Abstract
GABAA receptors, the major inhibitory receptors in the mammalian central nervous system, are affected by a number of drug compounds, including ethanol. The pharmacological effects of certain drugs have been shown to be dependent upon specific GABAA receptor subunits. Because benzodiazepines and ethanol have similar effect signatures, it has been hypothesized that these drugs share the γ2-containing GABAA receptors as a mechanism of action. To probe the involvement of the γ2 subunit in ethanol’s actions, spatial memory for the Morris water maze task was tested in γ2 heterozygous knockout mice and wild type littermate controls following ethanol administration at the following doses: 0.0, 1.25, 1.75, and 2.25 g/kg. While baseline learning and memory were unaffected by reduction of γ2 containing GABAA receptors, ethanol dose-dependently impaired spatial memory equally in γ2 heterozygous knockouts and wild type littermate controls.
Keywords: GABAA receptor knockout, γ2 subunit, ethanol, Morris water maze, spatial memory
Alcohol is one of the most abused drugs in the United States today. While research has demonstrated that alcohol produces a broad range of behavioral effects, the precise mechanisms underlying these effects are not completely understood. It is known that a number of ethanol’s behavioral effects, including impairment of learning and memory, mimic those elicited by drugs (i.e., benzodiazepines and barbiturates) known to work directly at the GABAA receptor (GABAA-R). As such, it has been suggested that ethanol modulation of GABAA-R is involved in mediating many of the behavioral effects of ethanol, including impairments in cognition.
GABAA-Rs are heteropentameric protein complexes that are present in an abundance of central nervous system neurons [Sieghart & Sperk, 2002]. It is through this ionotropic, ligand-gated receptor that GABA, the primary inhibitory neurotransmitter, exerts its effects via hyperpolarization of the postsynaptic neuron. Native GABAA-R subunit arrangements are drawn from a family of specific, subunit isoforms (α1–6, β1–4, γ1–3, δ, ε, θ, π, and ρ1–3) [Barnard, Skolnick, Olsen, Mohler, Sieghart, et al., 1998]. Despite the possibility of a vast number of subunit-isoform combinations, in reality, only a limited number of combinations actually exist. In the mammalian brain, the most frequent array is made up of two α1s, two β2s, and one γ2 [McKernan & Whiting, 1996].
It has been recognized for some time now that ethanol shares a similar pharmacological signature with benzodiazepines. Both potentiators of GABAA-R chloride influx, ethanol produces anxiolytic, sedative-hypnotic, and anticonvulsant effects in a concentration-dependant manner similar to that produced by benzodiazepines [Grobin, Matthews, Devaud, & Morrow, 1998; Sieghart & Sperk, 2002]. Ethanol and benzodiazepines have also been observed to develop cross-tolerance and cross-dependence [Morrow, 1995]. Additionally, benzodiazepines have been shown to reduce the effects of ethanol withdrawal and potentiate ethanol-induced anxiolysis (Grobin, et al., 1998; Morrow, 1995) Likewise, certain benzodiazepine receptor inverse agonists (i.e., Ro15-4513 and FG-7142) moderate behavioral signs of ethanol intoxication [Morrow, 1995; Koob, Percy, & Britton, 1988; Suzdak, et al., 1986; Suzdak, Paul, & Crawley, 1988; Lister, 1987; Lister 1988].
The sensitivity of GABAA-Rs to benzodiazepines has been shown to be moderated by particular subunits. For example, peak sensitivity requires the γ2 subunit while decreased sensitivity occurs when either the γ1 or γ3 is present [Wafford, et al., 2004; Wafford, Bain, Whiting, & Kemp, 1993; Hadingham, Wafford, Thompson, Palmer, & Whiting, 1995]. Similarly, global elimination of the γ2-containing GABAA-Rs in mice resulted in a ~94% reduction in benzodiazepine binding sites as well as resistance to diazepam-induced sedation and loss of righting reflex; however, most of these animals died within a few days after birth and none survived beyond postnatal day 18 [Gunther, et al., 1995]. Consequently, GABAA-R γ2 subunit heterozygous knockdown mice were developed that showed increases in anxiety-indicative behaviors yet normal hypnotic responses to certain benzodiazepines [Chandra, Korpi, Miralles, De Blas, & Homanics, 2005]. GABAA-R sensitivity to ethanol has also been proposed to be dependent upon the γ2 subunit; however, a great deal of the relevant research has focused its attention on the subunit’s variant forms (i.e., γ2S and γ2L), often yielding conflicting results [Wafford & Whiting, 1992; Wafford, et al., 1991; Wafford, Burnett, Harris, & Whiting, 1993; Sigel, Baur, & Malherbe, 1993; Marszalec, Kurata, Hamilton, Carter, & Narahashi, 1994; Mihic, Whiting, & Harris, 1994; Homanics, et al., 1999; Boehm, Ponomarev, Blednov, & Harris, 2006; Wick, et al., 2000].
The purpose of the present study was to investigate the role of γ2-containing GABAA-Rs in the mediation of ethanol-induced impairments of spatial memory. The hippocampus, a brain region having an abundance of γ2-containing GABAA-Rs [Sperk Schwarzer, Tsunashima, Fuchs & Sieghart W, 1997; Sieghart & Sperk, 2002], has been shown to be necessary for the normal learning and memory of certain spatial tasks, including the Morris water maze task, as evidenced by a reduction in swim latency and path length to reach a submerged platform that is consistently located in one spatial location [Morris, Garrud, Rawlins, & O’Keefe, 1982; Jarrard, 1983; Jarrard, 1993]. Previous work has also demonstrated that acute ethanol produces a selective, transient, spatial memory impairment for this task, as evidenced by significant increases in swim path lengths and latency, while failing to impair memory for the nonspatial Morris water maze task [Berry & Matthews, 2004]. In this study, the effects of acute ethanol administration on spatial memory for the Morris water maze task were assessed in γ2 heterozygous knockout mice. It was hypothesized that γ2 heterozygous knockout mice, compared to littermate control animals, would be less sensitive to ethanol-induced spatial memory impairment.
Materials and Methods
Subjects
GABAA-R γ2 subunit heterozygous knockouts (n = 43) and wild-type, littermate controls (n = 54) were created by crossing γ2 heterozygous knockout breeder males created at the University of Pittsburgh [Chandra, et al., 2005] with C57BL/6J females. Breeding occurred at the University of Memphis under IACUC approved protocols in an FDA approved facility in the Department of Psychology. Offspring had a mixed genetic background consisting of C57BL/6J, 129 S1/X1, and FVB/N mouse strains. We previously demonstrated (Chandra et al 2005) that homozygous γ2 knockout mice die before weaning age. Although γ2 protein levels were not directly measured in these mice, it has been demonstrated that heterozygous knockout of γ2 using a similar gene targeting strategy resulted in ~50% reduction in γ2 levels compared to wild type controls [Crestani, et al., 1999]. Post weaning (21 days of age), male offspring were genotyped via Southern blot analysis. Animals were housed in separate, individual cages and kept on a 12:12 hour light/dark cycle. Animals were allowed access to food and water ad libitum throughout the entire procedure. For all experiments, task and ethanol naïve, male mice between 45 and 60 days of age were used.
Drugs
Ethanol (10% w/v) was administered via intraperitoneal injection in one of three doses (1.25, 1.75, or 2.25 g/kg). Saline control injections were the same volume as the intermediate ethanol dose.
Blood Alcohol Concentration (BAC) Measurement
Thirty minutes following ethanol injection, animals (littermate controls, n = 10 for 1.25 g/kg, 1.75 g/kg, and 2.25 g/kg ethanol; knockouts, n = 6 for 1.25 g/kg, 1.75 g/kg, and 2.25 g/kg ethanol) were decapitated, and trunk blood was collected. Blood plasma samples were then separated, and BACs were determined via an Analox AV-1 (Analox Instruments, Lunenberg, MA.) according to the manufacturer’s instructions.
Morris Water Maze
The water maze was a circular, galvanized steel tank that was three feet in diameter and 24 inches high. A clear, cylindrical, Plexiglas escape platform measuring 15 inches high with a diameter of five and a half inches was rendered invisible by raising the water level to one-quarter inch above the surface of the platform and by clouding the water with the addition of white, non-toxic, water-based paint. Recording measurements were made using an HVS Image tracking system (HVS Image, Ltd., Buckingham, UK).
Spatial Task Procedure
Animals were trained for 9 days using the standard spatial version of the Morris water maze task (for a detailed protocol see Berry & Matthews, 2004). Briefly, mice were given four trials (once from each starting position), each with a ceiling time of 45 seconds and an inter-trial interval of approximately one minute. The escape platform location remained constant across training days. Those mice that achieved a particular Training Day 9 criterion (mean latency score of 20 seconds using all four trials or 15 seconds using three trials) were used for ethanol testing on the following day (4 knockouts and 1 control failed to meet this criterion; their data were excluded from spatial learning analysis). Ethanol testing procedure and start time were the same as those used during training. Thirty minutes prior to testing, intraperitoneal injections of ethanol were administered to each animal (littermate controls, n = 5 for saline and 1.25 g/kg ethanol and n = 7 for 1.75 g/kg and 2.25 g/kg ethanol; knockouts, n = 5 for saline, n = 6 for 1.25 g/kg ethanol, and n = 7 for 1.75 g/kg and 2.25 g/kg ethanol).
Probe trials were administered on the day following ethanol testing to assess basal (i.e., in the absence of ethanol exposure) spatial memory. The escape platform was removed from the water tank, forcing the animal to perform a search for the duration of the trial. Probe trials consisted of a single, 45-second trial originating from the starting position farthest from the learned escape platform location.
Results
Blood Alcohol Content
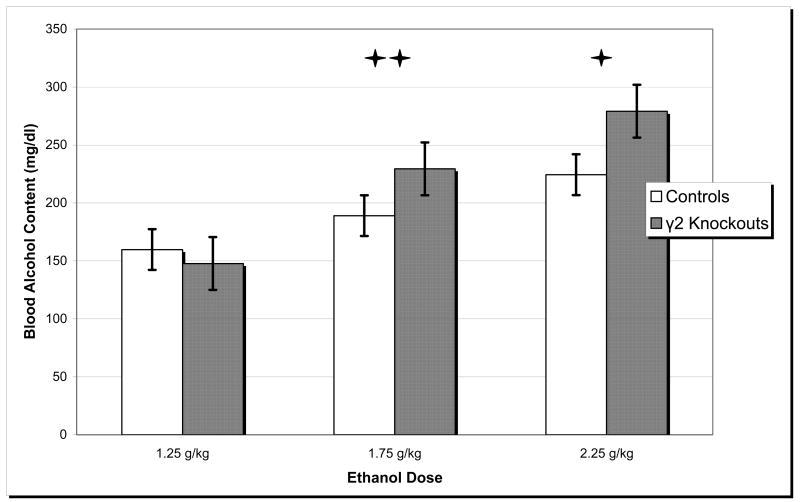
There was a significant effect of ethanol dose on BAC (univariate ANOVA, F(2, 42) = 11.639, p < 0.001), but there was no effect of genotype or interaction (see Figure 1). Post hoc analysis revealed that 2.25 g/kg ethanol resulted in higher BACs compared to 1.75 and 1.25 g/kg ethanol and that 1.75 g/kg ethanol resulted in higher BACs than 1.25g/kg ethanol (all p’s < 0.05).
Figure 1.
Blood Alcohol Content. Mean blood alcohol content values were measured in γ2 heterozygous knockout mice and wild type controls following acute ethanol administration for each dose. * 2.25 g/kg ethanol resulted in higher mean blood alcohol content than 1.75 g/kg and 1.25 g/kg ethanol (both p’s < 0.05). ** 1.75g/kg ethanol resulted in higher mean blood alcohol content than 1.25 g/kg ethanol (p < 0.05). Error bars represent S.E.M.
Spatial Learning
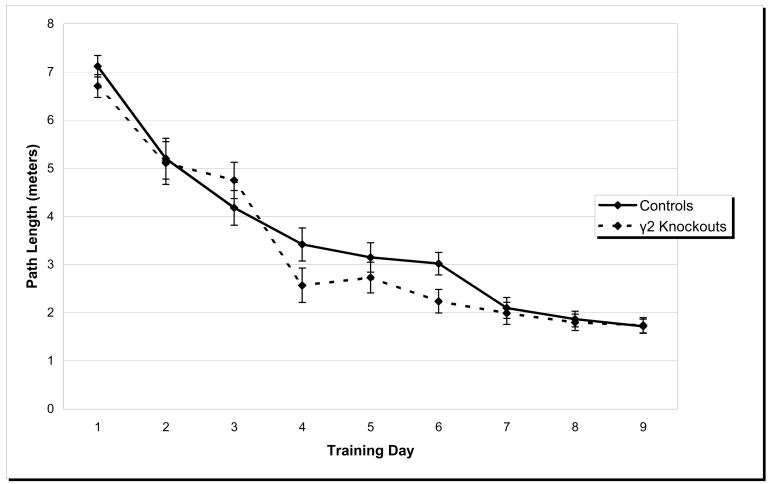
Heterozygous knockouts and wild type controls displayed significant decreases in path length (see Figure 2), latency (data not shown), and swim speed (data not shown) across training days (repeated measures ANOVA, all p’s < 0.05), but there were no genotypic differences or interactions. To further ensure that animals had achieved equal levels of learning, a separate analysis of path length scores on the final training day was done (data not shown). No differences in path length were observed.
Figure 2.
Spatial Learning. Mean path length values were measured for γ2 heterozygous knockout mice and wild type controls across training days in the Morris water maze spatial task. Mean path lengths decreased across training days (p < 0.05) but were not affected by genotype. Error bars represent S.E.M.
Spatial Memory (Ethanol)
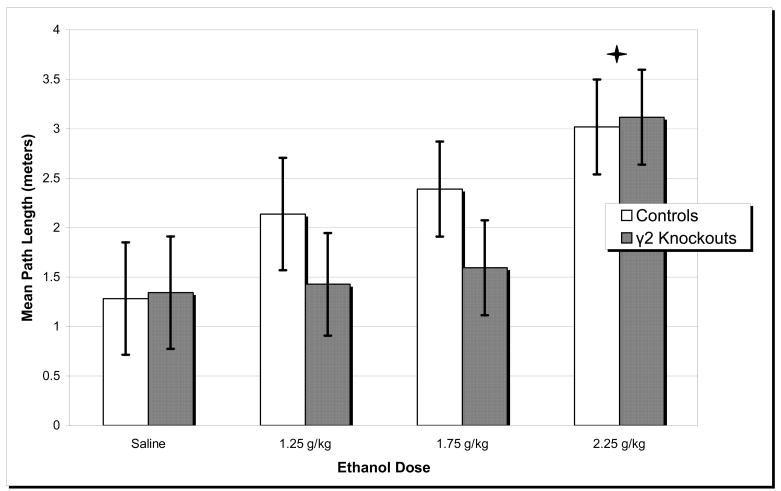
Analysis of test day path length scores showed neither a significant main effect of genotype nor a genotype by ethanol dose interaction. There was, however, a significant main effect of ethanol dose (univariate ANOVA, F(3, 41) = 4.247, p = 0.011). Post hoc analysis revealed that 2.25 g/kg ethanol resulted in higher path length scores compared to all other doses (all p’s < 0.05). See Figure 3. Likewise, an analysis of test day latency scores showed neither a significant main effect of genotype nor a genotype by dose interaction. A significant main effect of ethanol dose, however, was detected (univariate ANOVA, F(3, 41) = 5.732, p = 0.002). Post hoc analysis revealed that 2.25 g/kg ethanol resulted in higher latency scores compared to all other doses (all p’s < 0.05). Analysis of test day swim speed scores revealed neither significant main effects of either genotype or ethanol dose nor a significant genotype by dose interaction.
Figure 3.
Ethanol Test Day. Mean path length values were measured for γ2 heterozygous knockout mice and wild type control mice in the ethanol challenge. * 2.25 g/kg ethanol resulted in higher mean path length scores compared to all other doses (all p’s < 0.05) Error bars represent S.E.M.
Probe Trial
Control and knockout animals displayed equivalent spatial memory during the probe trial. Control and knockout animals made an equivalent number of counter passes across the missing escape platform’s training location (independent samples t-test, t(40) = −1.399, p = 0.170). Likewise, control and knockout animals had significantly higher scores (percentages of time and percentages of path length) in the quadrant that contained the escape platform during spatial training compared to the other quadrants (repeated measures ANOVAs, both p’s < 0.001, post hoc pairwise comparisons, all p’s < 0.001). There were no significant genotypic differences across these measures. See Table 1.
Table.
| Counter Passes | ||||
|
|
||||
| Control | M = 6.05, SEM = 0.64 | |||
| Knockouts | M = 7.25, SEM = 0.566 | |||
|
| ||||
| Percent Time in Quadrants | Quadrant 1 | Quadrant 2 | Quadrant 3 | Quadrant 4 |
|
| ||||
| Control | M = 14.436, SEM = 2.709 | M = 59.095, SEM = 3.438 | M = 13.014, SEM = 2.612 | M = 13.286, SEM = 2.686 |
| Knockouts | M = 12.99, SEM = 2.842 | M = 56.975, SEM = 3.606 | M = 16.93, SEM = 2.740 | M = 12.915, SEM = 2.817 |
|
| ||||
| Percent Path in Quadrants | Quadrant 1 | Quadrant 2 | Quadrant 3 | Quadrant 4 |
|
| ||||
| Control | M = 15.605, SEM = 2.578 | M = 56.732, SEM = 3.462 | M = 13.668, SEM = 2.637 | M = 13.818, SEM = 2.593 |
| Knockouts | M = 13.665, SEM = 2.704 | M = 55.335, SEM = 3.631 | M = 17.63, SEM = 2.766 | M = 13.2, SEM = 2.719 |
Discussion
The present study is the first to investigate if GABAA-R γ2-subunit heterozygote knockout alters ethanol-induced spatial memory impairments. Overall, it was demonstrated that both blood alcohol content and ethanol-induced spatial memory impairments in the water maze task, as evidenced by increases in swim path length and latency, were not influenced by the heterozygous knockout of γ2-containing GABAA-Rs. Heterozygous knockout and control animals displayed equivalent levels of spatial memory impairment for the water maze task following acute ethanol administration. However, it is possible that our study lacked sufficient power to detect subtle differences between genotypes. The data suggest that heterozygous knockout animals did display a smaller impairment by low dose ethanol (Figure 3) compared to controls, but this difference was not significant.
These results agree with previous work that employed genetically manipulated mice with an altered γ2 subunit population. Knockdown mice with ~35% γ2 subunit reduction failed to demonstrate an altered, ethanol-induced righting reflex response [Chandra, et al., 2005]. While it appears that behavioral sensitivity to ethanol is largely insensitive to genetic manipulation of the γ2 subunit, and that the γ2 subunit is not a key target of ethanol action, an alternative hypothesis might explain these results. As was proposed in Chandra et al. [2005], if ethanol and benzodiazepines exert their effects in the same manner at GABAA-Rs, then perhaps the issue is one of threshold. It is possible that the genetically altered animals in both studies retained sufficient levels of γ2-containing GABAA-Rs to exhibit normal ethanol sensitivity. The γ2 heterozygous knockout mice used in current study are predicted to have an ~50% reduction in γ2 protein levels based on studies of a nearly identical γ2 knockout mouse line (Gunther et al, 1995; Crestani et al 1999). However, γ2 protein levels were not measured in the mice used in the current study.
It was also demonstrated that basal learning and memory for the spatial Morris water maze task remained comparable between heterozygous knockouts and controls in that no significant difference in swim path length or latency was observed although a greater number of knockouts did not learn the task compared to control animals. The γ2 heterozygous knockouts used in Crestani et al. [1999] produced similar learning results in the spatial Morris water maze task as well as in contextual and delay fear conditioning tasks. The similar learning rates suggest the possibility that the hippocampus, a brain region shown to be necessary for normal learning and memory for the spatial water maze task, retained enough γ2-containing GABAA-Rs so as to allow for unaltered learning and memory. This seems plausible as Crestani’s knockout mice demonstrated unaltered LTP in CA1 hippocampal slices despite a marked reduction in γ2-containing GABAA-Rs in the CA1 region.
In summary, it was demonstrated that heterozygous knockout of the γ2 subunit of the GABAA-R did not alter ethanol-induced spatial memory impairment. Neither did the γ2 reduction impact basal learning and memory. Together these results suggest that the γ2 subunit is not involved in the learning and memory or the ethanol-induced cognitive impairments of the spatial water maze task. Additional studies, however, are needed in order to completely understand the role of γ2 subunit in the behavioral and pharmacological effects of ethanol including the determination of protein knockdown following genetic manipulations.
Acknowledgments
The authors would like to recognize and thank Carolyn Ferguson for her expert technical assistance, upon which this research depended. Supported by grants AA014588, AA013509 (DBM), DE14184 (DC) and AA10422 (GEH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: Classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Berry RB, Matthews DB. Acute ethanol administration selectively impairs spatial memory in C57BL/6J mice. Alcohol. 2004;32:9–18. doi: 10.1016/j.alcohol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: New perspective on GABAA receptor subunit selectivity of alcohol actions. Biochem Pharmacol. 2006;68:1581–1602. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- Chandra D, Korpi ER, Miralles CP, De Blas AL, Homanics GE. GABAA receptor γ2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005;6:30. doi: 10.1186/1471-2202-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:883–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role o f GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Günther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Wafford KA, Thompson SA, Palmer KJ, Whiting PJ. Expression and pharmacology of human GABAA receptors containing gamma 3 subunits. Eur J Pharmacol. 1995;291:301–309. doi: 10.1016/0922-4106(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Harrison NL, Quinlan JJ, Krasowski MD, Rick CEM, de Blas AL, et al. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the γ2 subunit of the γ-aminobutyrate type A receptor. Neuropharmacology. 1999;38:253–265. doi: 10.1016/s0028-3908(98)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place cells and cue tasks. Behav Neurosci. 1983;97:873–889. doi: 10.1037//0735-7044.97.6.873. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Percy L, Britton KT. The effects of Ro 15-4513 on the behavioral actions of ethanol in an operant reaction time task and a conflict test. Pharmacol Biochem Behav. 1988;31:757–760. doi: 10.1016/0091-3057(88)90261-4. [DOI] [PubMed] [Google Scholar]
- Lister RG. The benzodiazepine receptor inverse agonists FG 7142 and RO 15-4513 both reverse some of the behavioral effects of ethanol in a holeboard test. Life Sci. 1987;41:1481–1489. doi: 10.1016/0024-3205(87)90713-2. [DOI] [PubMed] [Google Scholar]
- Lister RG. Interactions of ethanol with benzodiazepine receptor ligands in tests of exploration, locomotion and anxiety. Pharmacol Biochem Behav. 1988;31:761–765. doi: 10.1016/0091-3057(88)90262-6. [DOI] [PubMed] [Google Scholar]
- Marszalec W, Kurata Y, Hamilton BJ, Carter DB, Narahashi T. Selective effects of alcohols on γ-aminobutyric acid A receptor subunits expressed in human embryonic kidney cells. J Pharmacology Exp Ther. 1994;269:157–163. [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Harris RA. Anaesthetic concentrations of alcohols potentiate GABAA receptor-mediated currents: Lack of subunit specificity. Eur J of Pharmacol. 1994;268:209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Regulation of GABAA receptor function and gene expression in the central nervous system. Int Rev Neurobiol. 1995;38:1–41. doi: 10.1016/s0074-7742(08)60523-1. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P. Recombinant GABAA receptor function and ethanol. FEBS Lett. 1993;324:140–142. doi: 10.1016/0014-5793(93)81380-i. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM, Crawley JN. Effects of Ro15-4513 and other benzodiazepine receptor inverse agonists on alcohol-induced intoxication in the rat. J Pharmacol Exp Ther. 1988;245:880–886. [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Whiting PJ, Kemp JA. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993;44:437–442. [PubMed] [Google Scholar]
- Wafford KA, Burnett D, Harris RA, Whiting PJ. GABAA receptor subunit expression and sensitivity to ethanol. Alcohol. 1993;2:327–330. [PubMed] [Google Scholar]
- Wafford KA, Burnett DM, Leidenheimer NJ, Burt DR, Wang JB, Kofuji P, et al. Ethanol sensitivity of the GABA-A receptor expressed in Xenopus oocytes requires eight amino acids contained in the γ2L subunit. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Macaulay AJ, Fradley R, O’Meara GF, Reynolds DS, Rosahl TW. Differentiating the role of γ-aminobutyric acid type A (GABAA) receptor subtypes. Biochem Soc Trans. 2004;32:553–556. doi: 10.1042/BST0320553. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Whiting PJ. Ethanol potentiation of GABA-A receptors requires phosphorylation of the alternatively spliced variant of the γ2 subunit. FEBS Lett. 1992;313:113–117. doi: 10.1016/0014-5793(92)81424-k. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Radcliffe RA, Bowers BJ, Mascia MP, Lüscher B, Harris RA, et al. Behavioral changes produced by transgenic overexpression of γ2L and γ2S subunits of the GABAA receptor. Eur J Neurosci. 2000;12:2634–2638. doi: 10.1046/j.1460-9568.2000.00160.x. [DOI] [PubMed] [Google Scholar]