Abstract
Introduction
To address the mechanisms by which apoE polymorphism affects functional outcome after intracerebral hemorrhage in humans, we tested the hypothesis that the presence of the APOE4 allele results in amplified inflammatory responses and increased cerebral edema.
Methods
We prospectively enrolled and collected data on 21 adult patients consecutively admitted to Duke University Hospital with supratentorial intracerebral hematoma including hemorrhage volume, midline shift, modified Rankin Score, Glasgow Outcome Score, and APOE genotype. Hemorrhage size (cm3) and midline shift (mm), at the level of the thalamus, were measured by computed tomography within 36 hours of admission. Rankin and Glasgow Scores were determined at discharge. Student’s t-test was used to analyze hemorrhage size, midline shift, and Glasgow Outcome Score and logistical regression was used to measure allele affect on modified Rankin Score. When analyzing modified Rankin Score, patients were grouped by favorable outcome (0–2) or unfavorable (3–6).
Results
Out of 21 patients, 11 possessed at least one APOE4 allele (APOE4+). There was no difference in hemorrhage volume (25.8 v. 38.3 mm for APOE4− v. APOE4+, respectively) between the groups, but there was a significant difference in midline shift (p=0.04, 0.7 v. 4mm). Functional outcomes were worse for the patients possessing at least one APOE4 allele (p=0.04)
Conclusion
The presence of APOE4 is associated with poor functional outcomes in humans after intracerebral hemorrhage. Our data suggest that the mechanism for this may be increased cerebral edema and not larger hematoma volume.
INTRODUCTION
Intracerebral hemorrhage (ICH) is a devastating and relatively common disease affecting as many as 50,000 people annually in the United States alone. Unfortunately, despite advances in supportive care ICH remains associated with poor outcome, 30-day mortality approaches 40 to 50%. (1) Recent preclinical data suggests a number of inflammatory mechanisms that may play a crucial role in the development of cerebral edema and secondary neuronal injury.(2) Additionally, the importance of genetic influences on functional recovery remains an active area of investigation. (3)
In particular, apolipoprotein E polymorphism (APOE: gene; apoE protein) has been found to play an important role in modifying outcomes after ICH. The three common human isoforms of apoE, designated apoE2, apoE3, and apoE4, are encoded on chromosome 19, and differ by single cysteine to arginine interchanges at positions 112 and 158: E3(Cys112Arg158); E4(Arg112Arg158); E2(Arg112Arg158). (4) Presence of the APOE4 allele has been demonstrated to adversely effect outcomes in a number of acute brain injuries, including ICH. (5) Although a full understanding of the mechanism(s) by which apoE affects the CNS response to injury remains elusive, there is increasing evidence supporting a role for apoE in down-regulating endogenous brain inflammatory responses in an isoform-specific fashion. This is consistent with the known immunomodulatory properties of apoE, (6–8) as well as with recent observations demonstrating isoform-specific effects of apoE in modifying secretion of inflammatory mediators and brain edema following closed head injury. (9)
Neuroinflammatory responses play an important role following ICH, and the release of inflammatory cytokines increases blood brain barrier permeability, cerebral edema and secondary neuronal injury. There is mounting pre-clinical data that cerebral edema, as a result of this inflammatory response, is due to a number of cellular mechanisms that likely conclude as a final common pathway to neuronal injury. (2) Thus, the isoform-specific effect of apoE on neuroinflammation may be particularly relevant in modifying outcomes after ICH, and several clinical studies have implicated the presence of APOE4 with poor outcome in this setting. (10) (11) Therefore, to further define the clinical relevance and possible mechanisms of these genotype effects, we evaluated commonly accepted radiographic surrogates of cerebral edema to investigate whether endogenous APOE genotype modifies the CNS inflammatory response after ICH with subsequent alteration in functional neurological outcome.
METHODS
After Institutional Review Board approval and obtaining informed consent, consecutive adult patients with computed tomography (CT)-proven supratentorial ICH admitted to the Duke University Hospital Neuroscience Intensive Care Unit (NICU) were prospectively enrolled between January, 2005 and December, 2006. Exclusion criteria included patients under the age of 18 years, known pregnancy, admitting diagnosis of subarachnoid hemorrhage, infratentorial hemorrhage, and known traumatic etiology. Upon entry into the study blood samples were obtained for APOE genotyping, demographics were recorded, and Glasgow Coma Score (GCS) and ICH Score (12) were tabulated. During the first 36 hours after admission, CT scans were obtained and evaluated for hemorrhage volume (cm3), midline shift (MLS, mm), an accepted surrogate for cerebral edema, and presence of intraventricular hemorrhage (IVH); additionally the latency from ictus to the study scan was recorded. Upon discharge from the hospital, modified Rankin score (mRS), Glasgow Outcome Score (GOS), hospital length of stay (LOS), and NICU LOS were recorded by a single observer blinded to genotyping. At analysis, patients were grouped by the presence of an APOE4 allele (i.e., patients with an APOE4 allele [APOE4+] v. patients without an APOE4 allele [APOE4−].
Determination of Radiographic Outcomes
All patients enrolled into the study were diagnosed with CT-proven ICH. The imaging scan used for the study protocol was the next subsequent scan that occurred within 36 hours after admission. A blinded neuroradiologist assessed CT scans in the following manner: On the CT slice with the largest area of ICH, the largest diameter (A) of the hematoma was measured in centimeters. The dimension of the hemorrhage perpendicular to the largest diameter represented the second diameter (B) in centimeters. The height of the hematoma was calculated by multiplying the number of slices involved by the slice thickness, providing the third diameter (C). The three diameters were multiplied and then divided by two (A×B×C/2) to obtain the volume of ICH in cubic centimeters. (13) For the purpose of determination of (C) diameter, the first and last slices where hematoma is first and last noted were not counted. Additionally, intraventricular hemorrhage (IVH) was defined as an intraventricular hyperdense image not attributable to calcification or the choroid plexus. MLS of the septum pellucidum were measured in millimeters by a blinded neuroradiologist and corrected for magnification by using the scale provided on each CT image. MLS was calculated as the distance from the center of the anterior horns of the lateral ventricles on the CT slice containing the third ventricle to a perpendicular line connecting the anterior and posterior insertions of the falx cerebri. Because individual measurements were recorded in millimeters, only MLS of >2 mm were considered to be significant and recorded.
Human apoE Genotyping
A polymerase chain reaction (PCR)-based assay was used to amplify a short polymorphic region residing within coding sequences of the human APOE gene. Each amplification reaction was performed using 20–100 ng of genomic DNA, 1.0 pmol/ml of each primer, 10% dimethylsulfoxide, 1.5 mM MgCl2, 200 mM of each dNTP, 0.05 U/ml Taq DNA polymerase (Promega, Madison, WI) and supplied buffer in a final volume of 15 μl. The forward primer was 5′ TAAGCTTGGCACGGCTGTCCAAGGA 3′ and reverse primer was 5′ACAGAATTCGCCCCGGCCTGGTACACTGCCA 3′. An initial denaturation at 94°C for 5 minutes was followed by 35 cycles of annealing at 65°C for 0.5 minutes, extension at 70°C for 45 sec, denaturation at 94°C for 0.5 minutes, and a final extension at 70°C for 10 minutes. Following amplification a 5 μl mixture composed of 2–5 U of the restriction enzyme Hha I (Promega, Madison, WI), 2.5 μl of Hha I 10X buffer, and dH2O to a final volume of 5 μl was added directly to each well and the reaction incubated for 1–2 hours at 37°C. Resultant DNA fragments were resolved on a 6% nondenaturing polyacrylamide gel via electrophoresis for one hour under constant current (45mA). DNA fragments were stained with SYBRGreen (FMC Bioproducts, Rockland, ME) and visualized using a Storm PhosphorImager and Image Quant TM version 5.0 software (Amersham Biosciences, Piscataway, NJ.) Hha I cleaves the 244 bp PCR product to yield smaller fragments that allow recognition of characteristic patterns following gel electrophoresis. Hha I cuts the APOE3 PCR product to generate 91 bp, 48 bp and 35 bp fragments, and the APOE4 allele produces fragments of 72 bp, 48 bp and 35 bp in length.
Statistical analysis
Student’s t-test was used to analyze patient demographics, hemorrhage volume, MLS, and GOS. Logistical regression was used to measure allele and extent of MLS affect on mRS. When evaluating the effect of APOE polymorphism and extent of MLS on mRS, patient outcomes were dichotomized to (mRS 0–2) or unfavorable outcome (mRS 3–6). Statistical significance was assumed with p < 0.05. All values are expressed as mean ± S.E.M.
RESULTS
To establish the effect of APOE polymorphism on neuroinflammatory responses following ICH, 21 consecutive patients with supratentorial ICH were enrolled and genotyped. Of these 21 patients, 11 possessed at least one APOE4 allele (APOE4+); the other 10 patients did not (APOE4−). Baseline demographic variables, including age, sex, race, body mass index, Glasgow Coma Score (GCS), ICH Score, (12) and presence of IVH were similar between groups (Table 1). Initial CT scans that were performed on presentation revealed similar hemorrhage volumes (APOE4−, 24.13 ± 11.5 v. APOE4+, 32.5 ± 13.3 cm3) and MLS (APOE4−, 0.5 ± 0.33 v. APOE4+, 0.8 ± 0.37 mm). This initial imaging data was associated with a similar latency from symptom onset (APOE4−, 8.38 ± 9.0 v. APOE4+, 8.63 ± 9.0 hrs). Follow-up imaging was performed at similar times in APOE4 and APOE4- groups (APOE4−, 21.2 ± 3.1 v. APOE4+, 18.4 ± 4.6 hrs), and also revealed no primary effect of APOE genotyope on hemorrhage size (APOE4−, 25.7 ± 11.4 v. APOE4+, 38.3 ± 12.7 cm3). Finally, NICU (6 ± 0.5 v. 6 ± 0.4 days) and hospital (14 ± 0.7 v. 14 ± 0.5 days) LOS were similar between the two groups.
Table 1.
Demographics for APOE4− and APOE4+ patients presenting with CT-proven supratentorial ICH
| APOE 4− | APOE 4+ | p | |
|---|---|---|---|
| N | 11 | 12 | NS |
| Age (yrs) | 62.3 ± 4.7 | 59.9±3.7 | NS |
| Sex | 45.4% Male | 50% Male | NS |
| Race | 36.4% Caucasion | 50% Caucasion | NS |
| BMI | 27.7 ± 0.9 | 26.4 ± 1.4 | NS |
| Latency from Ictus to Admit CT (hrs) | 8.38 ± 9.0 | 8.63 ± 9.0 | NS |
| Latency from Ictus to Follow-up CT (hrs) | 21.2 ± 3.1 | 18.4 ± 4.6 | NS |
| Admit GCS | 11 ± 1.4 | 10 ± 1.4 | NS |
| Admit ICH Volume (ml) | 24.13 ± 11.5 | 32.5 ± 13.3 | NS |
| ICH Volume by Follow-up CT (ml) | 27 ± 11.4 | 38 ± 12.8 | NS |
| ICH Score | 1.6 ± 0.5 | 1.45 ± 0.3 | NS |
| Presence of IVH | 6(55%) | 5(42%) | NS |
| MLS on Admit (mm) | 0.5 ± 0.33 | 0.8 ± 0.37 | NS |
| MLS by Follow-up CT (mm) | 0.7 ± 0.3 | 4 ± 1.4 | 0.04 |
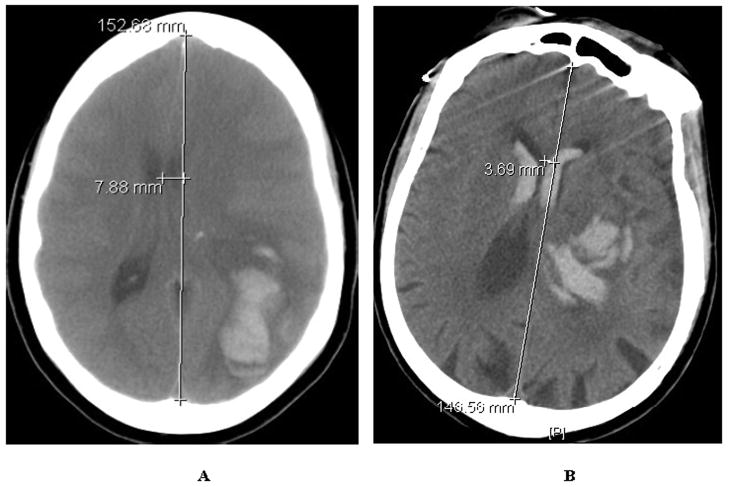
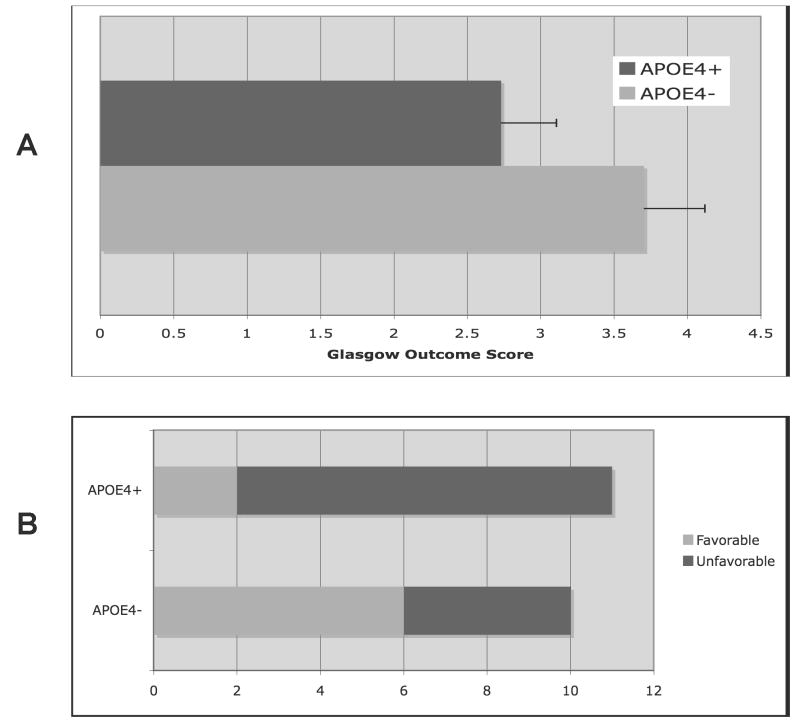
Despite the fact that the APOE4+ and APOE4− groups were similar with respect both to hematoma volume upon presentation and within the first 36 hours after injury, and MLS upon presentation, the APOE4+ patients exhibited a greater degree of MLS on follow-up imaging (APOE4−, 0.7 ± 0.37 v. APOE4+, 4.0 ± 1.41 mm; p < 0.05; Figure 1A & B) performed during the first 36 hours after hemorrhage. Consistent with previous reports, presence of the APOE4 allele was also associated with worse functional outcomes in the APOE4+ patients as assessed by GOS (3.7 ± 0.4 v. 2.7 ± 0.4, p < 0.05;) and mRS (p < 0.05, r2 = 0.19) at the time of discharge from the hospital (Figure 2A & B). Additionally, there was a significant correlation between MLS, an accepted surrogate marker for cerebral edema, and functional outcome by dichotomized mRS (p < 0.05, r2 = 0.26). Although this small population of patients was not powered to assess the effect of gene dose on outcome, the two patients who were homozygous for APOE4 had unfavorable outcomes (death and transfer to skilled nursing facility). These data indicate that, in accordance with previously published work, APOE genotype influences neurological outcome after supratentorial ICH and appears to do so through its effects on the generation of secondary cerebral edema. (5)
Figure 1.
Figure 1A & B. Representative CT scan of APOE4+ (A) vs. APOE4− (B) patients with measured midline shift at the level of the thalamus less than 36 hours after initial hematoma formation.
Figure 2.
Figure 2A & B. Functional neurological outcomes at hospital discharge for APOE4− v. APOE4+ after supratentorial ICH as measured by Glasgow Outcome Score (GOS) (A) and dichotomized modified Rankin Score (mRS; favorable mRS < 3 vs. unfavorable mRS ≥ 3) (B).
DISCUSSION
Although originally described in the context of cholesterol metabolism, clinical observations suggest that APOE polymorphism plays a uniquely important role in modifying the CNS response to acute and chronic injury. (14) In particular, presence of the APOE4 allele has been associated with poor neurological outcome after ICH (10, 11) and traumatic brain injury. (15, 16) Although the mechanism(s) by which apoE affects the CNS response to injury remains poorly defined, one unifying hypothesis is that APOE gene products modify the neuroinflammatory responses in an isoform-specific fashion by modulating microglial activation. (17) This function of apoE in the generation of inflammation in the brain has been well described in a number of in vitro and preclinical models, (18–20) and has recently been translated into the clinical setting, where APOE4 is associated with more robust inflammatory responses following cardiopulmonary bypass (21, 22) and in the critically ill population. (23, 24) Our data suggest that the enhanced neuroinflammatory response associated with APOE4 may be equally important in the setting of supratentorial ICH.
In particular, the isoform-specific effects of APOE gene products on modifying neuroinflammatory responses may be uniquely relevant in the setting of ICH, where inflammatory cascades contribute to BBB breakdown, the development of cerebral edema, and secondary neuronal injury resulting in delayed mortality and sustained neurological impairment. In the current study, we demonstrate that APOE4 is associated with enhanced neuroinflammatory responses and impaired functional outcomes independent of hematoma volume in. Therefore, one mechanism by which apoE may exert its isoform-specific effects is by altering neuroinflammatory responses, possibly by microglial modulation. (17, 25) Although this phenomenon has never been studied systematically in the setting of ICH, it is discrepant with a small retrospective study suggesting the lack of effect of APOE polymorphism on cerebral edema after ICH. (26) Though our findings are to some extent in contradiction to this finding, we note that, in contrast to the previous study, our data are prospective in nature and evaluate all patients presenting with supratentorial ICH. Although it is difficult to draw causative relationships from a retrospective study, McCarron et al. (26) found in their analysis of patients with ICH that the particular subset of nonsurviving subjects developed hemorrhages of such significant size that cerebral edema generation was limited, presumably by restrictions due to space and pressure in the intracranial vault. Therefore, we believe, as has been demonstrated in other forms of brain injury, apoE polymorphism appears to exhibit a neuroprotective effect after ICH due to amelioration of secondary inflammation as evidenced by decreasing cerebral edema.
Of note, allele frequency of APOE4 was nearly 50% in our study population, which is substantially higher than is seen in the general population. (27) This is not wholly unexpected, as independent from the allele affect on functional outcome, both APOE4 and APOE2 may predispose patients to the development of ICH. (28) In particular, APOE4 is associated with hemorrhage due to amyloid angiopathy. (29) This is a potential confounding factor, as amyloid associated hemorrhages tend to be larger, more superficial, and occur in an older patient population. However, we believe this is unlikely to have played a significant role in the current study, given the fact that the location of the hemorrhages was comparable (cortical vs. subcortical), as were patient ages, regardless of APOE4 allele presence.
There are several limitations in the current study that should be addressed. Although strongly suggestive of an APOE effect on inflammation and outcome after ICH, the current study was too small to assess the effect of all the different apoE isoforms. The small number of patients also made it impossible to assess whether there was an effect of gene dose on outcomes, although both patients who were homozygous for APOE4 experienced adverse outcomes. Clearly, a larger follow-up study would be necessary to confirm our findings. Moreover, we were unable to directly assess glial activation and neuroinflammation in this clinical study, and thus MLS was used as a radiographic surrogate of cerebral edema. We chose to use the surrogate measurement of MLS as it is a fairly concrete calculation and has been shown to be stable across interpreters. (30) In contrast, volumetric measurement of cerebral edema by CT can be inconsistent and unreliable after ICH. (31) In agreement with prior studies, (32, 33) MLS correlated with poor functional outcome, which in our cohort consisted of mRS and GOS at time of hospital discharge. Additionally, it appears that APOE associated differences in MLS are unrelated to hemorrhage volume as imaging upon presentation revealed similar MLS and lesion size despite similar latencies to imaging. Our findings in the current study are also consistent with prior preclinical work demonstrating the effect of endogenous apoE in modifying neuroinflammatory responses following traumatic brain injury (18, 34) and systemic inflammation. (19) Finally, the current study lacks long-term outcome measures. We chose a dichotomized endpoint of favorable or unfavorable outcome based on disposition (mRS) and GOS at time of discharge as this was an unambiguous and clearly defined outcome; however we are not able to address functional outcome following discharge from the hospital when it is logical to assume that patients will experience some level of rehabilitation. However, the degree of rehabilitation should be greatest in those patients with moderate to high levels of neurological function at the time of discharge from the hospital; therefore, it seems reasonable that long-term outcomes would follow short-term outcomes. Again, a larger trial would be necessary to definitively answer this question.
In summary, we find that in the first 36 hours following supratentorial ICH, the presence of the APOE4 allele is associated with increased cerebral edema, as assessed by the radiographic surrogate of MLS and, consistent with prior observations, with poor functional outcome. Additionally, these two outcomes measures appear to correlate with each other, irrespective of hemorrhage size. Therefore, our observations are in harmony with a growing body of data suggesting that apoE modifies neuroinflammatory responses in an isoform-specific fashion.
Acknowledgments
This study was made possible through funding by the Institute for the Study of Aging. Additionally, we would like to recognize the invaluable contributions to patient care made by the nurses and staff in the Neuroscience Intensive Care Unit at Duke University Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 2.James ML, Warner DS, Laskowitz DT. Preclinical Models of Intracerebral Hemorrhage: A Translational Perspective. Neurocrit Care. 2007 doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM. Genetics of primary intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2002;11:265–271. doi: 10.1053/jscd.2002.129615. [DOI] [PubMed] [Google Scholar]
- 4.Rall SC, Jr, Weisgraber KH, Mahley RW. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982;257:4171–4178. [PubMed] [Google Scholar]
- 5.Laskowitz DT, Vitek MP. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–969. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- 6.Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 7.Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 8.Colton CA, Brown CM, Czapiga M, Vitek MP. Apolipoprotein-E allele-specific regulation of nitric oxide production. Ann N Y Acad Sci. 2002;962:212–225. doi: 10.1111/j.1749-6632.2002.tb04070.x. [DOI] [PubMed] [Google Scholar]
- 9.Brown CM, Wright E, Colton CA, Sullivan PM, Laskowitz DT, Vitek MP. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med. 2002;32:1071–1075. doi: 10.1016/s0891-5849(02)00803-1. [DOI] [PubMed] [Google Scholar]
- 10.Alberts MJ, Graffagnino C, McClenny C, et al. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- 11.McCarron MO, Weir CJ, Muir KW, et al. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta Neurol Scand. 2003;107:106–109. doi: 10.1034/j.1600-0404.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- 12.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 13.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 14.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18:465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 16.Friedman G, Froom P, Sazbon L, et al. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–248. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- 17.Laskowitz DT, Thekdi AD, Thekdi SD, et al. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 18.Lynch JR, Pineda JA, Morgan D, et al. Apolipoprotein E affects the central nervous system response to injury and the development of cerebral edema. Ann Neurol. 2002;51:113–117. doi: 10.1002/ana.10098. [DOI] [PubMed] [Google Scholar]
- 19.Lynch JR, Tang W, Wang H, et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 20.Colton CA, Needham LK, Brown C, et al. APOE genotype-specific differences in human and mouse macrophage nitric oxide production. J Neuroimmunol. 2004;147:62–67. doi: 10.1016/j.jneuroim.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Grocott HP, Newman MF, El-Moalem H, Bainbridge D, Butler A, Laskowitz DT. Apolipoprotein E genotype differentially influences the proinflammatory and anti-inflammatory response to cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;122:622–623. doi: 10.1067/mtc.2001.115152. [DOI] [PubMed] [Google Scholar]
- 22.Drabe N, Zund G, Grunenfelder J, et al. Genetic predisposition in patients undergoing cardiopulmonary bypass surgery is associated with an increase of inflammatory cytokines. Eur J Cardiothorac Surg. 2001;20:609–613. doi: 10.1016/s1010-7940(01)00842-9. [DOI] [PubMed] [Google Scholar]
- 23.Moretti EW, Morris RW, Podgoreanu M, et al. APOE polymorphism is associated with risk of severe sepsis in surgical patients. Crit Care Med. 2005;33:2521–2526. doi: 10.1097/01.ccm.0000186368.96146.fb. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Girard TD, Shintani AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- 25.Laskowitz DT, Matthew WD, Bennett ER, et al. Endogenous apolipoprotein E suppresses LPS-stimulated microglial nitric oxide production. Neuroreport. 1998;9:615–618. doi: 10.1097/00001756-199803090-00010. [DOI] [PubMed] [Google Scholar]
- 26.McCarron MO, Hoffmann KL, DeLong DM, Gray L, Saunders AM, Alberts MJ. Intracerebral hemorrhage outcome: apolipoprotein E genotype, hematoma, and edema volumes. Neurology. 1999;53:2176–2179. doi: 10.1212/wnl.53.9.2176. [DOI] [PubMed] [Google Scholar]
- 27.Sudlow C, Martinez Gonzalez NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17, 965 controls. Stroke. 2006;37:364–370. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzourio C, Arima H, Harrap S, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008;70:1322–1328. doi: 10.1212/01.wnl.0000308819.43401.87. [DOI] [PubMed] [Google Scholar]
- 29.McCarron MO, Nicoll JA. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann N Y Acad Sci. 2000;903:176–179. doi: 10.1111/j.1749-6632.2000.tb06366.x. [DOI] [PubMed] [Google Scholar]
- 30.Bhattathiri PS, Gregson B, Prasad KS, et al. Reliability assessment of computerized tomography scanning measurements in intracerebral hematoma. Neurosurg Focus. 2003;15:E6. doi: 10.3171/foc.2003.15.4.6. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman RD, Maldjian JA, Brun NC, Horvath B, Skolnick BE. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by CT scan. AJNR Am J Neuroradiol. 2006;27:666–670. [PMC free article] [PubMed] [Google Scholar]
- 32.Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76:1534–1538. doi: 10.1136/jnnp.2004.055145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallevy C, Ifergane G, Kordysh E, Herishanu Y. Spontaneous supratentorial intracerebral hemorrhage. Criteria for short-term functional outcome prediction. J Neurol. 2002;249:1704–1709. doi: 10.1007/s00415-002-0911-1. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Durham L, Dawson H, et al. An apolipoprotein E-based therapeutic improves outcome and reduces Alzheimer’s disease pathology following closed head injury: evidence of pharmacogenomic interaction. Neuroscience. 2007;144:1324–1333. doi: 10.1016/j.neuroscience.2006.11.017. [DOI] [PubMed] [Google Scholar]