however, we did not verify depression presence by interview, and therefore there may have been some psychiatric misclassification in the limited binary comparisons that we made. The observed associations might be explained by the dose of insulin required, the subjective burden of using insulin, the personal meaning of requiring insulin for diabetes control, or other unmeasured factors. Our ethical obligation to intervene with suicidal participants may have altered associations by reducing depression levels. Finally, although the design was longitudinal, it was nonexperimental and thus cannot clearly determine whether glycemia plays a causal role in the observed association.
In closing, the findings indicate that under naturalistic circumstances, depressive symptoms are more likely to be an effect than a cause of poor glycemic control. Second, this relationship between glycemia and subsequent depressive symptoms occurs only among patients who are prescribed insulin. Potential mechanisms should be carefully studied. These include the potential depressogenic effects of poor glycemic control and the potential existence of a common factor that might explain poor outcomes in both depression and diabetes, whether it is biological, psychological, and/or related to health care delivery. Clinicians who provide diabetes care might be especially vigilant in terms of depression screening and management for diabetic patients who are prescribed insulin, inviting patients to discuss the potential impact of insulin on their lifestyle and mental health.
Acknowledgments
This work was funded by National Institutes of Health Grant R01DK066016 (to J.E.A.). It is also supported by the Behavioral, Clinical, and Health Systems Intervention Research Core of the Michigan Diabetes Research and Training Center (grant P60DK020572; National Institute of Diabetes and Digestive Kidney Disease). J.D.P. is a VA Senior Research Career Scientist.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Regier DA, Narrow WE, Rae DS: The de facto US mental and addictive disorders service system: epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50: 85– 94 [DOI] [PubMed] [Google Scholar]
- 2. Egede LE, Zheng D: Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care 2003; 26: 104– 111 [DOI] [PubMed] [Google Scholar]
- 3. Ali S, Stone MA, Peters JL, Davies MJ, Khunti K: Prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006; 23: 1165– 1173 [DOI] [PubMed] [Google Scholar]
- 4. Mezuk B, Eaton WW, Albrecht S, Golden SH: Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31( 12): 2383– 2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lustman PJ, Clouse RE, Nix BD, Freedland KE, Rubin EH, McGill JB, Williams MM, Gelenberg AJ, Ciechanowski PS, Hirsch IB: Sertraline for prevention of depression recurrence in diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry 2006; 63: 521– 529 [DOI] [PubMed] [Google Scholar]
- 6. Williams JW, Jr, Katon W, Lin EH, Noel PH, Worchel J, Cornell J, Harpole L, Fultz BA, Hunkeler E, Mika VS, Unutzer J. the IMPACT Investigators. The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med 2004; 140: 1015– 1024 [DOI] [PubMed] [Google Scholar]
- 7. Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T: The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004; 61: 1042– 1049 [DOI] [PubMed] [Google Scholar]
- 8. Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, Carney RM, McGill JB: Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med 1997; 59: 241– 250 [DOI] [PubMed] [Google Scholar]
- 9. Makine C, Karçsidağ C, Kadioğlu P, Ilkova H, Karçsidağ K, Skovlund SE, Snoek FJ, Pouwer F: Symptoms of depression and diabetes-specific emotional distress are associated with a negative appraisal of insulin therapy in insulin-naïve patients with type 2 diabetes mellitus: a study from the European Depression in Diabetes (EDID) Research Consortium. Diabet Med 2009; 26: 28– 33 [DOI] [PubMed] [Google Scholar]
- 10. Surwit RS, van Tilburg MA, Parekh PI, Lane JD, Feinglos MN: Treatment regimen determines the relationship between depression and glycemic control. Diabetes Res Clin Pract 2005; 69: 78– 80 [DOI] [PubMed] [Google Scholar]
- 11. Aikens JE, Perkins DW, Piette JD, Lipton B: Association between depression and concurrent type 2 diabetes outcomes varies by diabetes regimen. Diabet Med 2008; 25( 11): 1324– 1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV: Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005; 28: 2543– 2545 [DOI] [PubMed] [Google Scholar]
- 13. Spitzer RL, Kroenke K, Williams JB: Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study: Primary Care Evaluation of Mental Disorders: Patient Health Questionnaire. JAMA 1999; 10:282: 1737– 1744 [DOI] [PubMed] [Google Scholar]
- 14. Aikens JE, Nease DE, Jr, Nau DP, Klinkman MS, Schwenk TL: Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med 2005; 3: 23– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aikens JE, Rouse ME: Help-seeking for insomnia among adult patients in primary care. J Am Board Fam Pract 2005; 18: 257– 261 [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG: Development and validation of the diabetes care profile. Eval Health Prof 1996; 19: 209– 231 [DOI] [PubMed] [Google Scholar]
- 17. U.S. Bureau of the Census. Methodology and scores of the socioeconomic status, working paper No. 15. Fed Regist 2002; 67: 6931– 6933 [Google Scholar]
- 18. Pouwer F, Snoek FJ: Association between symptoms of depression and glycaemic control may be unstable across gender. Diabet Med 2001; 18: 595– 598 [DOI] [PubMed] [Google Scholar]
- 19. Surwit RS, van Tilburg MA, Parekh PI, Lane JD, Feinglos MN: Treatment regimen determines the relationship between depression and glycemic control. Diabetes Res Clin Pract 2005; 69: 78– 80 [DOI] [PubMed] [Google Scholar]
- 20. Pawaskar MD, Anderson RT, Balkrishnan R: Self-reported predictors of depressive symptomatology in an elderly population with type 2 diabetes mellitus: a prospective cohort study. Health Qual Life Outcomes 2007; 5: 50– 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aikens JE, Wallander JL, Bell DS, Cole JA: Daily stress variability, learned resourcefulness, regimen adherence, and metabolic control in adult type I diabetes mellitus: evaluation of a path model. J Consult Clin Psychol 1992; 60: 113– 118 [DOI] [PubMed] [Google Scholar]
- 22. Sachs G, Spiess K, Moser G, Kautzky A, Luger A, Pietschmann P, Schernthaner GS, Prager R: Hormonal and blood glucose responsiveness as an indicator of specific emotional arousal in type 1 diabetics. J Psychosom Res 1993; 37: 831– 841 [DOI] [PubMed] [Google Scholar]