Abstract
OBJECTIVE
To evaluate the safety and efficacy of ingested human recombinant interferon-α (hrIFN-α) for preservation of β-cell function in young patients with recent-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
Subjects aged 3–25 years in whom type 1 diabetes was diagnosed within 6 weeks of enrollment were randomly assigned to receive ingested hrIFN-α at 5,000 or 30,000 units or placebo once daily for 1 year. The primary outcome was change in C-peptide secretion after a mixed meal.
RESULTS
Individuals in the placebo group (n = 30) lost 56 ± 29% of their C-peptide secretion from 0 to 12 months, expressed as area under the curve (AUC) in response to a mixed meal. In contrast, children treated with hrIFN-α lost 29 ± 54 and 48 ± 35% (for 5,000 [n = 27] and 30,000 units [n = 31], respectively, P = 0.028, ANOVA adjusted for age, baseline C-peptide AUC, and study site). Bonferroni post hoc analyses for placebo versus 5,000 units and placebo versus 30,000 units demonstrated that the overall trend was determined by the 5,000-unit treatment group. Adverse events occurred at similar rates in all treatment groups.
CONCLUSIONS
Ingested hrIFN-α was safe at the doses used. Patients in the 5,000-unit hrIFN-α treatment group maintained more β-cell function 1 year after study enrollment than individuals in the placebo group, whereas this effect was not observed in patients who received 30,000 units hrIFN-α. Further studies of low-dose ingested hrIFN-α in new-onset type 1 diabetes are needed to confirm this effect.
Residual β-cell function was shown to correlate with decreased complication rates in the Diabetes Control and Complications Trial (1). As a result, preservation of β-cell function is an important treatment goal in patients with type 1 diabetes. Over the past 25 years, multiple clinical trials attempted to prevent progressive β-cell destruction after the diagnosis of type 1 diabetes using immunosuppressive or immunomodulatory agents such as cyclosporin (2,3), cyclosporin in combination with bromocriptine (4), azathioprine with or without glucocorticoids (5,6), nicotinamide with or without glucocorticoids (7,8), and parenteral interferon-α (IFN-α) (9). More recent intervention trials used monoclonal antibody–based therapies such as rituximab (anti-CD-20) (clinical trial reg. no. NCT00279205), daclizumab (anti-CD25) in combination with mycophenolate mofetil (clinical trial reg. no. NCT00100178), anti-CD3 (10–12), and GAD (13). Although several studies are still ongoing and thus the results are pending, trials of anti-CD3 antibodies and GAD have demonstrated delayed decline of endogenous insulin secretion. However, none of these intervention trials have yet been translated into common clinical practice for two reasons: 1) the β-cell protective effect has been temporary, and 2) some of the agents available were associated with unacceptable side effects, such as impairment of renal function in the case of calcineurin inhibitors.
The original observation that ingested type 1 interferon has an immunomodulatory effect was made in mice with chronic relapsing experimental autoimmune encephalomyelitis (14). Subsequently, a phase I trial demonstrated that 10,000–30,000 but not 100,000 units ingested IFN-α act as a biological response modifier without apparent toxicity in humans with multiple sclerosis (15), and a phase II trial suggested that 10,000 units may be more clinically effective than 30,000 units (16). The mechanism of action remains unclear. It is thought that ingested interferon, which is acid stable and therefore resists gastric digestion, binds to high-affinity receptors in the gut-associated lymphoid tissue where it transduces its signal. Serum levels do not change nor do other protein markers of interferon absorption (e.g., β2-microglobulin, neopterin, and 2-5A synthetase) (17).
A decade ago, ingested INF-α was reported to modify the onset of autoimmune diabetes in a commonly used animal model, the nonobese diabetic (NOD) mouse. Adoptive transfer of unstimulated splenocytes secreting interleukin (IL)-4 and IL-10 from mouse IFN-α–fed donors suppressed spontaneous diabetes in recipients, thus demonstrating the presence of ingested IFN-α–activated regulatory splenic cell populations that work via increased IL-4 or IL-10 production (18). A small, open-label pilot project in 10 children with recently diagnosed type 1 diabetes supported the safety of ingested interferon and helped with dose finding (19). The current study is the first randomized, controlled trial testing the safety and efficacy of ingested human recombinant (hr) IFN-α to preserve β-cell function in young patients with recent-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
The present clinical trial was designed as a prospective, randomized, placebo-controlled, double-blind, multicenter, parallel-group study. Eligible subjects were between 3 and 25 years old, had a diagnosis of type 1 diabetes by standard clinical criteria within the preceding 6 weeks, and had no other medically significant concurrent illness. Study approval was obtained from the institutional review boards at each participating center (a list of centers is found in an online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc08-2029/DC1). Written informed consent was obtained from subjects or their guardians before enrollment, and minors provided written or verbal assent.
A total of 128 patients were enrolled at the five participating centers (54 at the National Institutes of Health [NIH] and 74 at other sites) and were randomly assigned by block centrally with no stratification to one of the following three treatment arms: placebo, 5,000 units hrIFN-α, or 30,000 units hrIFN-α. At screening and every 3 months thereafter, patients underwent a mixed-meal study (Boost High Protein, 7 ml/kg, maximum 400 ml). Blood samples were obtained at –10, 0, 30, 60, 90, and 120 min.
Interferon preparation and administration
Medications were prepared at each center's pharmacy as either 5,000 or 30,000 units hrIFN-α. Roferon (Roche) was diluted in saline with 6 mg human serum albumin (HSA), and placebo was prepared as saline alone with 6 mg HSA. The initial dose of medication or placebo was administered under supervision. Patients received monthly supplies of vials containing frozen hrIFN-α or placebo and were instructed to store these vials in the freezer. Each night, one vial was transferred to the refrigerator and thawed. In the morning before breakfast, the content of each vial was swallowed with at least 150 ml of water. Neither taste nor general appearance differed between the placebo and interferon-containing saline solutions.
Biochemical measurements
NIH study participants' C-peptide levels were determined with a competitive chemiluminescence assay at the NIH Clinical Center laboratory. The normal range in healthy, nondiabetic individuals is 0.3–1.3 nmol/l (0.9–4.0 ng/ml) with an intra-assay precision of 3.4% at 1.44 nmol/l (4.34 ng/ml) and an interassay precision of 7.7 and 8.3% at 0.37 nmol/l (1.12 ng/ml) and 1.98 nmol/l (5.94 ng/ml), respectively. The lower limit of detection was 0.17 (0.5 ng/ml). All non-NIH participants' C-peptide concentrations were determined with a radioimmunoassay (Linco), which had an intra-assay precision of 3.4–6.4% and an interassay precision of 2.4–9.3%. The standard curve ranged from 0.03 to 1.67 nmol/l (0.1 to 5 ng/ml) and the lower limit of detection was 0.03 nmol/l (0.1 ng/ml). Serum glucose measurements were determined using the glucose oxidase method (intra-assay CV 2.9% at 2.4 mmol/l [44 mg/dl] and 0.4% at 22.1 mmol/l [397 mg/dl] and interassay CV 3.9% at 2.4 mmol/l [44 mg/dl] and 1.2% at 22.1 mmol/l [397 mg/dl]). A1C was determined by high-performance liquid chromatography (intra-assay CV 0.58% for normal A1C and 0.42% for elevated A1C; interassay CV 1.3% for normal A1C and 0.93% for elevated A1C).
Efficacy assessments
The primary outcome was the change in meal-stimulated C-peptide area under the curve (AUC) from the beginning to the end of the study. Because different C-peptide assays were used at the NIH versus the other participating centers, changes in C-peptide secretion are reported as percent change from baseline as well as absolute change.
Safety assessments
Safety was assessed by physical examinations, clinical laboratory measurements, and documentation of adverse events. Every 6 months during the study, a data safety and monitoring board reviewed all patients' data in an unblinded fashion.
Statistical analysis
C-peptide AUC following the mixed-meal test was calculated using the trapezoidal method. Repeated-measures mixed models (Proc Mix SAS) were used to assess the proportional differences in C-peptide AUC over time among treatment groups. All models were adjusted for age and included treatment, time, baseline C-peptide AUC, and interaction of treatment and time. Models that assessed C-peptide differences were also adjusted by site because two different C-peptide assays were used. An autoregressive covariance structure was used, and Bonferroni correction was used for multiple pairwise comparisons. The least-square means were estimated at each time point. Sensitivity analyses compared baseline differences in C-peptide levels by study site, by the assay used, and by completers among all three arms of the study. A completer was defined as a patient who completed at least 9 months follow-up.
In addition to the repeated-measures analyses, the absolute and relative changes from baseline to 12 months were assessed. The absolute difference of C-peptide AUC was defined as the AUC at screening minus the AUC at 12 months. The proportional loss of C-peptide AUC was calculated by dividing the absolute difference by C-peptide AUC at screening. Pearson χ2 tests and ANOVAs were used to assess the proportional loss of C-peptide AUC among the three arms of the study (placebo, 5,000 units hrIFN-α, or 30,000 units hrIFN-α). Secondary analyses included differences in A1C and insulin dose, over time and between treatment groups.
Data are presented as means ± SD. Statistical significance was assigned for P < 0.05. Data processing and statistical evaluations were completed using SAS (version 9.1, SAS Institute, Cary, NC).
RESULTS
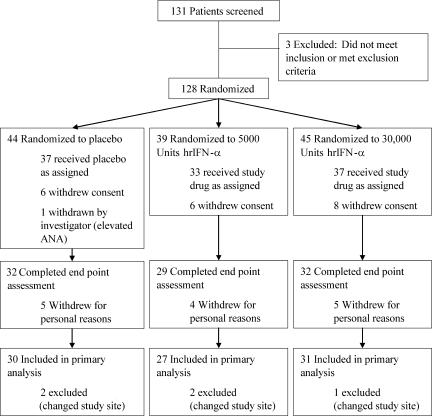
Of 128 subjects enrolled in the study, 107 received at least one dose of study drug, and 93 completed the follow-up (Fig. 1). Five patients who completed the study were excluded from the final analysis because they changed study centers (and thus C-peptide assays) during the study period. Thus, a total of 88 subjects generated data for the final analysis.
Figure 1.
Patient flow diagram. ANA, anti-nuclear antibodies.
Baseline characteristics of subjects included in the final analysis are shown in Table 1. Sensitivity analyses showed no significant differences between baseline characteristics of noncompleters and completers.
Table 1.
Baseline patient characteristics of 88 patients included in the final analysis
| Placebo | 5,000 units | 30,000 units | P | |
|---|---|---|---|---|
| n | 30 | 27 | 31 | |
| Study site | 0.56 | |||
| NIH | 16 (53) | 11 (41) | 13 (42) | |
| Non-NIH | 14 (47) | 16 (59) | 18 (58) | |
| Age (years) | 10.8 ± 4.8 | 10.5 ± 5.1 | 9.9 ± 4.4 | 0.78 |
| Sex | 0.88 | |||
| Male | 17 (57) | 17 (63) | 19 (61) | |
| Female | 13 (43) | 10 (37) | 12 (39) | |
| Ethnicity | 0.57 | |||
| Caucasian | 25 (83) | 26 (96) | 28 (90) | |
| Asian | 3 (10) | 1 (4) | 1 (3) | |
| Hispanic | 2 (7) | 0 (0) | 2 (6) | |
| Insulin dose (units · kg−1 · day−1) | 0.43 ± 0.27 | 0.46 ± 0.22 | 0.43 ± 0.19 | 0.89 |
| Diabetic ketoacidosis at diagnosis | 4 (13) | 4 (15) | 4 (13) | 1.00 |
| A1C (%) | 8.2 ± 1.6 | 8.0 ± 1.7 | 8.2 ± 1.2 | 0.86 |
| Positive GAD65 | 17 (77) | 14 (87) | 14 (74) | 0.66 |
| Fasting C-peptide (nmol/l) | 0.46 ± 0.33 | 0.39 ± 0.25 | 0.50 ± 0.47 | 0.53 |
| Screening C-peptide AUC (nmol · l−1 · 120 min−1) | 1.77 ± 1.11 | 1.81 ± 0.83 | 1.70 ± 1.02 | 0.91 |
| Screening glucose AUC (mmol · l−1 · 120 min−1) | 24.1 ± 8.2 | 21.9 ± 5.8 | 22.9 ± 6.2 | 0.46 |
Data are means ± SD or n (%). P values are based on Pearson's χ2 test, Fisher's exact test, Kruskal-Wallis test, or ANOVA as appropriate.
Safety
Three serious adverse events (SAEs) occurred during the study. An 18-year-old male patient developed Staphylococcus septicemia during his 9th month of treatment with 30,000 units hrIFN-α. His subsequent clinical course was complicated by multiple peripheral emboli, and he required prolonged hospitalization. The patient recovered fully but hrIFN-α was discontinued. One placebo-treated patient developed medulloblastoma, and another placebo-treated patient developed gastroenteritis resulting in diabetic ketoacidosis requiring hospitalization. All SAEs and adverse events were reviewed by the data safety and monitoring board, and no SAE was considered to be related to therapy.
There was no difference in the occurrence of adverse events among the three treatment groups. During the study, subjects had means ± SD of 2.1 ± 2.4, 1.6 ± 2.0, and 2.1 ± 2.1 adverse events each in the placebo, 5,000-unit hrIFN-α, and 30,000-unit hrIFN-α groups, respectively. Other than the SAEs, all adverse events were rated as mild to moderate (grades 1 or 2). A summary of adverse events is found in Table 2.
Table 2.
Adverse events by treatment group
| Treatment group |
|||
|---|---|---|---|
| 30,000 units | 5,000 units | Placebo | |
| n | 44 | 40 | 44 |
| Infection (viral) | 14 (31.8) | 9 (22.5) | 9 (20.5) |
| Infection (bacterial) | 7 (20.5) | 2 (12.5) | 4 (22.7) |
| Infection (upper airway) | 24 (54.5) | 20 (50.0) | 30 (68.2) |
| Dermatologic/skin | 2 (4.5) | 2 (5.0) | 3 (6.8) |
| Gastrointestinal | 5 (11.4) | 3 (7.5) | 4 (9.1) |
| Pancreatic/endocrine | 2 (4.5) | 6 (15.0) | 2 (4.5) |
| Bleeding | 2 (4.5) | 0 (0) | 0 (0) |
| Neurological | 1 (2.3) | 2 (5.0) | 5 (11.4) |
| Allergy/immunology | 8 (18.2) | 6 (15.0) | 3 (6.8) |
| Cancer | 0 (0) | 0 (0) | 1 (2.3) |
| Musculoskeletal | 0 (0) | 1 (2.5) | 4 (9.1) |
| Metabolic/laboratory | 20 (45.5) | 9 (22.5) | 8 (18.2) |
Data are n (%).
C-peptide secretion
C-peptide AUC at screening was lower in subjects evaluated at the NIH versus those evaluated at other study sites (1.5 ± 0.7 mmol/l for NIH vs. 2.0 ± 1.2 mmol/l for all other sites, P = 0.02). However, mixed models revealed no significant effect of study site for either absolute or relative change in C-peptide AUC from 0 to 12 months; hence, subjects from all sites were combined for analysis of the primary outcome.
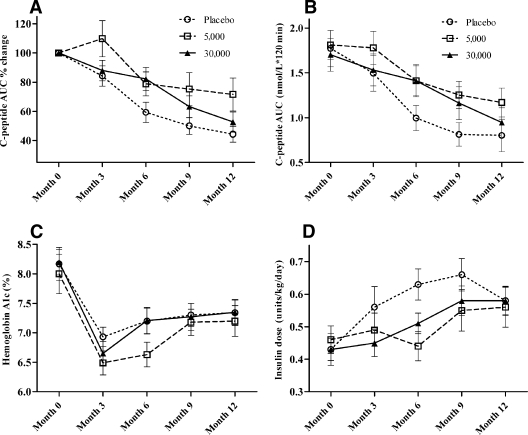
Figure 2 shows the decline in mixed-meal stimulated C-peptide AUC over the period of follow-up. Mixed-model analysis revealed a significant effect of treatment for percent loss (P = 0.046) (Fig. 2A) and near significance for absolute change (P = 0.064) (Fig. 2B) in C-peptide AUC. Treatment by time interactions in the repeated-measures analysis was not statistically significant (percent loss P = 0.116; absolute change P = 0.144). Subjects treated with hrIFN-α had less percent loss of C-peptide at 12 months relative to screening compared with placebo-treated control subjects (56 ± 29, 29 ± 54, and 48 ± 35% for placebo, 5,000 units, and 30,000 units, respectively, P = 0.028, ANOVA adjusted for age, study site, and baseline C-peptide AUC). Bonferroni post hoc analyses for placebo versus 5,000 units and placebo versus 30,000 units demonstrated that the overall trend was led by the 5,000-unit treatment group (placebo vs. 5,000 units P = 0.017; placebo vs. 30,000 units P = 0.599).
Figure 2.
Changes over time in 88 subjects with new-onset type 1 diabetes who completed 12 months of treatment with either once-daily placebo (n = 30) or 5,000 units (n = 27) or 30,000 units (n = 31) ingested hrIFN-α. A: Percent change in C-peptide AUC after a mixed-meal test. B: C-peptide AUC after a mixed-meal test. C: A1C. D: Insulin dose.
Age was a significant predictor of C-peptide loss, with older patients losing less C-peptide secretion (P = 0.002). However, post hoc analyses including age as a dichotomous variable (age ≤10 years [n = 47] vs. age >10 years [n = 41]) did not reveal a significant age by treatment interaction for either percent (P = 0.66) or absolute change (P = 0.63) in C-peptide AUC from 0 to 12 months.
Secondary study objectives
There was no difference among treatment groups for the decline in A1C from screening to 12 months (placebo 0.7 ± 1.6%, 5,000 units hrIFN-α 0.9 ± 2.1%, and 30,000 units hrIFN-α 0.8 ± 1.3%, P = 0.46) (Fig. 2C). There was also no significant difference between groups for rise in insulin dose from screening to 12 months (placebo 0.20 ± 0.28 units · kg−1 · day−1, 5,000 units hrIFN-α 0.10 ± 0.26 units · kg−1 · day−1, and 30,000 units hrIFN-α 0.14 ± 0.26 units · kg−1 · day−1, P = 0.71) (Fig. 2D).
CONCLUSIONS
Greater residual β-cell function lowers the risk of developing nephropathy, neuropathy, and retinopathy and markedly reduces the risk of hypoglycemia (1). As a result, preservation of as much residual endogenous insulin secretion as possible is predicted to have a major impact on health outcomes in patients with type 1 diabetes. In this study, we observed that ingested hrIFN-α was safe in children with new-onset type 1 diabetes. This finding is of crucial importance because we tested a novel treatment for children with a condition that can be managed well with insulin alone. Thus, the acceptable safety margin for any intervention in this population is very small. Some safety concerns had arisen due to reports about high-dose interferon treatment in adult patients with hepatitis who developed new-onset type 1 diabetes (20). However, the present diabetes treatment and the hepatitis therapy differ by an approximate factor of 10,000 regarding the interferon dose. Further concern could be based on the role of IFN-α as an initiator in the development of the autoimmune diabetes, which has only been shown in rodents (21).
In addition to the good safety profile, we found that children who had received the 5,000-unit hrIFN-α dose lost less C-peptide (expressed as percent loss) from study entry to 12 months. There was no significant treatment by time interaction observed on repeated-measures analysis, suggesting that, although the groups diverged early in the study, the rate of C-peptide loss was roughly equal among the groups after 3 months. This finding is consistent with those for other therapies for C-peptide preservation in type 1 diabetes, including anti-CD3 and GAD (11,13).
The superiority of the 5,000-unit dose of hrIFN-α over the 30,000-unit dose is consistent with human and animal data supporting greater efficacy of lower doses of ingested interferon in autoimmune disease (15,16,22). These findings suggest that ingested interferon may have a hormetic dose-response relationship (i.e., generally favorable biological responses to low exposures with the opposite effect at high doses). The biological basis of such biphasic dose-response relationships is poorly understood, but they appear to be common in immunology (23).
Although no statistically significant differences were found among treatment groups for secondary outcomes, including A1C and insulin dose, the general trend in these parameters supported the primary outcome. That is, patients in the 5,000-unit hrIFN-α group had smaller increases in insulin dose, accompanied by greater declines in A1C.
This study is limited by several important drawbacks. First, different C-peptide assays were used for NIH subjects versus subjects at all other study sites. We attempted to compensate for this limitation by determining relative loss of C-peptide secretion rather than using the absolute C-peptide measurements. In addition, we excluded from the analysis five subjects who changed study sites, for whom the 12-month C-peptide results could not be compared with the baseline values because of the different assays used. The validity of performing a combined analysis for primary outcome using subjects from all sites is supported by separate analyses for NIH and non-NIH subjects showing similar trends in C-peptide secretion (data not shown) and by mixed-model analyses that demonstrated no statistically significant differences by study site.
A second significant study drawback was the difference in chronic and acute blood glucose management among study participants. Primary diabetes care was provided by local pediatric endocrinologists. Thus, some patients may have benefited from more intense insulin therapy and reduced glucotoxicity, which by itself may have influenced β-cell preservation. However, there were no differences in A1C or insulin pump use among the three treatment groups, suggesting that differences in chronic blood glucose control were unlikely to have affected the study outcome. Most clinical trials in the field of type 1 diabetes are challenged by the differences in acute blood glucose levels at the time of provocative testing. Despite attempts to perform the mixed meals under reproducible conditions, blood glucose levels differed from one test to another. However, because this trial was performed in a randomized fashion, we believe that this factor did not play an important role regarding our main outcomes.
Although intent-to-treat analysis was not performed in this study, 87% of subjects who received even a single dose of hrIFN-α completed the study. In addition, sensitivity analyses did not demonstrate any significant differences between baseline variables in subjects who did versus did not complete the study. All but two subjects (one patient in the placebo group who developed a brain tumor and another patient in the 30,000-unit hrIFN-α group who developed Staphyloccocus sepsis) discontinued participation for personal reasons (largely because of distance to study centers), rather than for any medical reason.
In summary, we found that ingested hrIFN-α was a safe drug at the concentrations used, and a beneficial effect on β-cell function was observed in the 5,000-unit group. The long-term effects of hrIFN-α in type 1 diabetes are unknown, and further studies are needed to determine whether there is any sustained benefit.
Acknowledgments
This research was supported, in part, by the intramural research program of the National Institute of Diabetes, Digestive and Kidney Diseases (NIH Grant UL1 RR024148 to the University Clinical Research Center at the University of Texas—Houston), by the Children's Hospital of Minnesota Foundation, and by a grant from the Diabetes Action Research and Education Foundation.
S.A.B. is a patent holder (EP0752884) with the European Patent Office. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the annual meeting of the Federation of Clinical Immunology Societies, San Francisco, California, 11–14 June 2009.
Footnotes
A full list of investigators can be found in an online appendix.
Clinical trial reg. no. NCT00024518, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Steffes MW, Sibley S, Jackson M, Thomas W. β-Cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care 2003; 26: 832– 836 [DOI] [PubMed] [Google Scholar]
- 2. Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes 1990; 39: 1264– 1272 [DOI] [PubMed] [Google Scholar]
- 3. Cyclosporin-induced remission of IDDM after early intervention: association of 1 yr of cyclosporin treatment with enhanced insulin secretion. The Canadian-European Randomized Control Trial Group. Diabetes 1988; 37: 1574– 1582 [PubMed] [Google Scholar]
- 4. Atkison PR, Mahon JL, Dupre J, Stiller CR, Jenner MR, Paul TL, Momah CI. Interaction of bromocriptine and cyclosporine in insulin dependent diabetes mellitus: results from the Canadian open study. J Autoimmun 1990; 3: 793– 799 [DOI] [PubMed] [Google Scholar]
- 5. Cook JJ, Hudson I, Harrison LC, Dean B, Colman PG, Werther GA, Warne GL, Court JM. Double-blind controlled trial of azathioprine in children with newly diagnosed type I diabetes. Diabetes 1989; 38: 779– 783 [DOI] [PubMed] [Google Scholar]
- 6. Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med 1988; 319: 599– 604 [DOI] [PubMed] [Google Scholar]
- 7. Pozzilli P, Visalli N, Boccuni ML, Baroni MG, Buzzetti R, Fioriti E, Signore A, Cavallo MG, Andreani D, Lucentini L, Matteoli MC, Crinò A, Cicconetti CA, Teodonio C, Amoretti R, Pisano L, Pennafina MG, Santopadre G, Marozzi G, Multari G, Campea L, Suppa MA, De Mattia GC, Cassone Faldetta M, Marietti G, Perrone F, Greco AV, Ghirlanda G. Combination of nicotinamide and steroid versus nicotinamide in recent-onset IDDM: the IMDIAB II study. Diabetes Care 1994; 17: 897– 900 [DOI] [PubMed] [Google Scholar]
- 8. Lewis CM, Canafax DM, Sprafka JM, Barbosa JJ. Double-blind randomized trial of nicotinamide on early-onset diabetes. Diabetes Care 1992; 15: 121– 123 [DOI] [PubMed] [Google Scholar]
- 9. Koivisto VA, Aro A, Cantell K, Haataja M, Huttunen J, Karonen SL, Mustajoki P, Pelkonen R, Seppala P. Remissions in newly diagnosed type 1 (insulin-dependent) diabetes: influence of interferon as an adjunct to insulin therapy. Diabetologia 1984; 27: 193– 197 [DOI] [PubMed] [Google Scholar]
- 10. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005; 352: 2598– 2608 [DOI] [PubMed] [Google Scholar]
- 11. Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005; 54: 1763– 1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haller MJ, Gottlieb PA, Schatz DA. Type 1 diabetes intervention trials 2007: where are we and where are we going? Curr Opin Endocrinol Diabetes Obes 2007; 14: 283– 287 [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008; 359: 1909– 1920 [DOI] [PubMed] [Google Scholar]
- 14. Brod SA, Burns DK. Suppression of relapsing experimental autoimmune encephalomyelitis in the SJL/J mouse by oral administration of type I interferons. Neurology 1994; 44: 1144– 1148 [DOI] [PubMed] [Google Scholar]
- 15. Brod SA, Kerman RH, Nelson LD, Marshall GD, Jr, Henninger EM, Khan M, Jin R, Wolinsky JS. Ingested IFN-α has biological effects in humans with relapsing-remitting multiple sclerosis. Mult Scler 1997; 3: 1– 7 [DOI] [PubMed] [Google Scholar]
- 16. Brod SA, Lindsey JW, Vriesendorp FS, Ahn C, Henninger E, Narayana PA, Wolinsky JS. Ingested IFN-α: results of a pilot study in relapsing-remitting MS. Neurology 2001; 57: 845– 852 [DOI] [PubMed] [Google Scholar]
- 17. Witt PL, Goldstein D, Storer BE, Grossberg SE, Flashner M, Colby CB, Borden EC. Absence of biological effects of orally administered interferon-β ser. J Interferon Res 1992; 12: 411– 413 [DOI] [PubMed] [Google Scholar]
- 18. Brod SA, Malone M, Darcan S, Papolla M, Nelson L. Ingested interferon α suppresses type I diabetes in non-obese diabetic mice. Diabetologia 1998; 41: 1227– 1232 [DOI] [PubMed] [Google Scholar]
- 19. Brod SA, Atkinson M, Lavis VR, Brosnan PG, Hardin DS, Orlander PR, Nguyen M, Riley WJ. Ingested IFN-α preserves residual β cell function in type 1 diabetes. J Interferon Cytokine Res 2001; 21: 1021– 1030 [DOI] [PubMed] [Google Scholar]
- 20. Tanaka J, Sugimoto K, Shiraki K, Beppu T, Yoneda K, Fuke H, Yamamoto N, Ito K, Takei Y. Type 1 diabetes mellitus provoked by peginterferon α-2b plus ribavirin treatment for chronic hepatitis C. Intern Med 2008; 47: 747– 749 [DOI] [PubMed] [Google Scholar]
- 21. Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-α initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA 2008; 105: 12439– 12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brod SA, Khan M. Oral administration of IFN-α is superior to subcutaneous administration of IFN-α in the suppression of chronic relapsing experimental autoimmune encephalomyelitis. J Autoimmun 1996; 9: 11– 20 [DOI] [PubMed] [Google Scholar]
- 23. Calabrese EJ. Hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit Rev Toxicol 2005; 35: 89– 295 [DOI] [PubMed] [Google Scholar]