Abstract
Estrogens exert many of their behavioral effects by binding to nuclear estrogen receptor (ER) proteins, ERα and ERβ. Recent studies involving ER knockout mice and selective ER agonists suggest that estradiol’s anorexigenic effect is mediated via activation of ERα. To investigate this hypothesis, we examined whether the presumptive ERα antagonist, MPP, could block estradiol’s anorexigenic effect. In the first series of experiments, the effects of MPP on food intake and uterine weight were monitored in ovariectomized (OVX) rats treated with either a physiological dose of estradiol benzoate (EB) or a selective ERα agonist (PPT). In the final experiment, food intake was monitored following acute administration of MPP in ovarian-intact (cycling) female rats. Contrary to our hypothesis, MPP failed to attenuate either EB’s or PPT’s ability to decrease food intake and increase uterine weight in OVX rats. However, in ovarian-intact rats, a similar regimen of MPP treatment attenuated the phasic decrease in food intake that is associated with estrus. We conclude that MPP may be a useful tool to investigate the behavioral actions of endogenous estradiol, but may have limited utility in studying the behavioral effects of exogenous estradiol in OVX rats.
Keywords: SERM, Estradiol, Estrogen Receptor, MPP, PPT
INTRODUCTION
The anorexigenic action of estradiol is well characterized in the female rat. First, the pre-ovulatory increase in estradiol secretion promotes a phasic decrease in meal size that is apparent during estrus [1–4]. Second, ovariectomy promotes a sustained increase in meal size and weight gain [1] that can be prevented by treating ovariectomized (OVX) rats with a physiological regimen of estradiol treatment [5]. Progesterone treatment alone is not sufficient to normalize food intake and weight gain in OVX rats, and progesterone fails to alter the anorexigenic effect of estradiol [6]. Thus, of the ovarian hormones, only estradiol plays a crucial role in controlling food intake in the female rat. Acting as an indirect control of meal size, estradiol appears to decrease food intake by increasing the effects of anorexigenic compounds (e.g., cholecystokinin and serotonin) that signal meal termination and by decreasing the effects of orexigenic compounds (e.g., melanin-concentrating hormone, neuropeptides Y, and ghrelin) that sustain a meal [6–11].
As a steroid hormone, estradiol exerts many of its actions by coupling with nuclear estrogen receptor (ER) proteins, ERα and ERβ [12]. Studies involving ER knockout (ERKO) mice suggest that the estrogenic inhibition of food intake is mediated via ERα. The first indirect support for this hypothesis was a report that αERKO mice display age-related increases in body adiposity, relative to wild-type litter mates [13]. Although feeding was not examined in this study, it is likely that an increase in food intake contributed to the increased adiposity of αERKO mice. In a subsequent study, increased body adiposity was reported in αERKO and α/βERKO mice, but not in βERKO mice [14], suggesting minimal involvement of ERβ in the regulation of body weight and, presumably, control of food intake. Geary et al [15] provided the first direct evidence that activation of ERα appears necessary for the estrogenic inhibition of food intake. They reported that estradiol treatment failed to reduce food intake, weight gain, and body adiposity in ovariectomized (OVX) αERKO mice [15]. However, another group reported that estradiol treatment was sufficient to normalize the increases in food intake and body adiposity in ovariectomized αERKO mice [16], suggesting some involvement of ERβ.
In addition to ER knockout studies, which can be difficult to interpret due to possible developmental compensations of mice with deletions of ERα and/or ERβ, two recent studies involving the use of selective ERα/β agonists provide further support for the role of ERα in mediating the estrogenic inhibition of food intake. Chronic treatment with the ERα agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol, PPT, decreased 14-day food intake in OVX rats, whereas treatment with the ERβ agonist 2,3-bis(4-hyroxyphenyl)-propionitrile, DPN, had no effect [17]. These same ERα and ERβ agonists have also been administered via an acute injection schedule that more closely mimics the activation of ERs in cycling rats. In this study, OVX rats displayed a dose-dependent decrease in 24-h food intake following acute activation of ERα via PPT, but failed to display any alterations in 24-h food intake following acute activation of ERβ via DPN [18].
Contrary to the predominantly convergent evidence from ERKO mice and pharmacological studies, which supports a role for ERα in mediating estradiol’s anorexigenic effect, there is a single report that ventricular infusion of antisense oligodeoxynucleotides targeting ERβ, but not ERα, blocked estradiol’s anorexigenic effect in OVX rats [19]. Although this study provides some support for the role of ERβ in mediating estradiol’s anorexigenic effect, the majority of evidence suggests that activation of ERα is both sufficient and necessary for the anorexigenic effect of exogenous estradiol in OVX rats. It remains unclear, however, whether activation of ERα plays a physiological role in the control of food intake in cycling rats.
To address this question we investigated whether the presumptive ERα antagonist, methyl-piperidino-pyrazole (MPP), could attenuate the anorexigenic effects of either exogenous or endogenous estradiol. MPP is a non-steroidal, pyrazole compound that contains a basic side-chain addition that is reported to convert the pyrazole from an ERα agonist to an ERα antagonist [20]. Co-transfection and in vitro binding assays demonstrate that MPP displays a greater than 200-fold preferential binding affinity for ERα over ERβ [21]. Moreover, additional in vitro studies have shown that MPP functions as an ERα-selective antagonist on estrogen-regulated genes. For example, MPP was shown to downregulate interleukin-6 promotor activity mediated by ERα [22]. Although classified as a silent ER antagonist, two in vivo studies suggest that MPP may exert some estrogenic activity. In one study, acute administration of 50 μg MPP alone increased uterine weight [23]. This physiological change is a well characterized action of estradiol that appears to be mediated exclusively by activation of ERα [24]. In a second study, MPP augmented estradiol benzoate’s (EB’s) ability to increase the expression of multiple cancer-associated genes [25]. While MPP appeared to be a true ERα antagonist in cell-based, in vitro assays, emerging evidence suggests that it may more accurately resemble a selective estrogen receptor modulator (SERM) with mixed agonist/antagonist properties at ERα. In this regard, it is interesting that MPP was derived via the addition of a basic side chain to an ERα agonist of modest potency, methyl-pyrazole triol (MPT). This raises the possibility that, under certain conditions, metabolic cleavage of the basic side chain of MPP could result in MPT, which is known to have modest estrogenic activity [20]. Additional studies are required to determine the extent to which MPP may exert mixed estrogenic/antiestrogenic actions.
To the best of our knowledge, no studies have examined MPP’s ability to attenuate the behavioral (e.g., feeding) effects of estradiol. This, together with studies suggesting that MPP may function as a SERM rather than a silent ER antagonist, prompted us to examine MPP’s effect on food intake when administered both alone and in combination with either 17β-estradiol benzoate (EB) or the selective ERα agonist PPT in OVX rats. We then examined the effects of MPP on food intake in cycling rats. Our studies tested the hypothesis that MPP would attenuate the anorexigenic effects of both exogenous and endogenous estradiol in female rats.
METHODS
Animals and surgery
Female Long-Evans rats (Charles River Breeding Laboratory, Raleigh, NC), weighing 225–250 g at the onset of each experiment, were housed individually in Plexiglas, shoebox cages. Rats were given free access to chow (Purina 5001) and tap water. The testing rooms were maintained at 20 ± 2°C under a reverse 12:12-h light-dark cycle (dark onset = 1400 h). Animal usage and all procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
In Experiments 1–3, rats were anesthetized by intraperitoneal (i.p.) injections of a mixture of ketamine (50 mg/kg; Ketaset, Fort Dodge Animal Health, IA) and xylazine (4.5 mg/ml; Rompun, Mobay, Shawnee, KS) and then bilaterally OVX using an intra-abdominal approach. Following surgery, each rat received an i.p. injection of butorphanol (0.5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and a subcutaneous (s.c.) injection of gentamicin (10 mg/ml; Pro Labs Ltd, St. Joseph, MO) to minimize post-surgical pain and the risk of infection, respectively. After 1-week of postoperative recovery, rats were transferred to custom-designed cages equipped with feeding niches that provided access to spill-resistant food cups containing powdered chow. Each day at 0900 h, the rats’ body weights were recorded and food cups were weighed (± 0.1 g). Behavioral testing did not commence until stable levels of daily food intake were observed.
Experiment 1: Does acute administration of MPP alter food intake?
Because MPP has been reported to exert both estrogenic and antiestrogenic effects [22;23;25], it is possible that MPP administration alone might alter food intake in OVX rats. To investigate this possibility, 8 OVX rats received acute, s.c. injections (0.1 ml) of MPP in dimethyl sulfoxide (DMSO) vehicle (Sigma, St. Louis, MO) according to a within-subject design. The following doses of MPP were administered in a randomized order at 4-day intervals: 0, 5, 10, 25, 50 and 100 μg MPP (Tocris, Ellisville, MO). This regimen of MPP treatment (daily injection every fourth day) was chosen to coincide with an estradiol replacement protocol used in OVX rats that reinstates the cyclic pattern of food intake observed in cycling rats [5]. On test days, MPP/vehicle injections were administered at 1000 h and food intake was monitored during the 24-h period following drug treatment. Feeding data were collected during this interval based on a previous study in which a similar regimen of PPT (a selective ERα agonist) treatment reduced food intake for 24 h following its administration [18]. The range of doses was chosen based on a report that 50 and 100 μg of MPP exerted an estrogenic effect on uterine horn tissue [23].
Experiment 2A: Does MPP attenuate EB’s anorexigenic effect?
If EB’s anorexigenic effect is mediated solely via activation of ERα, as prevailing evidence suggests [17;18], then antagonism of ERα should attenuate the anorexigenic effect of EB. To investigate this hypothesis, one group of OVX rats (n = 8) received s.c. injections of the following drug treatments at 4 day intervals: DMSO immediately followed by DMSO, 1 μg EB (Sigma) followed by DMSO, 1 μg EB followed by 10 μg MPP, or 1 μg EB followed by 25 μg MPP. Food intake and body weight were monitored during the second 24-h period following drug treatment (i.e., at the time in which this regimen of EB treatment reduces food intake in OVX rats [5]). The doses of MPP were chosen because they failed to decrease food intake in Experiment 1.
Experiment 2B: Does MPP attenuate PPT’s anorexigenic effect?
To further test the pharmacological specificity of MPP, we examined its ability to attenuate the anorexigenic effect of the selective ERα agonist, PPT. Using a similar design as in Experiment 2A, the combined effects of PPT and MPP on food intake were examined in a group of OVX rats (n = 8) that received s.c. injections of the following drug treatments every fourth day: DMSO immediately followed by DMSO, 75 μg PPT (Tocris, Ellisville, MO) followed by DMSO, 75 μg PPT followed by 10 μg MPP, and 75 μg PPT followed by 25 μg MPP. Food intake and body weight were monitored during the first 24-h period following drug treatment (i.e., at a time in which this dose and regimen of PPT treatment reduces food intake and body weight in OVX rats [18].
Experiment 3: Does MPP attenuate EB or PPT’s ability to increase uterine weight?
As an additional (non-behavioral) test of the pharmacological specificity of MPP, we examined whether MPP would attenuate either EB or PPT’s ability to increase uterine weight. Using a between-subject design, OVX rats received s.c. injections of one of the following treatments, DMSO + DMSO, DMSO + 2 μg EB, DMSO + 25 μg MPP or 2 μg EB + 25 μg MPP (n = 7 per group). The rats’ uterine horns were removed bilaterally 48 h following drug treatment (i.e., at the time in which EB’s proliferative effect on uterine tissue is maximal). Two additional groups of OVX rats received s.c. injections of either DMSO + 75μg PPT (n = 6) or 25 μg MPP + 75 μg PPT (n = 7). The rats’ uterine horns were removed bilaterally 24 h following drug treatment (i.e., at a time in which PPT’s anorexigenic effect is maximal [18]). Following their removal, uterine horns were trimmed to 10 mm, blotted, and weighed (± 1 mg).
Experiment 4: Does MPP attenuate the estrous-related decrease in food intake in cycling rats?
Stage of the estrous cycle (diestrus 1, diestrus 2, proestrus, or estrus) was determined by examining the appearance and abundance of cells within vaginal cytology samples as described previously [2,26]. Cycle stage labels were assigned to the 24-h period ending at the time of sampling. Using this strategy, proestrus included the light-phase peak in estradiol and luteinizing hormone secretion, and estrus included the subsequent dark phase when female rats ovulate and display proceptive behaviors [2,26]. Data collection commenced when stable levels of food intake were observed and all rats had displayed a minimum of 2 consecutive 4-day estrous cycles. Using a within-subject, counterbalanced design, cycling female rats (n = 7) received acute s.c. injections of either DMSO or 25 μg MPP immediately after the dark phase of diestrus 2 and immediately prior to the dark phase of proestrus. These two time points were chosen because they correspond to the rise and subsequent peak in estradiol secretion in cycling rats [26]. Daily food intake was monitored for 2 consecutive estrous cycles.
Data analyses
The effects of drug treatment on food intake and body weight were analyzed via repeated-measures ANOVAs in Experiments 1 and 2. The effects of drug treatment on uterine weight (Experiment 3) were assessed via a one-way ANOVA. A two-factor, repeated-measures ANOVA (cycle stage × drug treatment) was used to assess the effect of MPP treatment on food intake in cycling rats (Experiment 4). A paired t-test was used to assess the magnitude of the estrous-related decrease in food intake between drug treatments. Tukey’s HSD multiple-comparison, post-hoc tests were used to assess group differences following significant (P < 0.05) ANOVA effects.
RESULTS
Experiment 1: Does acute administration of MPP alter food intake?
Food intake was influenced by acute administration of MPP, F(5,35) = 7.5, P < 0.05 (Fig. 1). A single injection of either 50 or 100 μg MPP decreased food intake relative to that observed in DMSO-treated OVX rats, Ps < 0.05. Food intake was not altered by smaller doses (5 – 25 μg) of MPP.
Fig. 1.
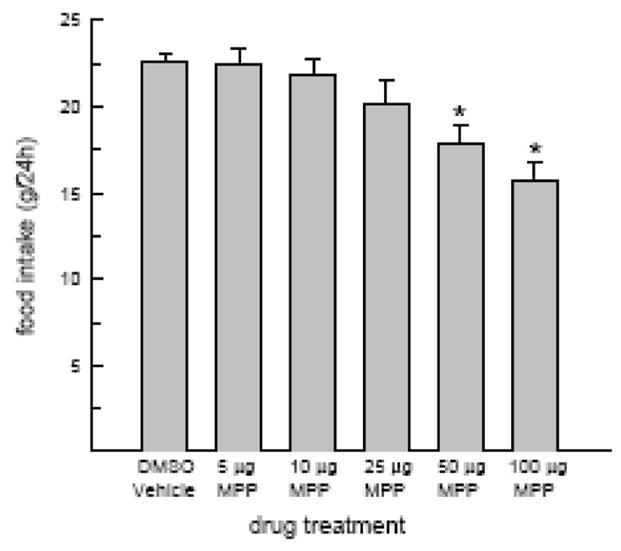
MPP treatment alone can reduce food intake in OVX rats. Food intake was monitored following acute injection of DMSO vehicle and multiple doses of MPP. The 50 and 100 μg doses of MPP decreased food intake in OVX rats. *Less than DMSO.
Experiment 2A: Does MPP attenuate EB’s anorexigenic effect?
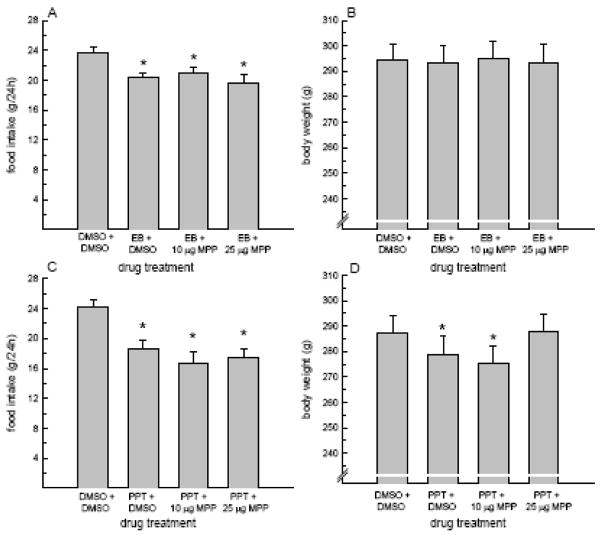
Food intake was influenced by our regimen of combined EB and MPP treatment, F(3,21) = 7.7, P < 0.05 (Fig. 2A). As expected, acute administration of EB decreased food intake, relative to that observed following DMSO treatment, P < 0.05. However, MPP failed to attenuate the anorexigenic effect of EB. Co-administration of EB and MPP produced similar decreases in food intake as that observed following co-administration of EB and DMSO (Ps < 0.05).
Fig. 2.
MPP failed to attenuate the anorexigenic effects of either EB or PPT but influenced weight loss following PPT, but not EB, treatment. (A) EB treatment decreased food intake in OVX rats. The anorexigenic effect of EB was not attenuated by either dose of MPP. (B) EB treatment alone or in combination with MPP had no effect on body weight in OVX rats. (C) PPT treatment decreased food intake in OVX rats. The anorexigenic effect of PPT was not attenuated by either dose of MPP. (D) PPT treatment decreased body weight in OVX rats. The larger (25 μg) dose of MPP abolished PPT-induced weight loss. *Less than DMSO.
Because the half-life of MPP is unknown, it is possible that MPP may have degraded more quickly than EB. This, in turn, could account for MPP’s inability to attenuate the anorexigenic effect of EB. Therefore, in light of our unexpected finding, we repeated Experiment 2A with a group of rats that received an additional injection of 25 μg MPP 24 h after combined 1 μg EB + 25 μg MPP treatment. This more sustained regimen of MPP treatment also failed to attenuate the anorexigenic effect of EB (data not shown).
Although our acute regimen of EB treatment produced a reliable decrease in food intake, it failed to reduce body weight. Similarly, combined MPP/EB treatment did not alter body weight, F(3,21) = 0.7, n.s. (Fig. 2B).
Experiment 2B: Does MPP attenuate PPT’s anorexigenic effect?
Food intake was influenced by our regimen of combined PPT and MPP treatment F(3,21) = 14.5, P < 0.05 (Fig. 2C). As expected, PPT treatment alone decreased 24-h food intake, relative to that observed following DMSO treatment, P < 0.05. However, MPP failed to attenuate the anorexigenic effect of PPT. Co-treatment with PPT and either 10 or 25 μg MPP produced decreases in food intake that were similar to that observed following PPT + DMSO treatment (Ps < 0.05). Interestingly, the larger dose of MPP abolished the body weight loss following PPT treatment F(3,21) = 18.7, P < 0.05 (Fig. 2D). PPT treatment alone decreased 24-h body weight, relative to that observed following DMSO treatment, P < 0.05. While 10 μg MPP was unable to attenuate the body weight loss following PPT, a dose of 25 μg MPP abolished the PPT-induced weight loss, P < 0.05.
Experiment 3: Does MPP attenuate EB or PPT’s ability to increase uterine weight?
Uterine weights varied by drug treatment, F(5,35) = 5.9, P < 0.05 (Fig. 3). PPT, EB, and MPP treatments alone increased uterine weight, relative to that observed following DMSO treatment, Ps < 0.05. In addition, MPP treatment failed to attenuate the increase in uterine weight in response to either EB or PPT treatment. That is, MPP treatment in combination with either EB or PPT increased uterine weight, relative to that observed in DMSO-treated rats, Ps < 0.05.
Fig. 3.
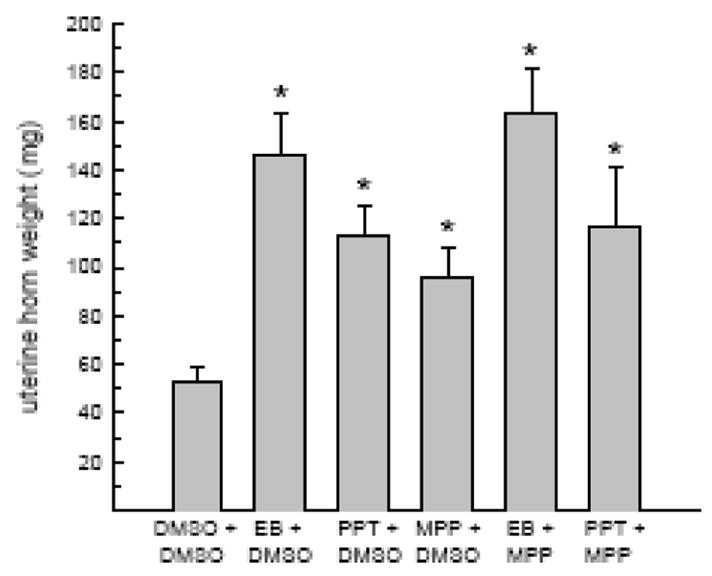
MPP failed to attenuate either EB or PPT’s ability to increase uterine horn weight. As expected, EB and PPT increased uterine horn weight, relative to DMSO treatment. A similar increase in uterine horn weight was observed in rats treated either with MPP alone or in combination with EB/PPT. *Greater than DMSO.
Experiment 4: Does MPP attenuate the estrous-related decrease in food intake in cycling rats?
In cycling rats, food intake was influenced by an interactive effect of cycle stage and drug treatment, F(1,6) = 9.8, P < 0.05 (Fig. 4). Following DMSO treatment, food intake was reduced during estrus, relative to diestrus (P < 0.05). This estrous-related decrease in food intake was attenuated in MPP-treated rats (P < 0.05). That is, the reduction in food intake during estrus was less in MPP-treated rats, relative to DMSO-treated rats (2.6 ± 0.6 g vs. 3.9 ± 0.6 g, respectively; t(7) = 3.1, P < 0.05). Importantly, this effect was not related to differences in baseline food intake in DMSO- and MPP-treated rats because diestrous-levels of food intake were the same between the two drug conditions, P > 0.05 (Fig. 4).
Fig. 4.
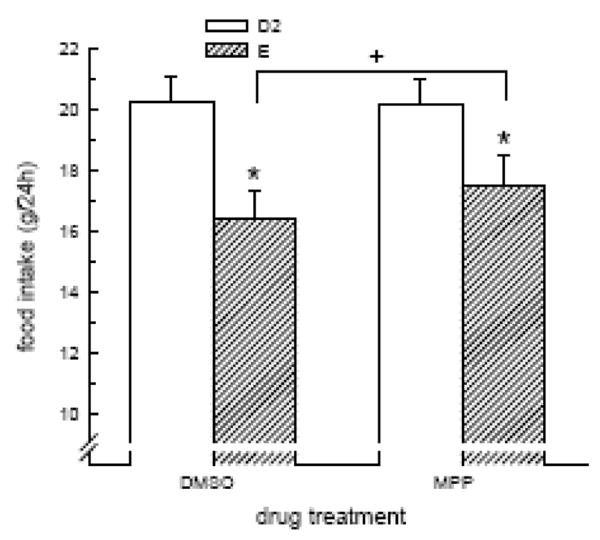
MPP attenuated the estrous-related decrease in food intake in cycling rats. The decrease in food intake during estrus was reduced in MPP-treated rats, relative to DMSO-treated rats. *Less than diestrus 2. +E/MPP greater than E/DMSO.
DISCUSSION
Most available data suggest that the estrogenic inhibition of food intake in OVX rats and mice is mediated via activation of ERα alone [13–15,17,18]. Here, we used the presumptive ERα antagonist, MPP, to investigate whether activation of ERα plays a physiological role in the control of food intake in EB-treated OVX and ovarian-intact (cycling) rats. Before testing this hypothesis directly, a series of experiments were conducted to assess the pharmacological specificity of MPP. This was necessary for two reasons. First, there is some evidence that MPP may function more like a SERM than a silent ERα antagonist [23,25]. SERMs typically exert their effects by competing with estrogens for estrogen binding sites. An extensive literature demonstrates that SERMs exhibit mixed estrogenic/antiestrogenic activity that appears to be tissue-, dose-, and species-specific. For example, tamoxifen is a SERM that acts as a full antagonist on mammary tissue, a partial agonist on uterine tissue, and a full agonist on bone and cholesterol metabolism in the rat [27]. Tamoxifen also exerts a potent inhibitory (i.e., estrogenic) effect on food intake in OVX rats [28]. A second reason for conducting a preliminary assessment of the pharmacological specificity of MPP is that this compound has not been used to study the involvement of ERα in mediating the behavioral actions of estradiol. Previous studies have focused on either in vitro assays or physiological actions of estradiol (e.g., its ability to increase the weight, proliferation, and differentiation of the luminal and glandular uterine epithelium) [21,22,25,29].
To assess the pharmacological specificity of MPP, food intake was monitored in OVX rats treated with MPP either alone or in combination with EB and a selective ERα agonist, PPT. In Experiment 1, we determined that the two largest doses of MPP, 50 and 100 μg, decreased food intake in OVX rats. This suggests that, in the absence of estradiol, MPP exerts some dose-related, estrogenic activity. Thus, within this specific dose range, MPP may function as a SERM, rather than a silent antagonist as previously reported. However, it is also possible that these high doses of MPP may have resulted in malaise sufficient to induce a conditioned taste aversion (CTA) and, thereby, reduced food intake. Both EB and PPT, when administered in large pharmacological doses, are capable of inducing a CTA [18,30]. Future studies will be necessary to address this issue. It is important to note that lower (5 – 25 μg) doses of MPP did not alter food intake. Thus, MPP may act as a silent antagonist within this lower dose range. To test this hypothesis, we examined whether 10 or 25 μg of MPP could attenuate that inhibition of feeding induced by acute administration of either EB or PPT. Consistent with previous studies [17,18], our regimen of EB and PPT treatment decreased food intake in OVX rats. However, neither dose of MPP was sufficient to attenuate the anorexigenic effects of either EB or PPT. While the inability of MPP to attenuate EB’s anorexigenic effect might suggest involvement of ERβ, such an interpretation is not consistent with prevailing pharmacological [17,18] and ERKO mouse data [13–15]. Given the fact that MPP also failed to attenuate the anorexigenic effect of the selective ERα agonist, PPT, an alternative explanation is that we may not have adopted an optimal schedule of MMP administration sufficient to block the inhibition of food intake by EB/PPT. Of course, it is also possible that MPP exerts an estrogenic effect on feeding and the doses of MPP tested in Experiment 2 were sub-threshold to reduce food intake, as suggested by Experiment 1.
While MPP failed to attenuate the anorexigenic effects of either EB or PPT, it did affect PPT-induced weight loss. That is, the 25 μg dose of MPP abolished the decrease in body weight observed following PPT treatment. In so far as a reduction in body weight reflects a reduction in adipose tissue, this finding suggests that MPP is capable of exerting an antagonistic effect on adipose tissue. This is plausible because functional ERs have been localized on adipocytes [31]. At the same time, large doses of MPP (50 and 100 μg) exerted an estrogenic effect on food intake that was mediated presumably via ERα-positive cells in the brain. Together, these findings provide further evidence that MPP may act as a SERM, since it is well established that the effects of SERMs are tissue specific [27,28]. Although EB can reduce body weight in OVX rats, it failed to do so in the present study. This is likely due to the fact that rats received acute injections of a low, physiological dose of EB that was sub-threshold to influence body weight. It remains to be determined whether MPP can attenuate the weight loss associated with a larger dose of EB that was used in the present study.
Because the results of Experiment 2A did not support the hypothesis that the inhibition of food intake following acute administration of either EB or PPT is mediated via ERα, another experiment assessing a different action of estradiol (i.e., increased uterine weight) was designed as a further test of the pharmacological specificity of MPP. As expected, increased uterine weights were observed in OVX rats treated with either EB or PPT. In addition, similar increases in uterine weights were observed in OVX rats treated with either 25 μg of MPP alone or a combination of MPP/EB and MPP/PPT. These findings are partially consistent with a previous study in which 50 μg of MPP alone increased uterine weight in mice [23]. However, in this same study, MPP attenuated the uterine horn proliferation induced by acute administration of EB. These discrepant findings may be related to a species difference, given the fact that other SERMs exhibit species-specific differences in their estrogenic/antiestrogenic properties [32]. Because MPP failed to attenuate either EB or PPT’s actions on food intake and uterine weight in the present study, it remains possible that MPP, at least at the doses and schedule of injection utilized here, functions as a SERM with some agonist activity in the OVX rat.
Despite questionable support for MPP’s ability to attenuate the actions of exogenous estradiol in OVX rats, we continued to investigate the possibility that MPP may be more efficacious in attenuating the anorexigenic effect of endogenous estradiol in cycling rats. MPP was administered at time points chosen to correspond to the initial rise and subsequent peak in estradiol secretion in cycling rats [26]. In contrast to our experiments involving OVX rats, this schedule of MPP treatment attenuated, but did not abolish, the estrous-related decrease in food intake in cycling rats. The fact that food intake was still suppressed during estrus in MPP-treated rats suggests that there may be some role of ERβ in mediating the estrous-related decrease in food intake in cycling rats. However, such an interpretation is not consistent with recent pharmacological data involving selective ERα and ERβ agonists [17;18]. An alternative interpretation is that this dose of MPP may exert only partial antagonist activity at ERα in cycling rats.
The present study suggests that the estrogenic effect of endogenous, but not exogenous, estradiol can be partially blocked by MPP. Further studies are clearly necessary to determine whether MPP has a differential mode of action that may be dependent upon the level of circulating estrogens, the relative expression of ER subtypes, or the relative availability of ER coactivator and corepressor protein complexes in OVX versus ovarian-intact (cycling) rats. Such studies should reveal whether MPP will prove to be a useful tool in evaluating the physiological role of ERα in the estrogenic inhibition of food intake as well as other estrogen-regulated behavioral and physiological responses.
Acknowledgments
This work was supported by grants from the NIH: DK-073936 (LAE) and NS-62667 (JS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973;21:772–780. doi: 10.1016/s0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 2.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 3.Laviano A, Meguid MM, Gleason JR, Yang ZJ, Renvyle T. Comparison of long-term feeding pattern between male and female Fischer 344 rats: influence of estrous cycle. Am J Physiol Regul Integr Comp Physiol. 1996;270:R413–R419. doi: 10.1152/ajpregu.1996.270.2.R413. [DOI] [PubMed] [Google Scholar]
- 4.Richter CP. A behavioral study of the activity of the rat. Comp Psychol Monog. 1922;1 [Google Scholar]
- 5.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 6.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56(2):281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 7.Clegg DJ, Brown LM, Kemp CJ, Strader AD, Benoit SC, Woods SC, et al. Estradiol-dependent decreases in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 8.Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Santollo J, Eckel LA. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol Behav. 2008;93:842–850. doi: 10.1016/j.physbeh.2007.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191(1):173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 15.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa N. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 16.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm Metab Res. 2002;34:758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 17.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol (Regulatory, Integrative, & Comp Physiol ) 2007;293(6):R2194–201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- 19.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Lijima K, et al. Estrogen receptor beta is involved in the anorectic action of estrogen. J Clin Endocrinol Metab. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 20.Zhou HB, Carlson KE, Stossi F, Katzenellenbogen BS, Katzenellenbogen JA. Analogs of methyl-piperidinopyrazole (MPP): antiestrogens with estrogen receptor alpha selective activity. Bioorganic & Medicinal Chemistry Letters. 2009;19:108–110. doi: 10.1016/j.bmcl.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonist selective for estrogen receptor alpha. Endocrinology. 2002;143(3):941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 22.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Molecular and Cellular Endocrinology. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 23.Davis AM, Ellersieck MR, Grimm KM, Rosenfeld CS. The effects of the selective estrogen receptor modulators, methyl-piperidino-pyrazole (MPP), and raloxifene in normal and cancerous endometrial cell lines and in the murine uterus. Molecular Reproduction and Development. 2006;73:1034–1044. doi: 10.1002/mrd.20520. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg MK, Weihau Z, Andersson N, Moverare S, Gao H, Vidal O, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002;174(2):167–178. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- 25.Davis AM, Mao J, Naz B, Kohl JA, Rosenfeld CS. Comparative effects of estradiol, methyl-piperidino-pyrazole, raloxifene, and ICI 182 780 on gene expression in the murine uterus. Journal of Molecular Endocrinology. 2008;41:205–217. doi: 10.1677/JME-08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker JB, Arnold AP, Berkley KB, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 27.Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45–52. doi: 10.3181/00379727-217-44204. [DOI] [PubMed] [Google Scholar]
- 28.Wade GN, Heller HW. Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am J Physiol Regul Integr Comp Physiol. 1993;264:R1219–R1223. doi: 10.1152/ajpregu.1993.264.6.R1219. [DOI] [PubMed] [Google Scholar]
- 29.Chen YJ, Lee MT, Yao HC, Hsiao PW, Ke FC, Hwang JJ. Crucial role of estrogen receptor-alpha interaction with transcription coregulators in follicle-stimulating hormone and transforming growth factor beta1 up-regulation of steroidogenesis in rat ovarian granulosa cells. Endocrinology. 2008;149(9):4658–4668. doi: 10.1210/en.2008-0063. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan-Cato LM, Grigson PS, King JL. Estrogen-induced suppression of intake is not mediated by taste aversion in female rats. Physiol Behav. 2001;72:549–558. doi: 10.1016/s0031-9384(01)00411-5. [DOI] [PubMed] [Google Scholar]
- 31.Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 32.Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens: Role of metabolism. Fed Proc. 1987;46:1870–1874. [PubMed] [Google Scholar]