Abstract
OBJECTIVE
To examine cardiovascular disease (CVD) mortality risk in men with diabetes only, metabolic syndrome only, and concurrent metabolic syndrome and diabetes.
RESEARCH DESIGN AND METHODS
We examined CVD mortality risk by metabolic syndrome and diabetes status in men from the Aerobics Center Longitudinal Study (ACLS) (mean ± SD age 45.1 ± 10.2 years). Participants were categorized as having neither diabetes nor metabolic syndrome (n = 23,770), metabolic syndrome only (n = 8,780), diabetes only (n = 532), or both (n = 1,097). The duration of follow-up was 14.6 ± 7.0 years with a total of 483,079 person-years of exposure and 1,085 CVD deaths.
RESULTS
Age-, examination year–, and smoking-adjusted CVD death rates (per 1,000 man-years) in men with neither metabolic syndrome nor diabetes, metabolic syndrome only, diabetes only, and both were 1.9, 3.3, 5.5, and 6.5, respectively. CVD mortality was higher in men with metabolic syndrome only (hazard ratio 1.8 [95% CI 1.5–2.0]), diabetes only (2.9 [2.1–4.0]), and both (3.4 [2.8–4.2]) compared with men with neither. The presence of metabolic syndrome was not associated (1.2 [0.8–1.7]) with higher CVD mortality risk in individuals with diabetes. In contrast, the presence of diabetes substantially increased (2.1 [1.7–2.6]) CVD mortality risk in individuals with metabolic syndrome.
CONCLUSIONS
The presence of diabetes was associated with a threefold higher CVD mortality risk, and metabolic syndrome status did not modify this risk. Our findings support the fact that physicians should be aggressive in using CVD risk–reducing therapies in all diabetic patients regardless of metabolic syndrome status.
Approximately 7.8% of the U.S. population has diabetes, and it is estimated that the number of adults with diabetes will increase to 48.3 million by 2050 in the U.S. and to 300 million worldwide in the year 2025, representing a 122% rise compared with 1995 (1–3). The public health importance is great, considering that individuals with diabetes have more than twice the risk for premature death, heart disease, and stroke compared with individuals without diabetes (1). Although clinical definitions differ slightly, metabolic syndrome is generally characterized as a clustering of abnormal levels of blood lipids (low HDL and high triglycerides), impaired fasting glucose, elevated blood pressure, and excess abdominal obesity (4–7). Approximately 25% of Americans and >50% of those aged >50 years meet the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III definition of metabolic syndrome (8). Similar to individuals with diabetes, individuals with metabolic syndrome have an increased risk for premature death, heart disease, and stroke (9–12).
Metabolic syndrome and diabetes share many common characteristics, so it is not surprising that 65–85% of individuals with diabetes also have metabolic syndrome (13–15). However, relativity few studies have examined the effect of the combination of metabolic syndrome and diabetes on cardiovascular disease (CVD) risk (11,13,14). A cross-sectional study using National Health and Nutrition Examination Survey data reported that the prevalence of coronary heart disease (CHD) among individuals with diabetes and without metabolic syndrome was similar to that in those without diabetes or metabolic syndrome (7.5 vs. 8.7%, respectively) (14). However, individuals with concurrent diabetes and metabolic syndrome had a substantially greater prevalence (19.2%) compared with these groups. This finding suggests that in individuals with diabetes there is an increased risk for CHD only when metabolic syndrome also is present. Similarly, in a prospective study Hunt et al. (16) reported that within individuals with diabetes, those with metabolic syndrome have an increased risk for CVD mortality, whereas individuals with diabetes but not metabolic syndrome do not. However, this study was relatively small (n = 2,815) with only 117 CVD deaths. Finally, the UK Prospective Diabetes Study (UKPDS) reported that in individuals with type 2 diabetes, the presence of metabolic syndrome (NCEP) increased the risk of CVD events (17). However, it was noted from a clinical perspective that the presence of metabolic syndrome in individuals with diabetes provided little information for detecting who has an increased risk of CVD.
Given the high prevalence of both metabolic syndrome and diabetes, it is of great clinical and public health importance that we develop a better understanding of the interactions of diabetes and metabolic syndrome on the risk of CVD. The primary aim of the current investigation is to examine the risk of CVD mortality in individuals with metabolic syndrome only, diabetes only, and concurrent metabolic syndrome and diabetes in a large prospective study population.
RESEARCH DESIGN AND METHODS
The Aerobics Center Longitudinal Study (ACLS) is a prospective study composed of patients who received preventive medical examinations at the Cooper Clinic in Dallas, Texas. The current analysis included 34,179 men aged 20 to 88 years who completed a clinical examination including fitness testing between 1979 and 2002. The mean ± SD duration of follow-up was 14.6 ± 7.0 years (range 1–25) with a total of 483,079 man-years of exposure. During this period there were 2,110 deaths, of which 1,085 (51.4%) were from CVD. The present study sample was limited to men because of the small number of women with metabolic syndrome or diabetes in the ACLS cohort. There were 9,692 women available for inclusion in this study, of which 252 (5 CVD deaths) had diabetes and 702 (17 CVD deaths) had metabolic syndrome. Participants were predominantly (>95%) non-Hispanic white and well educated and from middle-to-upper socioeconomic strata. Participants provided written informed consent to participate in the examination and follow-up study. The study protocol was approved annually by the Cooper Institute Institutional Review Board.
Clinical examination
The comprehensive health evaluation is described in detail elsewhere (18,19). A fasting antecubital venous blood sample was obtained, and serum samples were analyzed in a laboratory that participates in and meets the quality control standards of the Centers for Disease Control and Prevention Lipid Standardization Program. Health histories and medication use were obtained from a self-administered questionnaire and verified by a physician during the examination. Based on self-report, smoking was defined as never smoker, past smoker, and current smoker. Diabetes cases were defined as men who reported taking insulin, had a physician-diagnosed history of diabetes, or had a fasting plasma glucose concentration ≥126 mg/dl (≥7.0 mmol/l) at baseline (20). The metabolic syndrome was defined according to the criteria established by the NCEP ATP III but using the new definition of impaired fasting glucose from the American Diabetes Association (ADA) (4,20). Metabolic syndrome was diagnosed in participants who had three or more of the following five risk factors: high blood pressure (≥130 mmHg systolic or ≥85 mmHg diastolic), central obesity (waist circumference >102 cm); high triglycerides (≥1.69 mmol/l); low HDL cholesterol (<1.04 mmol/l); and high fasting plasma glucose (≥5.6 mmol/l). Men with normal blood pressure or fasting plasma glucose who indicated a history of physician-diagnosed hypertension (n = 1,446) or type 2 diabetes (n = 428), respectively, were also coded as positive for high blood pressure or high fasting plasma glucose, which added an additional 1,874 men to the metabolic syndrome category.
Mortality surveillance
We followed participants for mortality from their baseline examination until the date of death or until 31 December 2003 for survivors. The primary method of mortality surveillance was the National Death Index. The underlying cause of death was determined from the National Death Index report or by a nosologist's review of official death certificates obtained from the department of vital records in the decedent's state of residence. CVD mortality was defined if the primary cause of death or if any of the five underlying causes of death were listed as CVD, using ICD-9 codes 390–459 or ICD-10 codes I00–I99.
Statistical analyses
Participants were grouped into one of four metabolic syndrome–diabetes categories: 1) free of metabolic syndrome and diabetes, 2) having metabolic syndrome only, 3) having diabetes only, or 4) having both metabolic syndrome and diabetes. Descriptive statistics were used to summarize baseline characteristics separately by CVD mortality status and by metabolic syndrome–diabetes categories. Continuous variables were compared using Student's t tests, and categorical variables were compared using χ2 tests. Kaplan-Meier plots were used to compare survival curves, and Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs), associated 95% CIs, and CVD mortality rates (deaths/1,000 man-years of follow-up).
The proportional hazards assumption was confirmed by examining the log cumulative survival plots for exposure categories. P values are two-sided, and P < 0.05 was accepted as statistically significant. All analyses were performed using SAS (version 9.0; SAS Institute, Cary, NC).
RESULTS
Mean ± SD age of the study participants was 45.1 ± 10.2 years. Individuals who died of CVD were older and had less favorable cardiovascular risk factor profiles at baseline than survivors (Table 1). The baseline characteristics of participants by metabolic syndrome–diabetes category are described in Table 2. There were 23,770 men who had neither diabetes nor metabolic syndrome, 8,780 with metabolic syndrome only, 532 with diabetes only, and 1,097 with both metabolic syndrome and diabetes. Thus, 67% (1,097 of 1,629) of individuals with diabetes had concurrent metabolic syndrome. Follow-up was 14.6 ± 7.0 years for men without diabetes or metabolic syndrome, 13.4 ± 6.8 years for men with metabolic syndrome only, 9.7 ± 7.4 for men with diabetes only, and 10.8 ± 6.7 for men with both (P < 0.001).
Table 1.
Descriptive characteristics of 34,179 men grouped by survival status, ACLS, 1970–1997
| Survivors | CVD mortality | P | |
|---|---|---|---|
| n | 33,094 | 1,085 | |
| Years of follow-up | 14.2 ± 7.0 | 12.8 ± 6.1 | <0.001 |
| Age (years) | 44.7 ± 9.9 | 56.4 ± 10.8 | <0.001 |
| BMI (kg/m2) | 26.6 ± 3.9 | 27.1 ± 4.1 | <0.001 |
| Systolic blood pressure (mmHg) | 121.1 ± 13.5 | 128.9 ± 17.2 | <0.001 |
| Diastolic blood pressure (mmHg) | 81.2 ± 9.6 | 84.2 ± 10.7 | <0.001 |
| Waist circumference (cm) | 94.0 ± 11.0 | 97.4 ± 12.3 | <0.001 |
| Cholesterol (mmol/l [mg/dl]) | 5.4 ± 1.1 [208.6 ± 41.3] | 5.7 ± 1.1 [221.4 ± 41.2] | <0.001 |
| HDL cholesterol (mmol/l [mg/dl]) | 1.2 ± 0.3 [45.8 ± 12.0] | 1.1 ± 0.3 [42.8 ± 11.7] | <0.001 |
| Triglycerides (mmol/l [mg/dl]) | 1.6 ± 1.1 [137.9 ± 98.9] | 1.9 ± 1.4 [166.1 ± 121.3] | <0.001 |
| Fasting glucose (mmol/l [mg/dl]) | 5.6 ± 0.9 [100.5 ± 17.0] | 6.2 ± 2.0 [111.3 ± 36.7] | <0.001 |
| Cigarette smoking (%) | |||
| Never | 51.1 | 31.3 | |
| Past | 32.0 | 44.2 | |
| Current | 16.9 | 24.4 | |
| Personal history of CVD (%) | 1.7 | 15.2 | <0.001 |
| Family history of CVD (%) | 4.3 | 0.1 | <0.001 |
Data are means ± SD or %.
Table 2.
Baseline characteristics of 34,179 men grouped by metabolic syndrome and diabetes status, ACLS, 1970–1997
| Neither metabolic syndrome nor diabetes | Metabolic syndrome only | Diabetes only | Both metabolic syndrome and diabetes | |
|---|---|---|---|---|
| n | 23,770 | 8,780 | 532 | 1,097 |
| Years of follow-up | 14.6 ± 7.0 | 13.4 ± 6.8 | 9.7 ± 7.4 | 10.8 ± 6.7 |
| CVD deaths | 555 | 375 | 39 | 116 |
| Crude CVD death rate (per 1,000) | 1.6 | 3.2 | 7.5 | 9.8 |
| Age (years) | 44.0 ± 10.0 | 46.8 ± 9.8 | 49.2 ± 11.2 | 52.4 ± 9.6 |
| BMI (kg/m2) | 25.4 ± 2.9 | 29.4 ± 4.2 | 25.8 ± 2.8 | 31.0 ± 5.3 |
| Systolic blood pressure (mmHg) | 118.7 ± 12.6 | 127.2 ± 13.7 | 121.4 ± 13.4 | 131.7 ± 15.7 |
| Diastolic blood pressure (mmHg) | 79.3 ± 8.9 | 86.2 ± 9.6 | 79.9 ± 8.4 | 86.5 ± 9.8 |
| Waist circumference (cm) | 90.4 ± 8.5 | 102.5 ± 11.3 | 91.5 ± 8.5 | 107.0 ± 13.3 |
| Cholesterol (mmol/l [mg/dl]) | 5.3 ± 1.0 [204.4 ± 39.9] | 5.7 ± 1.1 [221.0 ± 42.2] | 5.2 ± 1.0 [201.7 ± 39.6] | 5.6 ± 1.2 [217.6 ± 44.7] |
| HDL cholesterol (mmol/l [mg/dl]) | 1.3 ± 0.3 [48.9 ± 11.6] | 1.0 ± 0.2 [37.7 ± 8.8] | 1.3 ± 0.3 [50.5 ± 10.8] | 1.0 ± 0.2 [37.3 ± 8.3] |
| Triglycerides (mmol/l [mg/dl]) | 1.2 ± 0.7 [106.5 ± 60.2] | 2.4 ± 1.4 [215.2 ± 122.7] | 1.1 ± 0.5 [101.0 ± 43.6] | 2.8 ± 1.9 [247.3 ± 168.3] |
| Fasting glucose (mmol/l [mg/dl]) | 5.4 ± 0.5 [96.6 ± 8.3] | 5.8 ± 0.5 [103.8 ± 8.6] | 7.4 ± 2.8 [132.6 ± 50.5] | 8.6 ± 3.0 [154.9 ± 54.1] |
| Cigarette smoking (%) | ||||
| Never | 52.3 | 45.4 | 60.0 | 47.0 |
| Past | 31.7 | 34.2 | 25.0 | 35.4 |
| Current | 15.9 | 20.4 | 15.0 | 17.6 |
| Personal history of CVD (%) | 1.5 | 3.1 | 2.8 | 6.7 |
| Family history of CVD (%) | 3.4 | 4.2 | 22.4 | 11.3 |
Data are means ± SD or %.
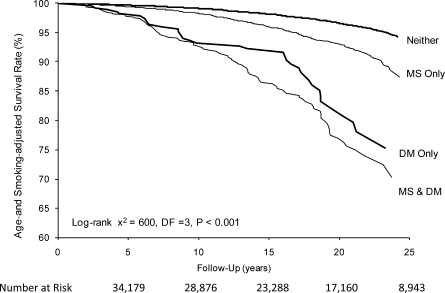
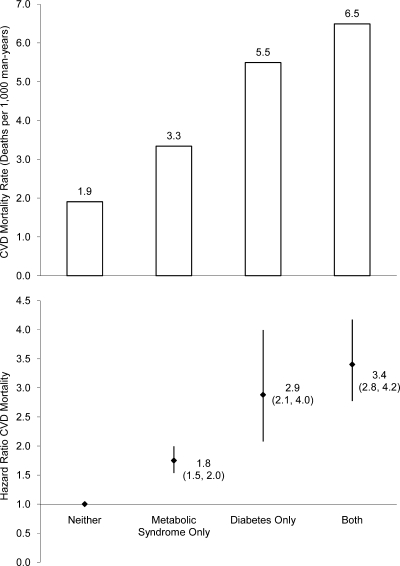
Age-, examination year–, and smoking-adjusted survival curves for CVD mortality by metabolic syndrome–diabetes category are depicted in Fig. 1. Removing current smokers from the dataset did not appreciably change the Kaplan-Meier plots (figure not presented). Age-, examination year–, and smoking-adjusted rates (top panel) and relative risk (bottom panel) of CVD mortality across metabolic syndrome–diabetes categories are presented in Fig. 2. The adjusted CVD death rates (per 1,000 man-years) in men with neither, metabolic syndrome only, diabetes only, and both were 1.9, 3.3, 5.5, and 6.5, respectively. The HRs followed a similar pattern because the adjusted risk of CVD mortality was higher in men with metabolic syndrome only (HR 1.8 [95% CI 1.5–2.0]), diabetes only (2.9 [2.1–4.0]), and both (3.4 [2.8–4.2]) compared with men with neither. Further adjustment for history of CVD had no substantive effect on any of the risks across groups because the adjusted risk of CVD mortality was 1.7 (95% CI 1.5–1.9), 3.1 (2.2–4.2), and 3.1 (2.5–3.8) for individuals with metabolic syndrome only, diabetes only, and both, respectively, compared with that for men with neither. In addition, limiting the sample to individuals not using insulin had no effect on any of the HRs. Last, we examined the risk for CVD death for men with metabolic syndrome compared with those for men with diabetes only and observed that men with diabetes had a significantly greater risk of CVD mortality (1.7 [1.2–2.3]).
Figure 1.
Data from the combined cohort of 34,179 men with 1,085 CVD deaths. Curves represent age- and smoking-adjusted survival rates with individuals categorized by diabetes (DM) and metabolic syndrome (MS) status: neither, MS only, DM only, or MS & DM.
Figure 2.
The risk of CVD mortality associated with having metabolic syndrome only, diabetes only, or both with the reference group being individuals free of metabolic syndrome or diabetes (neither) is depicted. Cox proportional hazards models were used to estimate age, examination year, and smoking-adjusted hazard ratios (bottom panel) and CVD mortality rates as deaths/1,000 man-years of follow-up (top panel). The error bars represent the 95% CIs.
To further explore the CVD risk associated with metabolic syndrome within individuals with diabetes, we conducted analyses in a subset of participants limited to men with diabetes. In individuals with diabetes (n = 1,629; 155 CVD deaths), the concurrent presence of metabolic syndrome was not associated (1.2 [95% CI 0.8–1.7]) with higher risk of CVD mortality compared with individuals who did not have metabolic syndrome. In contrast, when we examined the risk associated with diabetes on CVD mortality in individuals with metabolic syndrome (n = 9,877; 491 CVD deaths), we observed that the presence of diabetes increased (2.1 [1.7–2.6]) the risk of CVD mortality compared with that for individuals with no diabetes.
Because the prevalence of metabolic syndrome increases dramatically after age 50 years and previous reports have focused on older populations, we repeated the primary analysis in a subsample limited to participants aged >50 years at baseline (n = 10,901; 797 CVD deaths). Compared with data from the entire cohort, the CVD mortality rate in this older subsample more than doubled in each metabolic syndrome–diabetes category, yet the pattern of CVD mortality risk across metabolic syndrome–diabetes categories was similar to that from the entire cohort. Adjusted CVD death rates (per 1,000 man-years) in men aged ≥50 years with neither, metabolic syndrome only, diabetes only, and both were 4.8, 8.0, 12.9, and 14.8, respectively. Compared with men with neither (n = 6,602), individuals with metabolic syndrome only (n = 3,385), diabetes only (n = 242), and both (n = 672) had 1.7 (95% CI 1.4–1.9), 2.7 (1.9–3.8), and 3.1 (2.4–3.9) greater adjusted risk for CVD death, respectively.
We opted to use the most recent ADA definition of impaired fasting glucose (≥5.6 mmol/l). However, most previous studies used the original NCEP definition of metabolic syndrome that defines impaired fasting glucose as ≥6.1 mmol/l. To assure that the use of the more recent ADA impaired fasting glucose cut point did not influence our findings, we repeated all analyses with metabolic syndrome defined using the original NCEP definition. This change in metabolic syndrome definition produced no appreciable change in any of the findings. For example, by using the original NCEP definition of metabolic syndrome, the age-, examination year–, and smoking-adjusted risk of CVD mortality was 1.7 (95% CI 1.4–1.9) in men with metabolic syndrome only, 2.8 (2.0–4.0) in men with diabetes only, and 2.8 (2.2–3.5) in men with both compared with that in men with neither. In addition, when the metabolic syndrome–only group was limited to euglycemic individuals (fasting glucose <100 mg/dl), age-, examination year –, and smoking-adjusted CVD mortality was 1.7 (1.5–2.0) in men with metabolic syndrome only, 2.7 (1.9–3.7) in men with diabetes only, and 3.0 (2.5–3.7) in men with both compared with that in men with neither.
CONCLUSIONS
The primary finding of this study is that, compared with individuals free from metabolic syndrome and diabetes, individuals with diabetes only had approximately a threefold increased risk for CVD-associated mortality. Of particular interest is the finding that the addition of metabolic syndrome to diabetes did not significantly modify the CVD mortality risk even though metabolic syndrome alone was associated with a 1.5-fold increase in CVD mortality. The results were similar when the study sample was limited to individuals aged >50 years, the age-group in which the presence of metabolic syndrome and diabetes is most common and reflect similar increases in CVD mortality risk compared with that in individuals with neither diabetes nor the metabolic syndrome (10,12).
Data examining the interaction of diabetes and metabolic syndrome are limited, and these data are inconsistent with respect to both results and quality. Tong et al. (15) reported that the presence of metabolic syndrome (NCEP ATP III) was associated with increased risk (HR 2.1 [95% CI 1.8–3.5]) for CHD in native Chinese subjects with diabetes. A highly cited report by Alexander et al. (14), who used cross-sectional NHANES data showed that the prevalence of CHD was substantially higher in individuals with concurrent diabetes and metabolic syndrome compared with that in individuals with diabetes (19.2 vs. 7.5%, respectively). However, the authors noted that given the cross-sectional study design, the data should be considered hypothesis generating. Hunt et al.(15), using data from the San Antonio Heart Study, showed greater risk of CVD mortality in individuals with both diabetes and metabolic syndrome (NCEP ATP III), compared with those with diabetes alone (16). However, the results of this study must be interpreted with caution given the limited number of CVD deaths (n = 117). Cull et al. (17) reported that in type 2 diabetic subjects from the UKPDS, concurrent NECP-defined metabolic syndrome increases CVD risk by ∼33%. However, the authors noted that the addition of metabolic syndrome to diabetes had little positive predictive value (∼18% for ATP III criteria) and concluded that there is limited clinical value to assessing metabolic syndrome in individuals with diabetes. The above summary demonstrates the need for more datasets examining the interaction of diabetes and metabolic syndrome on future CVD risk.
Our findings are consistent with the joint ADA and European Association of Study of Diabetes statement on the clinical utility of metabolic syndrome, in that we found that diagnosing metabolic syndrome in individuals with diabetes offered no additional clinical insight into future CVD risk and that physicians should be aggressive in using recommended CVD risk–reducing therapies in all individuals with diabetes regardless of metabolic syndrome status (21). These include optimizing blood glucose, blood pressure, and lipids; the use of antiplatelet agents as appropriate; and counseling on diet, physical activity, and smoking cessation (22).
Our current study has a number of limitations that deserve mention. The ACLS cohort is predominantly male, Caucasian, well educated, and middle-to-upper class, limiting the generalizability of the study results. Data on duration of diabetes were not available, and this may be an important factor to consider in examining the diabetes-CVD mortality association because it has been noted that individuals who have had diabetes for a long period may have more subclinical diseases. Further, we lack detailed information on medication use. Although we used an updated definition of impaired fasting glucose, duplicate analysis using the older definition had no meaningful effect on our findings (data not shown). Strengths of this study include the large sample size with high internal validity, available clinical data and detailed medical histories, and a long follow-up period.
In conclusion, in this large cohort of Caucasian men, the presence of diabetes was associated with an approximately threefold higher risk for CVD mortality, and metabolic syndrome status did not modify this risk. Our findings support the hypothesis that in individuals with diabetes, physicians should be aggressive in using CVD risk–reducing therapies in all patients regardless of metabolic syndrome status.
Acknowledgments
This study was supported in part by U.S. Public Health Service Research Grant AG06945 from the National Institute on Aging (Bethesda, MD) and by the Communities Foundation of Texas on recommendation of Nancy Ann and Ray L. Hunt. P.T.K. is supported, in part, by the Louisiana Public Facilities Authority Endowed Chair in Nutrition.
No potential conflicts of interest relevant to this article were reported.
We thank our participants and the Cooper Clinic physicians, technicians, and staff.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 2. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414– 1431 [DOI] [PubMed] [Google Scholar]
- 3. Narayan KMV, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden; U.S., 2005–2050. Diabetes Care 2006; 29: 2114– 2116 [DOI] [PubMed] [Google Scholar]
- 4. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486– 2497 [DOI] [PubMed] [Google Scholar]
- 5. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539– 553 [DOI] [PubMed] [Google Scholar]
- 6. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet 2005; 366: 1059– 1062 [DOI] [PubMed] [Google Scholar]
- 7. Balkau B, Charles MA. Comment on the provisional report from the WHO consultation: European Group for the Study of Insulin Resistance (EGIR). Diabet Med 1999; 16: 442– 443 [DOI] [PubMed] [Google Scholar]
- 8. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356– 359 [DOI] [PubMed] [Google Scholar]
- 9. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288: 2709– 2716 [DOI] [PubMed] [Google Scholar]
- 10. Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med 2004; 164: 1066– 1076 [DOI] [PubMed] [Google Scholar]
- 11. Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003; 108: 414– 419 [DOI] [PubMed] [Google Scholar]
- 12. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004; 110: 1245– 1250 [DOI] [PubMed] [Google Scholar]
- 13. Koehler C, Ott P, Benke I, Hanefeld M. Comparison of the prevalence of the metabolic syndrome by WHO, AHA/NHLBI, and IDF definitions in a German population with type 2 diabetes: the Diabetes in Germany (DIG) Study. Horm Metab Res 2007; 39: 632– 635 [DOI] [PubMed] [Google Scholar]
- 14. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003; 52: 1210– 1214 [DOI] [PubMed] [Google Scholar]
- 15. Tong PC, Kong AP, So WY, Yang X, Ho CS, Ma RC, Ozaki R, Chow CC, Lam CW, Chan JC, Cockram CS. The usefulness of the International Diabetes Federation and the National Cholesterol Education Program's Adult Treatment Panel III definitions of the metabolic syndrome in predicting coronary heart disease in subjects with type 2 diabetes. Diabetes Care 2007; 30: 1206– 1211 [DOI] [PubMed] [Google Scholar]
- 16. Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation 2004; 110: 1251– 1257 [DOI] [PubMed] [Google Scholar]
- 17. Cull CA, Jensen CC, Retnakaran R, Holman RR. Impact of the metabolic syndrome on macrovascular outcomes in type 2 diabetes mellitus, United Kingdom Prospective Diabetes Study 78. Circulation 2007; 116: 2119– 2126 [DOI] [PubMed] [Google Scholar]
- 18. Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004; 27: 83– 88 [DOI] [PubMed] [Google Scholar]
- 19. Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000; 132: 605– 611 [DOI] [PubMed] [Google Scholar]
- 20. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160– 3167 [DOI] [PubMed] [Google Scholar]
- 21. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005; 28: 2289– 2304 [DOI] [PubMed] [Google Scholar]
- 22. Standards of medical care in diabetes—2007. Diabetes Care 2007; 30 ( Suppl. 1): S4– S41 [DOI] [PubMed] [Google Scholar]