Abstract
OBJECTIVE
Aldose reductase inhibitors (ARIs) are potential disease modifiers for diabetes complications. We aimed to determine whether ranirestat, an ARI, could slow or reverse the course of diabetic sensorimotor polyneuropathy (DSP).
RESEARCH DESIGN AND METHODS
A total of 549 patients with DSP were randomly assigned to treatment with placebo or 10, 20, or 40 mg/day ranirestat for 52 weeks in this multicenter, double-blind study. Efficacy was evaluated by nerve conduction studies, the modified Toronto Clinical Neuropathy Score (mTCNS), and quantitative sensory tests (QSTs).
RESULTS
At week 52, the summed sensory (bilateral sural plus proximal median sensory) nerve conduction velocity (NCV) did not show significant changes from baseline (2.0 m/s for placebo compared with 3.2–3.8 m/s for ranirestat). Significant improvement in the summed motor (peroneal, tibial, and median) NCV was observed with 20 and 40 mg/day ranirestat treatment at week 12 (P ≤ 0.05) and at weeks 24 and 36 and in peroneal motor NCV at weeks 36 and 52 (P ≤ 0.05) for the 20 mg/day ranirestat group. The mTCNS and QST results did not differ among the groups during the study. Ranirestat was well tolerated with no pertinent differences in drug-related adverse events or in effects on clinical laboratory parameters, vital signs, or electrocardiograms among the four groups.
CONCLUSIONS
Treatment with ranirestat appears to have an effect on motor nerve function in mild to moderate DSP, but the results of this study failed to show a statistically significant difference in sensory nerve function relative to placebo.
Sensorimotor polyneuropathy is one of the major complications of diabetes with a prevalence of ∼50% in both type 1 and type 2 diabetes (1,2). Although the biochemical mechanisms underlying the development of diabetic sensorimotor polyneuropathy (DSP) are complex and still controversial, the polyol pathway is an important factor. Elevated blood glucose in diabetic patients leads to increased activity of aldose reductase, an enzyme that converts glucose to sorbitol, one of the alcohol sugars. The result is accumulation of sorbitol within nerves, which is associated with oxidative stress and nerve damage (3). Aldose reductase inhibitors (ARIs) block the polyol pathway and should be effective in preventing the progression of DSP. In fact, an earlier study showed that inhibition of nerve sorbitol levels was associated with improved motor nerve conduction velocity (NCV) and an increase in the density of small-diameter sural nerve myelinated fibers (4).
Although a number of ARIs have been developed, none have achieved clinical success for diverse reasons, one being that not all ARIs penetrate human peripheral nerves (5,6). Ranirestat (previously known as AS-3201), an ARI developed by Dainippon Sumitomo Pharma (Osaka, Japan), has demonstrated 65 and 84% inhibition of sorbitol accumulation in sural nerves from patients treated for 12 weeks with 5 and 20 mg/day, respectively (P < 0.001) (7). In a 48-week extension study, the sensory NCV improved by ≥1 m/s relative to baseline (P < 0.05) (8). Building on these phase II study results, we aimed to determine whether ranirestat would safely slow or reverse the progression of DSP compared with placebo treatment for 52 weeks.
RESEARCH DESIGN AND METHODS
We performed a multicenter, double-blind, randomized, placebo-controlled study in which patients were assigned to 10, 20, or 40 mg/day ranirestat or placebo administered as a once-daily dose for 52 weeks. The 40 mg/day dose was selected to determine whether a higher ranirestat dose, with presumed greater sorbitol inhibition, would improve the efficacy observed in the phase II study with a maximum dose of 20 mg/day (7,8). The institutional review boards at the participating centers reviewed and approved the study before the start of any study procedures. All patients provided written informed consent before screening procedures.
A total of 549 patients were enrolled in the study using the Interactive Voice Response System. Entry criteria were age 18–70 years, type 1 or 2 diabetes for at least 6 months, stable glycemic control for at least 3 months before entry, A1C ≥7.0%, and the presence of bilateral sural nerve potential amplitude responses of at least 1.0 μV. DSP was diagnosed by the modified San Antonio criteria requiring the presence of two of the following four criteria: 1) symptoms of DSP, 2) signs of DSP, 3) abnormal nerve conduction studies (NCS) with at least two abnormal nerves, and 4) abnormal vibration perception threshold (VPT). The presence of either of the latter two criteria was required (9). Patients with nondiabetic neuropathy or severe neuropathy (sural nerve amplitude <1.0 μV) were excluded. The results of NCS and VPT and the entry criteria for each patient were reviewed and approved by the Central Core Laboratory before a patient could be randomly assigned to ensure consistency of study procedures and high-quality data (10).
Procedures
Screening included a medical history, physical and neurological examinations, NCS, and quantitative sensory tests (QSTs) including VPT, cold detection threshold (CDT), and monofilament sensitivity. The modified Toronto Clinical Neuropathy Score (mTCNS) was used as a potentially more sensitive measure for clinical change than the validated TCNS (11). At weeks 12, 24, 36, and 42, NCS and QSTs were repeated and the mTCNS was again determined.
The primary end point was the summed change in sensory NCV from baseline of bilateral sural and proximal median sensory nerves. Secondary end points were the changes for individual sensory NCV, summed and individual motor NCV, F wave latencies, QST results, and the mTCNS.
Electrophysiology measurements.
Testing was standardized for temperature, side of testing, stimulation protocol, averaging of sensory potentials, and measurement of latencies and amplitudes. Unilateral NCS were performed on the nondominant median motor, dominant peroneal motor, and nondominant median sensory nerves. Bilateral sural NCS were performed. Sensory NCS were performed antidromically. Distances, response latencies, and amplitudes were measured using onset latencies and baseline-to-peak amplitudes. Measurements from the initial positive peak, if present, to the negative peak were made for sensory NCS. F waves were obtained for all motor nerves, and the minimal reproducible latency was measured. Conduction velocities were calculated for motor and sensory nerves.
mTCNS.
The mTCNS consists of graded symptom and sensory test scores associated with DSP in the judgment of the examiner (12). Details of the mTCNS have been presented previously in a validation study (12). The individual symptoms and signs evaluated are identical with those for the original, validated TCNS (11). The scale varies from 0 (no signs or symptoms of DSP) to 33 (maximal symptoms and signs of DSP) with a maximum of 18 symptom points and 15 sensory test points.
QSTs.
The VPT and CDT were measured at the first toe by the method of limits using the TSA-II NeuroSensory Analyzer (Medoc, Ramat, Yishai, Israel).
Biochemical measurements.
Tandem Laboratories (Salt Lake City, UT) performed the ranirestat plasma assays. Samples were prepared by a solid-phase extraction procedure and were analyzed by liquid chromatography/tandem mass spectrometry. Calibration standards were prepared by spiking blank, homogenized human plasma with the appropriate spiking solutions provided by Dainippon Sumitomo Pharma. The API 3000 system was operated in the selected-reaction monitoring mode under optimized conditions for detection of ranirestat negative ions formed by TurboIonSpray ionization (13).
Statistical analyses
Demographic and baseline characteristics were analyzed for homogeneity using a Kruskal-Wallis χ2 test. An intention-to-treat analysis was performed. Within-group comparisons between the baseline and end of treatment value were assessed using a Student's paired t test. ANCOVA was constructed to test for effects of treatment. Groups were compared using an ANCOVA model including baseline values as covariates. Medically meaningful predefined covariates were not included in the model if they were found to be homogeneous at baseline. Because of differences in the number of patients between dose groups and between centers, the changes are expressed as least-squares means (LSMs) and were statistically analyzed with baseline as a covariate. P values were adjusted for multiplicity using Dunnett's procedure. Missing observations were handled by the last observation carried forward method.
RESULTS
Of 1,645 patients screened, 549 patients fulfilled the entry criteria and were randomly assigned: 134 to placebo, 138 to 10 mg/day ranirestat, 132 to 20 mg/day ranirestat, and 145 to 40 mg/day ranirestat. Two patients assigned to 10 mg/day ranirestat were not included in safety and efficacy evaluation because they did not take any study medication. The patient demographic data are shown in Table 1. No significant differences were observed among the treatment groups for sex, age, BMI, type of diabetes, duration of diabetes, duration of DSP, or A1C. For all patients, the mean baseline BMI was 33.1 kg/m2, and most patients (83%) had type 2 diabetes for 14.5 years and DSP for 4.9 years. Their baseline A1C was 8.3%. NCS and mTCNS data at screening are also shown in Table 1. The mean value for each symptom in the mTCNS was ∼1 and was somewhat higher for each sensory test, introducing a floor effect.
Table 1.
Patient baseline demographic information
| Placebo | Ranirestat |
All patients | P | |||
|---|---|---|---|---|---|---|
| 10 mg/day | 20 mg/day | 40 mg/day | ||||
| n | 134 | 136 | 132 | 145 | 547 | |
| Male sex | 74 (55.2) | 94 (69.1) | 83 (62.9) | 91 (62.8) | 342 (62.5) | 0.134 |
| Age (years) | 56.1 ± 8.9 | 56.2 ± 8.5 | 55.7 ± 9.2 | 54.5 ± 9.5 | 55.6 ± 9.0 | 0.342 |
| BMI (kg/m2) | 32.9 ± 6.9 | 32.9 ± 6.9 | 32.9 ± 6.0 | 33.5 ± 7.5 | 33.1 ± 6.8 | 0.821 |
| Type 2 diabetes (%) | 111 (82.8) | 120 (88.2) | 104 (78.8) | 119 (82.1) | 454 (83.0) | 0.222 |
| Duration of diabetes (years) | 14.6 ± 9.0 | 13.4 ± 8.2 | 16.0 ± 10.7 | 14.1 ± 9.0 | 14.5 ± 9.3 | 0.136 |
| A1C (%) | 8.3 ± 1.3 | 8.1 ± 1.4 | 8.3 ± 1.4 | 8.4 ± 1.4 | 8.3 ± 1.4 | 0.219 |
| Duration DSP (years) | 4.6 ± 3.2 | 4.9 ± 3.8 | 5.3 ± 4.2 | 4.7 ± 4.0 | 4.9 ± 3.8 | 0.536 |
| Symptoms | 4.7 ± 3.7 | 5.1 ± 3.6 | 4.7 ± 3.3 | 5.2 ± 4.2 | ||
| Sensory tests | 5.6 ± 3.6 | 6.1 ± 3.2 | 5.5 ± 3.5 | 6.4 ± 3.9 | ||
| Right sural CV (m/s) | 42.5 ± 5.2 | 41.0 ± 5.1 | 42.0 ± 5.7 | 41.7 ± 4.8 | ||
| Peroneal CV (m/s) | 40.5 ± 5.2 | 39.7 ± 3.9 | 40.1 ± 4.8 | 39.5 ± 4.7 | ||
Data are n (%) for sex and type of diabetes and means ± SD for other parameters. P values for sex and type of diabetes were obtained from χ2 tests. P values for the other parameters were obtained from ANOVA. Symptoms and sensory tests are domains of the mTCNS. Symptoms range from 0 to 18 and sensory tests from 0 to 15. CV, conduction velocity.
Patient commitment was shown by the 85.7% who completed the entire 12-month study. The most common cause for discontinuation was voluntary withdrawal in 5.5%. Other reasons for not completing the study were adverse events in 1.8%, lack of compliance in 2.2%, lost to follow-up in 2.2%, failed to continue to meet entry criteria in 2.2%, and other in 0.4%. There was no difference in reasons for withdrawal across the different treatment groups. The average compliance per visit was greater than 94% in each treatment group.
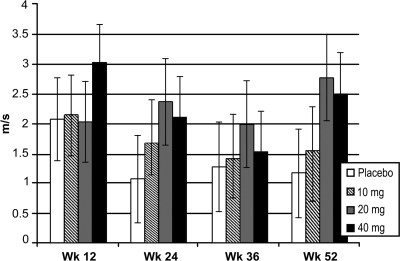
For the summed sensory NCV of the bilateral sural and proximal median sensory nerves, the observed mean ± SD changes from baseline to week 52 were 2.0 ± 7.98 m/s in the placebo group, 3.2 ± 7.98 m/s in the 10 mg/day group, 3.8 ± 8.45 m/s in the 20 mg/day group, and 3.6 ± 9.33 m/s in the 40 mg/day group. For the purposes of inferential statistical analysis by the LSM method, these changes are 1.17, 1.55, 2.55, and 2.50 m/s for placebo and 10, 20, and 40 mg/day ranirestat, respectively, at week 52. These differences did not achieve statistical significance between treatment groups. Figure 1 shows the LSM change from baseline for the summed sensory NCV.
Figure 1.
Changes from baseline in the summed sensory NCV. Summed sensory NCV includes bilateral sural and proximal median sensory nerves. Data shown are LSM ± SEM change from baseline for last observation carried forward. Adjusted P values: placebo vs. 10 mg (P = 0.962), 20 mg (P = 0.246), and 40 mg (P = 0.369) at week 52.
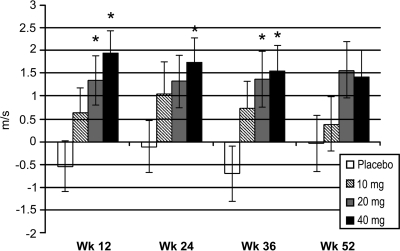
Figure 2 shows the LSM change from baseline in the summed motor NCV of the tibial, peroneal, and median nerves. A mean decrease (deterioration) in the summed motor NCV occurred at week 12 in the placebo group compared with mean increases (improvement) in all ranirestat groups. There were significant differences between 20 mg/day ranirestat and placebo (P = 0.028) and between 40 mg/day ranirestat and placebo (P = 0.002) at week 12, when the LSM changes from baseline were –0.54 m/s in the placebo group, 0.65 m/s in the 10 mg/day group, 1.35 m/s in the 20 mg/day group, and 1.94 m/s in the 40 mg/day group. These results remained consistent at subsequent visits.
Figure 2.
Changes from baseline in the summed motor NCV. The summed motor NCV includes the median, peroneal, and tibial nerves. Data shown are LSM ± SEM change from baseline for last observation carried forward. Adjusted P values: placebo vs. 10 mg (P = 0.247), 20 mg (P = 0.028), and 40 mg (P = 0.002) at week 12; placebo vs. 10 mg (P = 0.296), 20 mg (P = 0.152), and 40 mg (P = 0.036) at week 24; placebo vs. 10 mg (P = 0.188), 20 mg (P = 0.029), and 40 mg (P = 0.013) at week 36; placebo vs. 10 mg (P = 0.913), 20 mg (P = 0.123), and 40 mg (P = 0.162) at week 52.
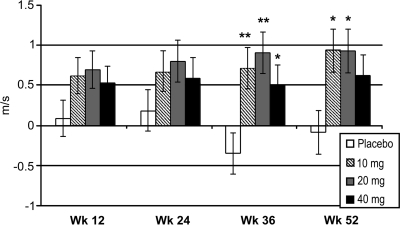
The peroneal motor NCV improved with ranirestat treatment significantly at weeks 36 (P < 0.05 to P < 0.01) and 52 (P = 0.014, 0.015, and 0.108 for 10, 20 and 40 mg/day ranirestat, respectively) with ranirestat treatment compared with a slight deterioration of the LSM 0.1 m/s at week 52 in the placebo group (Fig. 3). The F-wave latencies also increased (worsened) in the placebo group compared with decreased latencies (improvement) in the 20 and 40 mg/day ranirestat groups at most visits (data not shown).
Figure 3.
Changes from baseline in peroneal motor NCV. *P < 0.05; **P < 0.01. Data shown are LSM ± SEM change from baseline for last observation carried forward. Adjusted P values: placebo versus ranirestat all doses at week 36 (P ≤ 0.035); placebo vs. 10 mg (P = 0.014), 20 mg (P = 0.015), and 40 mg (P = 0.108) at week 52.
The mTCNS total, symptom, and sensory test scores tended to decrease (improve) in all treatment groups (including placebo), resulting in no statistically significant differences. A decrease in mTCNS (improvement) was observed in all groups as early as 12 weeks, and the decrease in mTCNS persisted (or decreased more) during the study in all groups to week 52 without significant differences between groups. The decrease in the mTCNS at week 52 was –2.75 points in the placebo group and varied from –2.4 to –2.75 in ranirestat groups (data not shown). Both VPT and CDT improved at every visit in all groups, but the differences did not achieve statistical significance between ranirestat and placebo.
Ranirestat plasma levels were proportional to dose, an indication of linear pharmacokinetics. The mean values were consistent over time, without evidence of ranirestat accumulation or autoinduction.
The incidence of adverse events was comparable in all groups and led to premature withdrawal of 2.2% patients in the placebo group, 1.5% patients in the 10 mg/day group, 2.3% patients in the 20 mg/day group, and 1.4% patients in the 40 mg/day group. Ranirestat had no relevant effects on clinical laboratory parameters including liver and renal function tests.
CONCLUSIONS
The results of this study did not show a significant difference between ranirestat and placebo treatment in the primary efficacy end point (change in summed sensory NCV) perhaps because of the unexpected placebo improvement. In the previous phase II study, the changes in the summed sensory NCV with placebo treatment at week 12 ranged from –0.21 to 0.20 m/s, whereas a change from baseline of 2.1 m/s was observed at 12 weeks in the placebo group in the current study (7). The explanation for the differences in behavior of the placebo groups between the two studies is unclear because the patient populations are similar with the same entry criteria and only a small difference in BMI between the two study populations was noted. A placebo response has been observed in other recent studies of DSP and may result from lifestyle modification by the patients as they enter a clinical trial (14,15). Lifestyle behavior (such as regular assessment of weight and BMI during the study) needs to be examined in much more detail in future studies than was done in the current study to determine whether this was the full explanation for the differences in placebo group behavior. A phase II proof-of-concept study completed in Japan demonstrated a significant improvement in the summed sensory NCV with ranirestat compared with placebo after 26 weeks of treatment although the placebo group in this study also improved (T.-H., personal communication).
The changes in motor and sensory nerve conduction parameters differed in this study, and there is no good explanation for this observation. Both summed and individual motor NCS parameters showed improvements with ranirestat treatment compared with deterioration in patients treated with placebo. The improved peroneal motor NCV with ranirestat treatment suggests clinical benefit because an abnormal peroneal motor NCV has been associated with the development of foot ulcers and mortality (16,17). One possible reason for the differences observed in sensory and motor NCV results in this study compared with those in the earlier phase II study is that sensory NCS are more technically challenging to perform than motor NCS because of the magnitude of the signals (sensory potential amplitudes are measured in microvolts compared with motor potential amplitudes measured in millivolts). As a result, factors such as limb edema may have more of an impact on sensory recordings than on motor recordings. The sural nerve is the most challenging (of the nerves tested in this study) to evaluate because of anatomic variations and the small size of the sural nerve response. As the number of study sites increased and the BMI of the patients increased from the phase II study, it is possible that these factors played a role in the results consistent with the observed higher standard deviations of the baseline parameters in this study. The use of a central core laboratory can minimize, but not eliminate, technical barriers in DSP studies; standardization of procedures across centers is also essential. The balance between including many centers to decrease the recruitment time for the study and including fewer centers with tightly controlled procedures needs to be kept in mind for future studies on DSP.
The mTCNS did not show a difference between the placebo and ranirestat treatment groups. In addition to an unexpected placebo effect perhaps due to lifestyle modification, another possible explanation for the lack of differences in the mTCNS response is the floor effect. Six symptoms are evaluated in the mTCNS, and the mean symptom score on entry was ∼5. This score means that each symptom was ∼1 at entry, a level that leaves very little room for a significant change with treatment; i.e., there is a floor effect. Similar comments apply to the sensory tests. Current criteria are set to capture patients at a stage of DSP that will respond to treatment. Abnormalities in NCS and/or QSTs are required for entry, but symptoms and signs are not essential, although the majority of patients have both when they enter these clinical trials. DSP is at a mild to moderate stage using these entry criteria as shown by sural nerve morphology from prior studies, so the study population desired has been captured, but it is likely that clinical changes cannot be expected after only 12 months of treatment and that longer treatment intervals are essential to demonstrate clinical effects (4,11). Alternatively, only patients with marked symptoms may be recruited, but then it is uncertain whether severity would be uniform, as symptoms are a poor guide to the underlying pathological changes in DSP. Determination of a drug effect on harder end points (such as frequency of foot ulceration) requires much larger study cohorts, patients with more advanced stages of DSP when foot ulceration is more likely to occur, and much longer durations of treatment such as 5-year studies.
The results of this study lend support to the importance of the polyol pathway in the pathophysiology of DSP and show that inhibition of the polyol pathway by the ARI ranirestat improves motor nerve function parameters. The placebo response and appropriate efficacy variables remain continuing challenges in ARI development, and important lessons have been learned from the current study. Ranirestat continues to hold promise for the treatment of patients with DSP.
Acknowledgments
V.B. is a paid consultant for Dainippon Pharmaceuticals, which provided funds to her laboratory to conduct studies on AS-3201. R.A.B. is a paid consultant for Dainippon Sumitomo Pharma. T.H. and S.T. are paid employees of Dainippon Sumitomo Pharma. No other potential conflicts of interest relevant to this article were reported.
We thank David Liang for assistance in preparation of the figures.
APPENDIX
Members of the Ranirestat Study Group are the following: R. Bedlack, Durham, NC; A. Belanger, Laval, QC, Canada; T. Brannagan, New York, NY; D. Brunet, Quebec, QC, Canada; P. Dandona, Buffalo, NY; J. Ervin, Kansas City, MO; R. Freeman, Boston, MA; I. Grant, Halifax, NS, Canada; P. Hollander, Dallas, TX; L. Klaff, Renton, WA; D. Lau, Calgary, AB, Canada; M. Liebowitz, New York, NY; J. Liljenquist, Idaho Falls, ID; D. Lorber, Flushing, NY; T. Lyons, Oklahoma City, OK; M. Mollen, Phoenix, AZ; P. Raskin, Dallas, TX; M. Rendell, Omaha, NE; S. Schwartz, San Antonio, TX; J. Selam, Tustin, CA; A. Shaibani, Houston, TX; A. Vinik, Norfolk, VA; R. Weinstein, Walnut Creek, CA; and J. Wymer, Albany, NY.
Footnotes
Clinical trial reg. no. NCT00101426, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein JM, Pach D, Wilson DM, O'Brien PC, Melton LJ, III. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817– 824 [DOI] [PubMed] [Google Scholar]
- 2. De Wytt C, Jackson RV, Hockings GI, Joyner JM, Strakosch CR. Polyneuropathy in Australian outpatients with type II diabetes mellitus. J Diabetes Complications 1999; 13: 74– 78 [DOI] [PubMed] [Google Scholar]
- 3. Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol 2002; 50: 325– 392 [DOI] [PubMed] [Google Scholar]
- 4. Greene DA, Arezzo JC, Brown MB. Zenarestat Study Group. Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Neurology 1999; 53: 580– 591 [DOI] [PubMed] [Google Scholar]
- 5. Pfeifer MA, Schumer MP. Clinical trials of diabetic neuropathy: past, present, and future. Diabetes 1995; 44: 1355– 1361 [DOI] [PubMed] [Google Scholar]
- 6. Pfeifer MA, Schumer MP, Gelber DA. Aldose reductase inhibitors: the end of an era or the need for different trial designs? Diabetes 1997; 46: S82– S89 [DOI] [PubMed] [Google Scholar]
- 7. Bril V, Buchanan RA. AS-3201 Study Group. Aldose reductase inhibition by AS-3201 in sural nerve from patients with diabetic sensorimotor polyneuropathy. Diabetes Care 2004; 27: 2369– 2375 [DOI] [PubMed] [Google Scholar]
- 8. Bril V, Buchanan RA. Ranirestat Study Group. Long-term effects of ranirestat (AS-3201) on peripheral nerve function in patients with diabetic sensorimotor polyneuropathy. Diabetes Care 2006; 29: 68– 72 [DOI] [PubMed] [Google Scholar]
- 9. Report and recommendations of the San Antonio Conference on Diabetic Neuropathy. Diabetes 1988; 37: 1000– 1004 [DOI] [PubMed] [Google Scholar]
- 10. Bril V, Ellison R, Ngo M, Bergstrom B, Raynard D, Gin H. Roche Neuropathy Study Group. Electrophysiological monitoring in clinical trials. Muscle Nerve 1998; 21: 1368– 1373 [DOI] [PubMed] [Google Scholar]
- 11. Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for Diabetic Polyneuropathy. Diabetes Care 2002; 25: 2048– 2052 [DOI] [PubMed] [Google Scholar]
- 12. Bril V, Tomioka S, Buchanan RA, Perkins BA. mTCNS Study Group. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 2009; 26: 240– 246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niwa T, Tohyama K, Kato Y. Analysis of polyols in uremic serum by liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry. J Chromatogr 1993; 613: 9– 14 [DOI] [PubMed] [Google Scholar]
- 14. Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ, 3rd, MBBQ Study Group. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C β-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther 2005; 27: 1164– 1180 [DOI] [PubMed] [Google Scholar]
- 15. Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care 2006; 29: 2365– 2370 [DOI] [PubMed] [Google Scholar]
- 16. Carrington AL, Shaw JE, Van Schie CHM, Abbott CA, Vileikyte L, Boulton AJM. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care 2002; 25: 2010– 2015 [DOI] [PubMed] [Google Scholar]
- 17. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ERE, Whalley AM, Widdows P, Williamson S, Boulton AJM. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002; 19: 377– 384 [DOI] [PubMed] [Google Scholar]