Abstract
OBJECTIVE
To evaluate the brain's responses to painful visceral and somatic stimuli in diabetic patients with gastrointestinal symptoms.
RESEARCH DESIGN AND METHODS
The sensitivity to electrical esophageal and median nerve stimulations was assessed in 15 healthy volunteers and 14 type 1 diabetic patients with autonomic neuropathy and gastrointestinal symptoms using a euglycemic-hyperinsulinemic clamp. Evoked brain potentials were recorded.
RESULTS
Patients had reduced sensitivity to esophageal (48%; P < 0.001) and median nerve (80%; P < 0.001) stimulations. They also had increased (8.8%; P = 0.007) and nonreproducible (P = 0.006) latencies of evoked potentials in response to esophageal stimulations, with 26% reduction in amplitude (P = 0.011). No potential differences were seen to median nerve stimulations. In diabetic patients, the topographic location of the first peak in potentials was more central (P < 0.001) and gastrointestinal symptoms correlated with characteristics of brain potentials (P = 0.049).
CONCLUSIONS
This study supports that diabetes induces changes in peripheral visceral nerves as well as in the central nervous system.
Gastrointestinal symptoms are more prevalent in diabetic individuals than in the general population (1,2). The pathogenesis is undoubtedly multifactorial, including motor dysfunction, glycemic control, psychological factors, etc. (3,4). However, diabetic autonomic neuropathy (DAN) seems to play a central role (1,3,5). The aim of this study, in healthy control subjects and patients with long-standing diabetes and gastrointestinal symptoms, was to describe the following: 1) the sensory thresholds for electrical esophageal and median nerve stimulation and 2) the evoked brain potentials (EPs) recorded with a high-resolution electroencephalogram system.
RESEARCH DESIGN AND METHODS
Fourteen type 1 diabetic patients (12 female; mean age 34.4 years [range 20–51]) and 15 healthy control subjects (10 female; mean age 33.5 years [21–50]) participated. Primary gastrointestinal and other diseases were ruled out with endoscopy where appropriate. The patients' diabetes lasted 14–40 years (mean 22 years) and was managed with multiple insulin injection regimens or insulin pumps. Mean A1C level was 9.6% (range 7.1–14.1). DAN was verified by abnormal gastric emptying breath tests, heart rate variability (HRV), and modified Ewing tests (6,7). An HRV index was calculated based on the mean of R-R intervals, the SD of all normal-to-normal (NN) R-R intervals, the mean of the SDs of all NN intervals, the SD of sequential 5-min R-R interval means, and the square root of the mean of the sum of the squares of differences between adjacent NN intervals (6). An autonomic score was calculated as the sum of orthostatic blood pressure changes, R-R variability upon deep respiration, and corrected QT (7). All patients had one or more gastrointestinal symptoms, and a gastrointestinal symptom score was calculated based on the sum of the following symptom scores: nausea, vomiting, early satiety, bloating, abdominal pain, diarrhea, and constipation, all ranging from no (0) to moderate (1) to severe (2) symptoms. No medication (except insulin) was allowed 24 h before the study.
After 6 h fasting, the blood glucose level was adjusted in all subjects to 6 mmol/l using a hyperinsulinemic-hyperglycemic clamp technique (8). A 64 surface electrode electroencephalogram cap was mounted (according to the international 10–20 system of electrode placement). A 3-mm catheter was swallowed with the ring electrodes positioned in the distal esophagus. Electrical stimulations were applied as single stimuli consisting of a series of five short 1-ms square pulses at 200 Hz. The sensations were rated on a 0–10 visual analog scale (VAS): 0, no perception; 3, vague perception of moderate sensation; and 5, pain detection threshold. Afterward, right median nerve stimulation was performed with identical protocol settings. The electrocardiogram was monitored during all stimulations.
Fifty identical esophageal and 30 median nerve stimulations were applied twice at 0.2 Hz with 5-min breaks. The electroencephalogram signals were recorded with a sampling rate of 1,000 Hz (SynAmps; Neuroscan, El Paso, TX). The EPs were processed offline, and the mean of the two stimulation runs was computed (version 4.3.1; Neuroscan) (Fig. 1A). The primary topographic location of each component was identified on topographic maps. The latencies and amplitudes of each component (N1-P1-N2-P2-N3) at Cz (vertex) were identified blindly using topographic brain maps as guidance. For correlation analysis, a total electroencephalogram index was calculated based on the average of the N1, P1, and N2 latencies (mean 100) divided by the average of the N1, P1, and N2 amplitudes (mean 100).
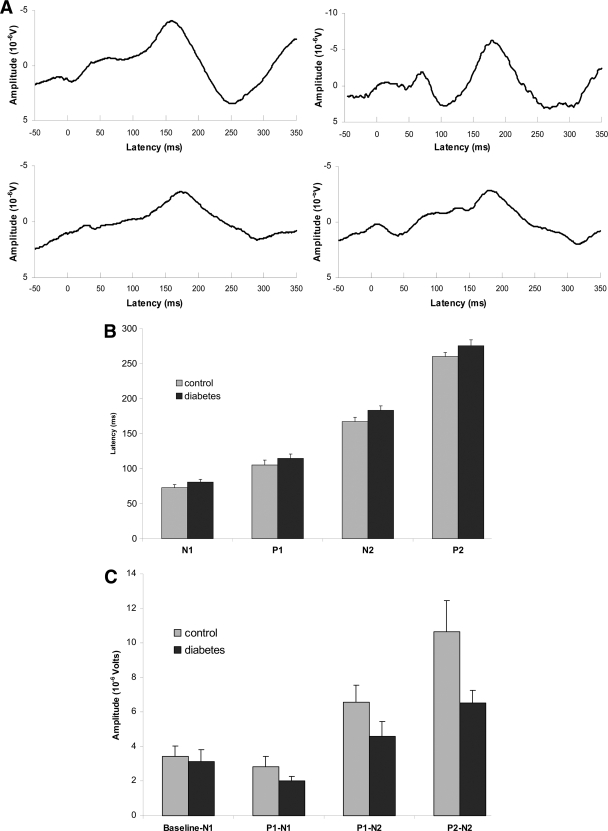
Figure 1.
A: EPs at vertex (Cz) in response to painful esophageal electrical stimulation. The grand mean (left panel) and typical examples (right panel) are illustrated for both healthy control subjects (top panel) and diabetic patients (bottom panel) with verified autonomic neuropathy and related gastrointestinal symptoms. The latencies (B) and amplitudes (C) of the EPs at Cz are illustrated. Including the first four components (N1–P2), the latencies were increased and the amplitudes reduced in the diabetic patients. Error bars indicate SE of the mean. *Significant results of the post hoc test.
RESULTS
The blood glucose level was adjusted to a mean of 6.0 ± 0.1 mmol/l for the control subjects and 6.2 ± 0.2 mmol/l for the patients. With all three sensory levels combined, the diabetic patients had reduced sensitivity to esophageal (F = 16, P < 0.001; post hoc VAS 1, P = 0.03; VAS 3, P = 0.01; and VAS 5, P = 0.03) and median nerve (F = 26, P < 0.001; post hoc VAS 1, P = 0.15; VAS 3, P = 0.048; and VAS 5, P < 0.001) stimulation. There were no significant correlations between esophageal and median nerve thresholds in either group (all P > 0.3).
When the two stimulation runs for EPs in control subjects were compared, they were reproducible (latencies: F = 3.3, P = 0.11; amplitudes: F = 0.3, P = 0.62). The EPs were more variable in the diabetic patients, with longer latencies in the second run (F = 11, P = 0.006).
When the two stimulation runs were combined and analyzed (N1-P1-N2-P2), we found that the patients had increased latencies (F = 7.6; P = 0.007) and reduced amplitudes (F = 6.7; P = 0.01) of the EPs (Fig. 1B and C). For median nerve stimulation, neither the latencies (F = 0.7; P = 0.4) nor the amplitudes (F = 0.5; P = 0.5) were different from those of the healthy control subjects. The location of the first positive deflection of the EP in patients was shifted more centrally compared with the frontal location in the healthy control subjects (χ2 = 17; P < 0.001), whereas no differences in the topographic distribution of the other peaks were found (all P > 0.5).
The gastrointestinal symptom score only correlated with the total electroencephalogram index (r = 0.55; P = 0.049); increasingly abnormal esophageal EP correlated positively with the symptom. The total electroencephalogram index did not correlate with the diabetes duration (P = 0.3), A1C level (P = 0.6), HRV index (P = 0.8), or autonomic score (P = 0.5).
CONCLUSIONS
Diabetic patients had reduced esophageal sensitivity, with reduced quality and nonreproducible EPs having increased latencies and reduced amplitudes. The electroencephalogram findings correlated with the gastrointestinal symptoms.
Our results are comparable with previous findings in small samples of patients (9–11). The observed decrease in sensation and increase in latencies of EPs indicate altered sensory processing. DAN induces a decrease in conduction velocity of both peripheral and spinal nerve fibers, contributing to an increase in latency (10). However, the visceral sensory system is not hardwired, and neuroplastic changes could be expected in diabetic individuals involving spinal and supraspinal reorganization with activation of latent ascending pathways as well as descending inhibitory or facilitatory control mechanisms (12). A combination of all these mechanisms is probably involved, resulting in the final configuration of the EPs. Structural cerebral changes could also explain the findings. Reduced density of cortical gray matter, cortical atrophy, and deep-white-matter lesions are seen in patients with long-lasting diabetes (13). Aging has shown to increase sensory thresholds, reduce amplitude, and increase latencies of somatic EPs (14). Similar aging-like changes may be present in long-lasting diabetes.
The reduced upper gastrointestinal sensitivity with altered and delayed brain responses seems somehow surprising given that diabetic patients have an increased prevalence of gastrointestinal symptoms (1,2). However, the topographic findings indicate changes in the primary pain processing of visceral pain, and such central changes may result in gastrointestinal events being perceived in an abnormal way (15). This theory is supported by gastrointestinal symptoms being correlated with the degree of abnormal EPs. Hence, viscero-sensory EPs may be useful biomarkers of diabetes-induced neuropathic-like mechanisms, which are not assessed using traditional cardiovascular tests.
Supplementary Material
Acknowledgments
The Danish Council for Independent Research/Medical Sciences, the Spar Nord Foundation, the Obelske Family Foundation, the Danish Diabetes Association, the Karen Elise Jensen Foundation, and Heinrich Kopp's Grant are acknowledged for providing funds for this project.
Novo Nordisk Foundation also funded this project. No other potential conflicts of interest relevant to this article were reported.
The Centre for Clinical Research at Haukeland University Hospital, Bergen, Norway, is acknowledged for invaluable help and facilitation.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Spångéus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol 1999; 34: 1196– 1202 [DOI] [PubMed] [Google Scholar]
- 2. Ricci JA, Siddique R, Stewart WF, Sandler RS, Sloan S, Farup CE. Upper gastrointestinal symptoms in a U.S. national sample of adults with diabetes. Scand J Gastroenterol 2000; 35: 152– 159 [DOI] [PubMed] [Google Scholar]
- 3. Horowitz M, Samsom M. (Eds): Gastrointestinal Function in Diabetes Mellitus. Chichester, U.K., John Wiley & Sons, 2004 [Google Scholar]
- 4. Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001; 24: 371– 381 [DOI] [PubMed] [Google Scholar]
- 5. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003; 26: 1553– 1579 [DOI] [PubMed] [Google Scholar]
- 6. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996; 17: 354– 381 [PubMed] [Google Scholar]
- 7. Ewing DJ, Clarke BF. Autonomic neuropathy: its diagnosis and prognosis. Clin Endocrinol Metab 1986; 15: 855– 888 [DOI] [PubMed] [Google Scholar]
- 8. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 9. Rathmann W, Enck P, Frieling T, Gries FA. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care 1991; 14: 1086– 1089 [DOI] [PubMed] [Google Scholar]
- 10. Tougas G, Hunt RH, Fitzpatrick D, Upton AR. Evidence of impaired afferent vagal function in patients with diabetes gastroparesis. Pacing Clin Electrophysiol 1992; 15: 1597– 1602 [DOI] [PubMed] [Google Scholar]
- 11. Kamath MV, Tougas G, Fitzpatrick D, Fallen EL, Watteel R, Shine G, Upton AR. Assessment of the visceral afferent and autonomic pathways in response to esophageal stimulation in control subjects and in patients with diabetes. Clin Invest Med 1998; 21: 100– 113 [PubMed] [Google Scholar]
- 12. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999; 353: 1959– 1964 [DOI] [PubMed] [Google Scholar]
- 13. Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006; 55: 326– 333 [DOI] [PubMed] [Google Scholar]
- 14. Gibson SJ, Gorman MM, Helme RD. Assessment of pain in the elderly using event-related cerebral potentials. In Proceedings of the VIth World Congress on Pain, Amsterdam, 1991. Bond MR, Charlton JE, Woolf CJ. Eds. Shannon, Ireland, Elsevier Science Publishers, 1991, p. 527– 533. [Google Scholar]
- 15. Drewes AM, Krarup AL, Detlefsen S, Malmstrøm ML, Dimcevski G, Funch-Jensen P. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut 2008; 57: 1616– 1627 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.