An International Expert Committee with members appointed by the American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation was convened in 2008 to consider the current and future means of diagnosing diabetes in nonpregnant individuals. The report of the International Expert Committee represents the consensus view of its members and not necessarily the view of the organizations that appointed them. The International Expert Committee hopes that its report will serve as a stimulus to the international community and professional organizations to consider the use of the A1C assay for the diagnosis of diabetes.
Diabetes is a disease characterized by abnormal metabolism, most notably hyperglycemia, and an associated heightened risk for relatively specific long-term complications affecting the eyes, kidney, and nervous system. Although diabetes also substantially increases the risk for cardiovascular disease, cardiovascular disease is not specific to diabetes and the risk for cardiovascular disease has not been incorporated into previous definitions or classifications of diabetes or of subdiabetic hyperglycemia.
Background
Diagnosing diabetes based on the distribution of glucose levels
Historically, the measurement of glucose has been the means of diagnosing diabetes. Type 1 diabetes has a sufficiently characteristic clinical onset, with relatively acute, extreme elevations in glucose concentrations accompanied by symptoms, such that specific blood glucose cut points are not required for diagnosis in most clinical settings. On the other hand, type 2 diabetes has a more gradual onset, with slowly rising glucose levels over time, and its diagnosis has required specified glucose values to distinguish pathologic glucose concentrations from the distribution of glucose concentrations in the nondiabetic population. Virtually every scheme for the classification and diagnosis of diabetes in modern times has relied on the measurement of plasma (or blood or serum) glucose concentrations in timed samples, such as fasting glucose; in casual samples independent of prandial status; or after a standardized metabolic stress test, such as the 75-g oral glucose tolerance test (OGTT).
Early attempts to standardize the definition of diabetes relied on the OGTT, but the performance and interpretation of the test were inconsistent and the number of subjects studied to define abnormal values was very small (1–6). Studies in the high-risk Pima Indian population that demonstrated a bimodal distribution of glucose levels following the OGTT (7,8) helped establish the 2-h value as the diagnostic value of choice, even though most populations had a unimodal distribution of glucose levels (9). Of note, a bimodal distribution was also seen in the fasting glucose samples in the Pimas and other high-risk populations (10,11). However, a discrete fasting plasma glucose (FPG) or 2-h plasma glucose (2HPG) level that separated the bimodal distributions in the Pimas was difficult to identify, with potential FPG and 2HPG cut points ranging from 120 to 160 mg/dl (6.7–8.9 mmol/l) and from 200 to 250 mg/dl (11.1–13.9 mmol/l), respectively.
In 1979, the National Diabetes Data Group (NDDG) provided the diagnostic criteria that would serve as the blueprint for nearly two decades (12). The NDDG relied on distributions of glucose levels, rather than on the relationship of glucose levels with complications, to diagnose diabetes despite emerging evidence that the microvascular complications of diabetes were associated with a higher range of fasting and OGTT glucose values (11,13–15). The diagnostic glucose values chosen were based on their association with decompensation to “overt” or symptomatic diabetes.
When selecting the threshold glucose values, the NDDG acknowledged that “there is no clear division between diabetics and nondiabetics in the FPG concentration or their response to an oral glucose load,” and consequently, “an arbitrary decision has been made as to what level justifies the diagnosis of diabetes.” The diagnosis of diabetes was made when 1) classic symptoms were present; 2) the venous FPG was ≥140 mg/dl (≥7.8 mmol/l); or 3) after a 75-g glucose load, the venous 2HPG and levels from an earlier sample before 2 h were ≥200 mg/dl (≥11.1 mmol/l). An intermediate group was classified as having “impaired glucose tolerance” (IGT) with FPG <140 mg/dl (7.8 mmol/l) and a 2HPG value between 140 and 200 mg/dl (7.8–11.1 mmol/l). IGT was identified on the basis of its relatively higher risk of progression to diabetes compared with that of “normal” glucose tolerance, low frequency of “diabetic symptoms,” high probability of reverting to normal glucose tolerance or continuing to have IGT, and rarity of “clinically significant” microvascular disease. The NDDG recommendations were also promulgated by the contemporaneous report of the World Health Organization (WHO) (16).
Diagnosing diabetes based on the relationship between glucose levels and long-term complications
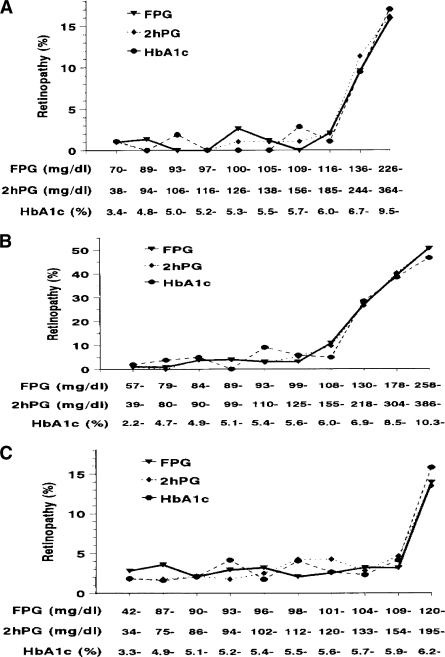
In 1997, the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (17) reexamined the basis for diagnosing diabetes. This committee made two seminal contributions: First, they refocused attention on the relationship between glucose levels and the presence of long-term complications as the basis for the diagnosis of diabetes. Second, they summarized data negating the widespread hypothesis that the 2HPG was the gold-standard test for diagnosing diabetes. The committee examined data from three cross-sectional epidemiological studies that included an Egyptian population (n = 1,018), Pima Indians (n = 960), and the U.S. National Health and Nutrition Examination Survey (NHANES) population (n = 2,821). Each assessed retinopathy with fundus photography or direct ophthalmoscopy and measured glycemia as FPG, 2HPG, and A1C. These studies demonstrated glycemic levels below which there was little prevalent retinopathy and above which the prevalence of retinopathy increased in an apparently linear fashion (Fig. 1). When the prevalence of retinopathy was expressed by deciles of glycemia for each of the three measures, the deciles at which retinopathy began to increase were the same for each measure within each population. Moreover, the glycemic values above which retinopathy increased were similar among the populations. These data showed a clear relationship between glycemia and the risk for retinopathy that would supplant the previous notion of risk for progression to overt, symptomatic diabetes as the basis for diagnosing diabetes.
Figure 1.
Prevalence of retinopathy by deciles of the distribution of FPG, 2HPG, and A1C in Pima Indians (A), Egyptians (B), and 40- to 74-year-old participants in NHANES III (C). Adapted with permission from ref. 17.
In comparing the relationship between FPG and 2HPG values and retinopathy, it was apparent that the previous FPG cut point of ≥140 mg/dl (7.8 mmol/l) was substantially above the glucose level at which the prevalence of retinopathy began to increase. As a result, the committee recommended that the FPG cut point be lowered to ≥126 mg/dl (7.0 mmol/l) so that this cut point would represent a degree of hyperglycemia that was “similar” to the 2HPG value and diagnosis with either measure would result in a similar prevalence of diabetes in the population. The 1997 committee report acknowledged that even at the lower FPG cut point, the FPG and OGTT (2HPG) were not perfectly concordant. An individual could have diabetes using one test but not the other. This discrepancy has been confirmed in numerous subsequent reports and may be due, in part, to the fact that although both tests are measures of glycemia, they reflect different physiological measures of acute glucose metabolism (18). The debate regarding the relative roles of FPG and 2HPG in the diagnosis of diabetes in the nonpregnant adult has continued (19–21).
The 1997 report also recommended that the FPG level, rather than the 2HPG, be the preferred test to diagnose diabetes because it was more convenient for patients and less costly and time consuming and the repeat-test reproducibility was superior (17). In addition, the committee introduced the term “impaired fasting glucose” (IFG) to differentiate the metabolic state between a normal state (FPG <110 mg/dl or <6.1 mmol/l) and diabetes (≥126 mg/dl or ≥7.0 mmol/l) when the FPG test was used. If an OGTT was performed, the intermediate glycemic state continued to be called IGT, with the 2HPG (between 140 and 200 mg/dl [7.8 and 11.1 mmol/l]) the same as that as in the NDDG report. A WHO consultation (22) adopted most of the above recommendations except they concluded that, whenever feasible, individuals with IFG should be given an OGTT to exclude the presence of diabetes that would otherwise be missed and that the OGTT should remain the “gold standard.” A 2003 follow-up report from the expert committee refined the fasting glucose value range for IFG from ≥110 but <126 mg/dl to ≥100 but <126 mg/dl (≥6.1 but <7.0 mmol/l to ≥5.6 but <7.0 mmol/l) to make it more comparable with the IGT value (21). The WHO did not change its previous recommendations (23).
Can the A1C test be used to diagnose diabetes?
If chronic hyperglycemia sufficient to cause diabetes-specific complications is the hallmark of diabetes, common sense would dictate that laboratory measures that capture long-term glycemic exposure should provide a better marker for the presence and severity of the disease than single measures of glucose concentration. Observational studies that have assessed glycemia with measures that capture longer-term exposure (i.e., A1C) or with single or longitudinal measurements of glucose levels have consistently demonstrated a strong correlation between retinopathy and A1C (24–26) but a less consistent relationship with fasting glucose levels (27). In one study that measured both FPG and A1C, there was a stronger correlation between A1C and retinopathy than between fasting glucose levels and retinopathy (25). The correlation between A1C levels and complications has also been shown in the setting of controlled clinical trials in type 1 (28) and type 2 (29) diabetes, and these findings have been used to establish the widely accepted A1C treatment goals for diabetes care (30).
All of these observations suggest that a reliable measure of chronic glycemic levels such as A1C, which captures the degree of glucose exposure over time (31,32) and which is related more intimately to the risk of complications than single or episodic measures of glucose levels, may serve as a better biochemical marker of diabetes and should be considered a diagnostic tool. Although the 1997 expert committee report considered this option, it recommended against using A1C values for diagnosis in part because of the lack of assay standardization (17). The 2003 follow-up report noted that, while the National Glycohemoglobin Standardization Program (33) had succeeded in standardizing the vast majority of assays used in the U.S., the use of A1C for diagnosis still had “disadvantages,” and it reaffirmed the previous recommendation that A1C not be used to diagnose diabetes (21).
An updated examination of the laboratory measurements of glucose and A1C by the current International Expert Committee indicates that with advances in instrumentation and standardization, the accuracy and precision of A1C assays at least match those of glucose assays. The measurement of glucose itself is less accurate and precise than most clinicians realize (34). A recent analysis of the performance of a variety of clinical laboratory instruments and methods that measure glucose revealed that 41% of instruments have a significant bias from the reference method that would result in potential misclassification of >12% of patients (35). There are also potential preanalytic errors owing to sample handling and the well-recognized lability of glucose in the collection tube at room temperature (36,37). Even when whole blood samples are collected in sodium fluoride to inhibit in vitro glycolysis, storage at room temperature for as little as 1 to 4 h before analysis may result in decreases in glucose levels by 3–10 mg/dl in nondiabetic individuals (36–39).
By contrast, A1C values are relatively stable after collection (40), and the recent introduction of a new reference method to calibrate all A1C assay instruments should further improve A1C assay standardization in most of the world (41–43). In addition, between- and within-subject coefficients of variation have been shown to be substantially lower for A1C than for glucose measurements (44). The variability of A1C values is also considerably less than that of FPG levels, with day-to-day within-person variance of <2% for A1C but 12–15% for FPG (45–47). The convenience for the patient and ease of sample collection for A1C testing (which can be obtained at any time, requires no patient preparation, and is relatively stable at room temperature) compared with that of FPG testing (which requires a timed sample after at least an 8-h fast and which is unstable at room temperature) support using the A1C assay to diagnose diabetes.
In summary, compared with the measurement of glucose, the A1C assay is at least as good at defining the level of hyperglycemia at which retinopathy prevalence increases; has appreciably superior technical attributes, including less preanalytic instability and less biologic variability; and is more clinically convenient. A1C is a more stable biological index than FPG, as would be expected with a measure of chronic glycemia levels compared with glucose concentrations that are known to fluctuate within and between days (Table 1).
Table 1.
Advantages of A1C testing compared with FPG or 2HPG for the diagnosis of diabetes
|
What is the most appropriate A1C cut point for the diagnosis of diabetes?
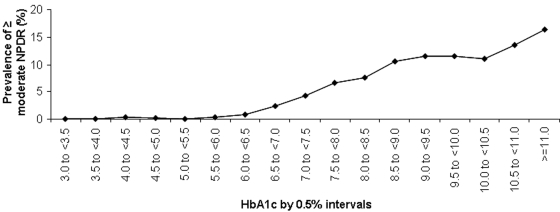
As shown in the 1997 committee report, the prevalence of retinopathy increases substantially at A1C values starting between 6.0 and 7.0% (17) (Fig. 1). A recent analysis derived from DETECT-2 (48) and including the 3 that were included in the 1997 report examined the association between A1C and retinopathy, objectively assessed and graded by fundus photography (S. Colagiuri, personal communication). This analysis included ∼28,000 subjects from nine countries and showed that the glycemic level at which the prevalence of “any” retinopathy begins to rise above background levels (any retinopathy includes minor changes that can be due to other conditions, such as hypertension), and for the more diabetes-specific “moderate” retinopathy, was 6.5% when the data were examined in 0.5% increments (Fig. 2). Among the >20,000 subjects who had A1C values <6.5%, “moderate” retinopathy was virtually nonexistent. The receiver operating characteristic curve analysis of the same data indicated that the optimal cut point for detecting at least moderate retinopathy was an A1C of 6.5%.
Figure 2.
Prevalence of retinopathy by 0.5% intervals and severity of retinopathy in participants aged 20–79 years. NPDR, nonproliferative diabetic retinopathy. Adapted with permission from (S. Colagiuri, personal communication).
In summary, the large volume of data from diverse populations has now established an A1C level associated with an increase in the prevalence of moderate retinopathy and provides strong justification for assigning an A1C cut point of ≥6.5% for the diagnosis of diabetes. A recently published population-based study of 3,190 adults of Malay ethnicity independently concluded that A1C levels “in the range 6.6 to 7% were optimal for detecting microvascular complications” (26).
Any suggestion that the relationship between chronic glycemic levels and the long-term complications of diabetes may be better expressed as a continuum, rather than as a strictly dichotomous relationship, is belied by the retinopathy findings presented herein. There is a low prevalence of “any” retinopathy at A1C levels <6.5% that may reflect a continuum of risk; alternatively, retinopathy related to conditions other than diabetes (e.g., hypertension) or inaccurate assessment of long-term glycemic levels with a single A1C measurement may contribute to this observation. However, the substantial increase in the prevalence of moderate retinopathy at A1C levels ≥6.5% supports a threshold level of glycemia that results in retinopathy most characteristic of diabetes.
This cut point should not be construed as an absolute dividing line between normal glycemia and diabetes; however, the A1C level of 6.5% is sufficiently sensitive and specific to identify individuals who are at risk for developing retinopathy and who should be diagnosed as diabetic. The A1C level is at least as predictive as the current FPG and 2HPG values. In selecting a diagnostic A1C level ≥6.5%, the International Expert Committee balanced the stigma and costs of mistakenly identifying individuals as diabetic against the minimal clinical consequences of delaying the diagnosis in someone with an A1C level <6.5%. The committee agreed to emphasize specificity rather than sensitivity. This decision was aided by the parallel decision to recommend effective prevention strategies for the highest at-risk group with an A1C between 6.0 and 6.5%. (See below.)
Limitations of A1C as the recommended means of diagnosing diabetes
The A1C assay is the test of choice for the chronic management of diabetes and is now being recommended for its diagnosis; however, there are parts of the world where the costs of providing the assay preclude its routine use. In such circumstances, clinicians should continue to use the previously recommended approaches to diagnose diabetes based on glucose measurements. The International Expert Committee encourages clinicians worldwide to move as quickly as possible to A1C testing using standardized methods and instrumentation. However, the decision to change to A1C assays as the means of diagnosing diabetes should take into account the performance of local A1C assays and the local prevalence of conditions that may interfere with the assay. (See below.)
Although the discussion above argues for using the A1C assay for the diagnosis of diabetes in nonpregnant individuals, there are patient conditions that either will require a specific A1C assay method or will preclude A1C testing. First, some hemoglobin traits, such as HbS, HbC, HbF, and HbE, interfere with some A1C assay methods (49). Currently, many assay methods can correct for the presence of the most common hemoglobin traits (www.ngsp.org), and affinity assays that are unaffected by hemoglobin traits may be used (49). Second, any condition that changes red cell turnover, such as hemolytic anemia, chronic malaria, major blood loss, or blood transfusions, will lead to spurious A1C results. Clinicians must be aware of these conditions, particularly in populations in which they are more prevalent. As in the setting where A1C assays are unavailable, the traditional diagnostic tests (e.g., FPG, 2HPG) must be used in individuals in whom interpreting the A1C is problematic. Third, A1C levels appear to increase with age (50), but the extent of the change, whether it relates to factors other than glucose metabolism, and the effect of the age-related increases on the development of complications are not sufficiently clear to adopt age-specific values in a diagnostic scheme. Similarly, racial disparities in A1C, based on putative differences in the relationship between glucose levels and A1C, have been suggested (51); however, here too, their etiology and significance are unclear, and it is premature to establish race-specific diagnostic values. Finally, there are rare clinical settings, such as rapidly evolving type 1 diabetes, where the A1C level will not have had time to “catch up” with the acute elevations in glucose levels; however, in these very rare cases, diabetes should be diagnosable with typical symptoms and casual glucose levels >200 mg/dl (11.1 mmol/l) despite a nondiagnostic A1C level.
Notwithstanding the above limitations of A1C testing, the assay has numerous important advantages compared with the currently used laboratory measurements of glucose (Table 1). The prevalence of diabetes in some populations may not be the same when diagnosis is based on A1C compared with diagnosis with glucose measurements, and one method may identify different individuals than the other. Because the measurements of glucose levels and A1C reflect different aspects of glucose metabolism, this is to be expected. However, establishing identical prevalences should not be the goal in defining a new means of diagnosing diabetes. The ultimate goal is to identify individuals at risk for diabetes complications so that they can be treated. The A1C diagnostic level of 6.5% accomplishes this goal.
Can A1C measurements define a specific subdiabetic “high-risk” state?
The 2003 International Expert Committee report reduced the lower bound of IFG from 110 mg/dl (6.1 mmol/l) to 100 mg/dl (5.6 mmol/l) on the grounds that the lower level optimized the sensitivity and specificity for predicting future diabetes and also increased the proportion of those with IGT who could be identified with an FPG test (21). While previous studies have shown a powerful effect of IFG and/or IGT on the subsequent development of diabetes diagnosed with glucose values (52–54), recent reports have demonstrated a graded risk of diabetes development at glycemic levels well within what was previously considered “normal,” i.e., FPG <100 mg/dl (5.6 mmol/l) and A1C <6.0% (55,56). In addition, metabolic derangements related to diabetes have been documented at similarly low glycemic levels, increasing in severity with higher glucose values within the nondiabetic range (57,58).
As with measures of glucose, a continuum of risk for the development of diabetes based on A1C levels has been demonstrated (59–61). Thus, while there appears to be an approximate glycemic threshold above which the risk for retinopathy escalates, there does not appear to be a specific level at which risk for diabetes clearly begins. A continuum of risk for the development of diabetes across a wide range of subdiabetic A1C levels may make the classification of individuals into categories similar to IFG and IGT equally problematic for A1C, as it implies that we actually know where risk begins or becomes clinically important. The continuum of risk in the subdiabetic glycemic range argues for the elimination of dichotomous subdiabetic classifications, such as “pre-diabetes,” IFG, and IGT. However, as A1C levels approach the diagnostic level for diabetes, the risk of developing diabetes becomes greatest (59,60,62).
Should A1C testing be used to identify individuals at high risk for diabetes?
The screening tests to identify individuals at elevated risk for diabetes are the same as the diagnostic tests; therefore, the technical advantages of A1C testing compared with glucose testing apply to the detection of individuals at high risk as they do to the diagnosis of diabetes. Therapeutic decisions should be based on how close A1C levels are to the diagnosis of diabetes. In the absence of a specific identifiable lower threshold defining when prevention efforts should be implemented, and with potentially limited resources taken into consideration, individuals whose A1C values are close to the 6.5% A1C threshold of diabetes (i.e., ≥6.0%) should receive demonstrably effective interventions (63,64). By identifying this very high-risk subdiabetic group, the International Expert Committee is implying not that populations at lower A1C levels are not at risk but, rather, that they are at lower risk. All individuals at risk for diabetes should receive counseling to maintain normal weight, lose weight if necessary, and become more physically active.
Other risk factors for diabetes development in addition to A1C have been identified, including elevated levels of triglycerides, blood pressure, BMI, and family history of diabetes (59,60), and these should be taken into account in determining when to initiate interventions in individuals with A1C <6.0%. The use of well-validated risk assessment tools may be valuable in that regard. At the population level, the A1C value at which prevention services are provided will depend on the resources available, the size of the target population, and the nature of the intervention.
What are the practical issues related to A1C testing?
A1C tests to diagnose diabetes should be performed using clinical laboratory equipment. Point-of-care instruments have not yet been shown to be sufficiently accurate or precise for diagnosing diabetes. Although this International Expert Committee has concluded that the attributes of the A1C assay with regard to diagnosing diabetes and detecting individuals at high risk support its use over the FPG or 2HPG tests, the superiority of A1C testing does not invalidate the diagnostic criteria based on glucose testing. In circumstances when A1C testing cannot be performed, the diagnostic glucose tests are acceptable alternatives.
Whichever of the three different tests now available to diagnose diabetes (A1C, FPG, and 2HPG) is used, both initial and confirmatory testing should be performed with the same test. As the three tests are not completely concordant, using different tests could easily lead to confusion. The only exception to the need to confirm the diagnosis of diabetes with the same test would be the presence of clinical symptoms characteristic of diabetes and glucose levels >200 mg/dl (>11.1 mmol/l). Confirmatory testing is also not required to establish risk status in individuals identified as in the highest-risk group for diabetes (A1C of 6.0 to <6.5%).
Most cases of type 1 diabetes, particularly in children and adolescents, are diagnosed by the classical symptoms of polyuria, polydipsia, polyphagia, unexplained weight loss, and a casual glucose >200 mg/dl. If diabetes is suspected in the absence of those conditions, A1C testing is warranted.
Recommendations and conclusions
Based on the above discussion, the International Expert Committee has concluded that the best current evidence supports the following recommendations, summarized in Table 2.
Table 2.
Recommendation of the International Expert Committee
For the diagnosis of diabetes:
|
For the identification of those at high risk for diabetes:
|
For the diagnosis of diabetes
There is no single assay related to hyperglycemia that can be considered the gold standard, as it relates to the risk for microvascular or macrovascular complications.
A measure that captures chronic glucose exposure is more likely to be informative regarding the presence of diabetes than is a single measure of glucose.
The A1C assay provides a reliable measure of chronic glycemia and correlates well with the risk of long-term diabetes complications.
The A1C assay (standardized and aligned with the Diabetes Control and Complications Trial/UK Prospective Diabetes Study assay) has several technical, including preanalytic and analytic, advantages over the currently used laboratory measurements of glucose.
For the reasons above, the A1C assay may be a better means of diagnosing diabetes than measures of glucose levels.
The diagnosis of diabetes is made if the A1C level is ≥6.5%. Diagnosis should be confirmed with a repeat A1C test unless clinical symptoms and glucose levels >200 mg/dl (>11.1 mmol/l) are present.
If A1C testing is not possible owing to patient factors that preclude its interpretation (e.g., hemoglobinopathy or abnormal erythrocyte turnover) or to unavailability of the assay, previously recommended diagnostic measures (e.g., FPG and 2HPG) and criteria should be used. Mixing different methods to diagnose diabetes should be avoided.
In children and adolescents, A1C testing is indicated when diabetes is suspected in the absence of the classical symptoms or a plasma glucose concentration >200 mg/dl (>11.1 mmol/l).
The diagnosis of diabetes during pregnancy, when changes in red cell turnover make the A1C assay problematic, will continue to require glucose measurements.
For the identification of individuals at high risk for diabetes
Individuals with an A1C level ≥6% but <6.5% are likely at the highest risk for progression to diabetes, but this range should not be considered an absolute threshold at which preventative measures are initiated.
The classification of subdiabetic hyperglycemia as pre-diabetes is problematic because it suggests that all individuals so classified will develop diabetes and that individuals who do not meet these glycemia-driven criteria (regardless of other risk factor values) are unlikely to develop diabetes—neither of which is the case. Moreover, the categorical classification of individuals as high risk (e.g., IFG or IGT) or low risk, based on any measure of glycemia, is less than ideal because the risk for progression to diabetes appears to be a continuum. The glucose-related terms describing subdiabetic hyperglycemia will be phased out of use as clinical diagnostic states as A1C measurements replace glucose measurements for the diagnosis of diabetes.
When assessing risk, implementing prevention strategies, or initiating a population-based prevention program, other diabetes risk factors should be taken into account. In addition, the A1C level at which to begin preventative measures should reflect the resources available, the size of the population affected, and the anticipated degree of success of the intervention. Further analyses of cost-benefit should guide the selection of high-risk groups targeted for intervention within specific populations.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
APPENDIX
International Expert Committee members: David M. Nathan, MD (Chair); Beverly Balkau, PhD; Enzo Bonora, MD, PhD; Knut Borch-Johnsen, MD, DMSc; John B. Buse, MD, PhD; Stephen Colagiuri, MD; Mayer B. Davidson, MD; Ralph DeFronzo, MD; Saul Genuth, MD; Rury R. Holman, FRCP; Linong Ji, MD; Sue Kirkman, MD; William C. Knowler, MD, Dr PH; Desmond Schatz, MD; Jonathan Shaw, MD; Eugene Sobngwi, MD; Michael Steffes, MD, PhD; Olga Vaccaro, MD; Nick Wareham, MD; Bernard Zinman, MD; and Richard Kahn, PhD.
Footnotes
See accompanying editorial on p. 1344.
References
- 1. Moyer JH, Womack CR. Glucose tolerance: comparison of four types of diagnostic tests in 103 control subjects and 26 patients with diabetes. Am J Med 1950; 219: 161– 173 [DOI] [PubMed] [Google Scholar]
- 2. Mosenthal HO, Barre E. Criteria for and interpretation of normal glucose tolerance test. Ann Intern Med 1950; 33: 1175– 1194 [DOI] [PubMed] [Google Scholar]
- 3. Fajans SS, Conn JW. The early recognition of diabetes mellitus. Ann N Y Acad Sci 1959; 82: 208– 218 [DOI] [PubMed] [Google Scholar]
- 4. Hayner NS, Kjelsberg MD, Epstein FH, Francis T. Carbohydrate tolerance and diabetes in a total community, Tecumseh, Michigan: effects of age, sex, and test conditions on one-hour glucose tolerance in adults. Diabetes 1965; 14: 413– 423 [DOI] [PubMed] [Google Scholar]
- 5. Sisk CW, Burnham CE, Steward J, McDonald GW. Comparison of the 50 and 100 gram oral glucose tolerance test. Diabetes 1970; 19: 852– 862 [DOI] [PubMed] [Google Scholar]
- 6. West KM. Substantial differences in the diagnostic criteria used by diabetes experts. Diabetes 1975; 24: 641– 644 [DOI] [PubMed] [Google Scholar]
- 7. Rushforth NB, Bennett PH, Steinberg AG, Burch TA, Miller M. Diabetes in the Pima Indians: evidence of bimodality in glucose tolerance distributions. Diabetes 1971; 20: 756– 765 [DOI] [PubMed] [Google Scholar]
- 8. Rushforth NB, Bennett PH, Steinberg AG, Miller M. Comparison of the value of the two- and one-hour glucose levels of the oral GTT in the diagnosis of diabetes in Pima Indians. Diabetes 1975; 24: 538– 546 [DOI] [PubMed] [Google Scholar]
- 9. Gorden T. Glucose Tolerance of Adults. Series 11, No. 2. National Center for Health Statistics, U.S. Public Health Service, 1964 [Google Scholar]
- 10. Zimmet P, Whitehouse S. Bimodality of fasting and two-hour glucose tolerance distributed in a Micronesian population. Diabetes 1978; 27: 793– 800 [DOI] [PubMed] [Google Scholar]
- 11. Rushforth NB, Miller M, Bennett PH. Fasting and two-hour post-load glucose levels for the diagnosis of diabetes: the relationship between glucose levels and complications of diabetes in the Pima Indians. Diabetologia 1979; 16: 373– 379 [DOI] [PubMed] [Google Scholar]
- 12. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28: 1039– 1057 [DOI] [PubMed] [Google Scholar]
- 13. Jarrett RJ, Keen H. Hyperglycemia and diabetes mellitus. Lancet 1976; 1: 1009– 1011 [DOI] [PubMed] [Google Scholar]
- 14. Sayegh HAI, Jarrett RJ. Oral glucose tolerance tests and the diagnosis of diabetes: results of a prospective study based on the Whitehall Survey. Lancet 1979; 2: 431– 433 [DOI] [PubMed] [Google Scholar]
- 15. Pettitt DJ, Knowler WC, Lisse , Bennett PH. Development of retinopathy and proteinuria in relation to plasma-glucose concentrations in Pima Indians. Lancet 1980; 2: 1050– 1052 [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. World Health Organization Expert Committee on Diabetes Mellitus: Second Report. Geneva, World Health Org., 1980. ( Tech. Rep. Ser., no. 646) [PubMed] [Google Scholar]
- 17. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183– 1197 [DOI] [PubMed] [Google Scholar]
- 18. Abdul-Ghani MA, Jenkinson C, Richardson D, Tripathy D, DeFronzo RA. Insulin secretion and insulin action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006; 55: 1430– 1435 [DOI] [PubMed] [Google Scholar]
- 19. Tuomilehto J. Point: a glucose tolerance test is important for clinical practice. Diabetes Care 2002; 25: 1880– 1882 [DOI] [PubMed] [Google Scholar]
- 20. Davidson MB. Counterpoint: the oral glucose tolerance test is superfluous. Diabetes Care 2002; 25: 1883– 1885 [DOI] [PubMed] [Google Scholar]
- 21. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160– 3167 [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999 [Google Scholar]
- 23. World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva, World Health Org., 2006 [Google Scholar]
- 24. van Leiden HA, Dekker JM, Moll AC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol 2003; 121: 245– 251 [DOI] [PubMed] [Google Scholar]
- 25. Tapp RJ, Tikellis G, Wong TY, Harper C, Zimmet PZ, Shaw JE. Longitudinal association of glucose metabolism with retinopathy. Diabetes Care 2008; 31: 1349– 1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, Wong TY. Relationship between glycated hemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes. Diabetologia. 22 April 2009. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27. Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, Klein R, Klein BEK, Zimmet P, Shaw J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population based cross-sectional studies. Lancet 2008; 371: 736– 743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DCCT Research Group. The association between glycemic exposure and long-term diabetes complications in the Diabetes Control and Complications Trial. Diabetes 1995; 44: 968– 9837622004 [Google Scholar]
- 29. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes: prospective observational study (UKPDS 35). BMJ 2000; 321: 405– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia 2009; 52: 17– 30 [DOI] [PubMed] [Google Scholar]
- 31. Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007; 50: 2239– 2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. the A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31: 1473– 1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. The national glycohemoglobin standardization program: a five year progress report. Clin Chem 2001; 47: 1985– 1992 [PubMed] [Google Scholar]
- 34. Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem 2007; 53: 2040– 2041 [DOI] [PubMed] [Google Scholar]
- 35. Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Ehlers GW, Hassemer D, Lo SF, Secombe D, Siekmann L, Thienpont LM. State of the art in trueness and interlaboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med 2008; 132: 838– 846 [DOI] [PubMed] [Google Scholar]
- 36. Lin YL, Smith CH, Dietzler DN. Stabilization of blood glucose by cooling with ice: an effective procedure for preservation of samples from adults and newborns. Clin Chem 1976; 22: 2031– 2033 [PubMed] [Google Scholar]
- 37. Murphy JM, Browne RW, Hill L, Bolelli GF, Abagnato C, Berrino F, Freudenheim J, Trevisan M, Muti P. Effects of transportation and delay in processing on the stability of nutritional and metabolic biomarkers. Nutr Cancer 2000; 37: 155– 160 [DOI] [PubMed] [Google Scholar]
- 38. Gambino R, Piscitelli J, Ackattupathil TA, Theriault JL, Andrin RD, Sanfilippo ML, Etienne M. Acidification of blood is superior to sodium fluoride alone as an inhibitor of glycolysis. Clin Chem 2009; 55: 1019– 1021 [DOI] [PubMed] [Google Scholar]
- 39. Bruns DE, Knowler WC. Stabilization of glucose in blood samples: why it matters. Clin Chem 2009; 55: 850– 852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Little RR, Rohlfing CL, Tennill AL, Connolly S, Hanson S. Effects of sample storage conditions on glycated hemoglobin measurement: evaluation of five different high performance liquid chromatography methods. Diabetes Technol Ther 2007; 9: 36– 42 [DOI] [PubMed] [Google Scholar]
- 41. Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, Hoshino T, John WG, Kobold U, Little R, Mosca A, Mauri P, Paroni R, Susanto F, Takei I, Theinpont L, Umemoto M, Wiedmeyer HM. the IFCC Working Group on HbA1c Standardization. IFCC reference system for measurement of hemoglobin A1C in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 2004; 50: 166– 174 [DOI] [PubMed] [Google Scholar]
- 42. Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care 2007; 30: 2399– 2400 [DOI] [PubMed] [Google Scholar]
- 43. Weykamp C, John WG, Mosca A, Hoshino T, Little R, Jeppsson J-O, Goodall I, Miedema K, Myers G, Reinauer H, Sacks DB, Slingerland R, Siebelder C. The IFCC reference measurement system for HbA1c: a 6-year progress report. Clin Chem 2008; 54: 240– 248 [DOI] [PubMed] [Google Scholar]
- 44. Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, England J, Madsen R, Goldstein D. Biological variation of glycohemoglobin. Clin Chem 2002; 48: 1116– 1118 [PubMed] [Google Scholar]
- 45. Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR. Day-to-day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care 1999; 22: 394– 398 [DOI] [PubMed] [Google Scholar]
- 46. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002; 48: 436– 472 [PubMed] [Google Scholar]
- 47. Petersen PH, Jorgensen LG, Brandslund I, Olivarius DF, Stahl M. Consequences of bias and imprecision in measurements of glucose and HbA1c for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl 2005; 240: 51– 60 [DOI] [PubMed] [Google Scholar]
- 48. Colagiuri S, Borch-Johnsen K. DETECT-2: early detection of type 2 diabetes and IGT. Diabetes Voice 2003; 48: 11– 13 [Google Scholar]
- 49. Roberts WL, Safa-Pour S, De BK, Rohlfing CL, Weykamp CW, Little RR. Effects of hemoglobin C and S traits on glycohemoglobin measurements by eleven methods. Clin Chem 2005; 51: 776– 778 [DOI] [PubMed] [Google Scholar]
- 50. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM. Effects of aging on A1C levels in individuals without diabetes. Diabetes Care 2008; 31: 1991– 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Conner E. the Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007; 30: 2756– 2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gabir M, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes mellitus. Diabetes Care 2000; 23: 1108– 1112 [DOI] [PubMed] [Google Scholar]
- 53. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003; 52: 1475– 1484 [DOI] [PubMed] [Google Scholar]
- 54. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Population-based incidence rates and risk factors for type 2 diabetes in caucasians: the Bruneck Study. Diabetes 2004; 53: 1782– 1789 [DOI] [PubMed] [Google Scholar]
- 55. Tirosh A, Shai I, Tekes-Manova DT, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005; 353: 1454– 1462 [DOI] [PubMed] [Google Scholar]
- 56. Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med 2008; 121: 519– 524 [DOI] [PubMed] [Google Scholar]
- 57. Piche M-E, Arcand-Bosse J-F, Despres J-P, Peruse L, Lemieux S, Weisnagel SJ. What is a normal glucose value? Differences in indexes of plasma glucose homeostasis in subjects with normal fasting glucose. Diabetes Care 2004; 27: 2470– 2477 [DOI] [PubMed] [Google Scholar]
- 58. Meigs JB, Nathan DM, Wilson PWF, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance. The Framingham Offspring Study. Ann Intern Med 1998; 128: 524– 533 [DOI] [PubMed] [Google Scholar]
- 59. Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, Eschwege E. the DESIR Study Group. Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006; 29: 1619– 1625 [DOI] [PubMed] [Google Scholar]
- 60. Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Utility of hemoglobin A1c in predicting diabetes risk. J Gen Intern Med 2004; 19: 1175– 1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Little RR, England JD, Wiedemeyer HM, Madsen RW, Pettitt DJ, Knowler WC, Goldstein DE. Glycated haemoglobin predicts progression to diabetes mellitus in Pima Indians with impaired glucose tolerance. Diabetologia 1994; 37: 252– 256 [DOI] [PubMed] [Google Scholar]
- 62. Ko GT, Chan JC, Tsang LW, Cockram CS. Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care 2000; 23: 1770– 1773 [DOI] [PubMed] [Google Scholar]
- 63. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343– 1350 [DOI] [PubMed] [Google Scholar]
- 64. Knowler WC, Barrett-Connor E, Fowler S, Hamman R, Lachin J, Walker E, Nathan DM. the DPP Research Group. Reduction in incidence of type 2 diabetes with life-style intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.