Abstract
Elimination of the helminth parasite Nippostrongylus brasiliensis from infected mice is mediated by IL-4 or IL-13 and dependent on the IL-4Rα chain and the transcription factor Stat6 in non-hematopoietic cells. However, it is not clear which Stat6-dependent effector molecules mediate worm expulsion. We identified intelectin-1 and -2 as Stat6-dependent genes that are induced during infection. Intelectins can bind galactofuranose, a sugar present only in microorganisms and might therefore serve as microbial pattern element. To analyze whether constitutive expression of intelectin-1 or -2 leads to accelerated pathogen clearance, transgenic mice were generated which express high levels of these genes selectively in the lung. Infection with N. brasiliensis or Mycobacterium tuberculosis did not result in accelerated pathogen clearance in transgenic as compared to wild-type mice. Further, no significant modulation of the immune response in lung or lymph nodes was observed. Thus, under these conditions, intelectins did not enhance pathogen clearance.
Index descriptors: Nippostrongylus brasiliensis, nematode, Mycobacterium tuberculosis
Introduction
Helminth parasites and allergens induce strong type 2 immune responses characterized by recruitment of IL-4- and IL-13-expressing effector cells like Th2 cells, eosinophils and basophils to the site of infection or allergen encounter, increased mucus production by goblet cells, high serum IgE levels and smooth muscle cell hyperreactivity (Finkelman, 1997). These effector mechanisms have likely evolved to efficiently eliminate parasites from the host organism. IL-4 and IL-13 are the main cytokines involved in the induction of this defense program (Kopf, 1993; McKenzie, 1999). Both cytokines signal through the IL-4Rα chain, which constitutes the IL-4 receptor (IL-4R) by pairing with the common γ chain or the IL-13 receptor 1 (IL-13R1) by pairing with the IL-13Rαl chain. The main signaling pathway from both receptors involves activation of Stat6, which gets recruited to the IL-4Rα chain where it is phosphorylated by Jak kinases (reviewed in (Nelms, 1999)). Activated Stat6 translocates to the nucleus where it binds to regulatory sites in many different genes. Mice deficient in IL-4/IL-13, IL-4Rα or Stat6 have largely overlapping phenotypes, i.e. impaired worm expulsion, low serum IgE levels, defective effector cell recruitment and resistance in mouse models of allergen-induced airway hyperreactivity (Kaplan, 1996; Takeda, 1996; Urban, 1998). However, only a small fraction of Stat6-dependent genes that mediate these effector mechanisms are currently known.
Lung and intestine are both organs with large mucosal surfaces exposed to inhaled or ingested pathogens, respectively. Interestingly, both organs develop from the same primordial tissue consisting of meso- and endoderm of the foregut (Slack, 2004) and might therefore use similar effector mechanisms to defend the host from invading pathogens. The helminth Nippostrongylus brasiliensis (N. brasiliensis) has been used for many years to study type 2 immune responses in mice and rats. L3 infective larvae migrate through the skin to the blood stream and first accumulate in the lung, where they break through the capillaries, are coughed up and swallowed, and finally mature to adult worms in the small intestine. Wild-type mice expel the parasites within 10 days after infection. Many human nematode parasites, like hookworms and roundworms, have a similar developmental program which involves migration of L3 larvae through the lung and maturation in the intestines. N. brasiliensis expulsion requires Stat6 expression in non-hematopoietic cell types (Voehringer, 2004). However, the critical Stat6-dependent gene(s) required for worm expulsion have thus far not been identified. Stat6 expression is required in intestinal epithelial cells to increase mucosal permeability, decrease glucose absorption and decrease chloride secretion during helminth infection (Madden, 2002). Furthermore, expression of Fizz2/resistin-like beta in the small intestine has been shown to be dependent on IL-4 receptor signaling and might interfere with the chemosensory apparatus of helminth parasites in the intestine, thereby leading to enhanced worm expulsion (Artis, 2004). Interestingly, Fizz2 has also been described as a Stat6-dependent gene induced in the lung of mice during an experimental asthma model indicating that common effector mechanisms exist at both sites (Stutz, 2003).
We used global gene expression profiling of lung and small intestine after infection with N. brasiliensis to identify intelectin-1 and -2 as Stat6-dependent genes that are strongly induced at both sites. We over-expressed these genes in the lung of transgenic mice and studied their potential role for worm expulsion or modulation of immune responses against N. brasiliensis. In addition, since intelectins have been shown to bind galactofuranose, an essential cell wall component of Mycobacteria, we determined whether intelectins show bactericidal activity against Mycobacterium tuberculosis infection in vivo.
Results
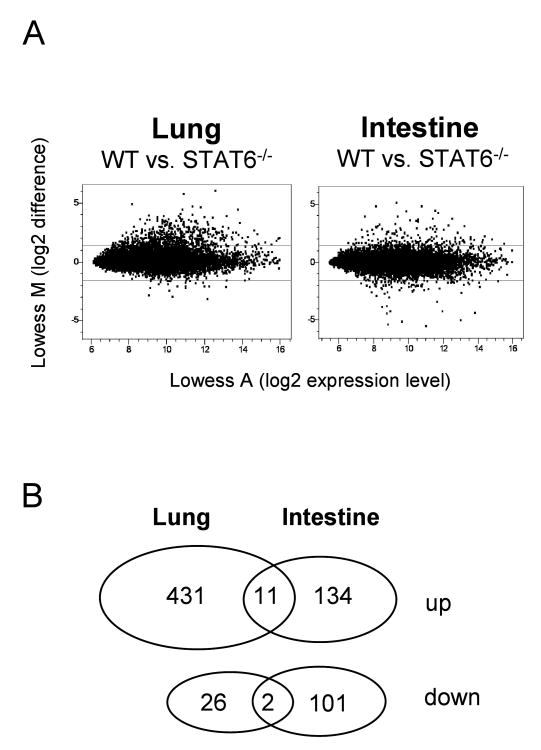
The transcription factor Stat6 integrates signals from the IL-4 and IL-13 receptors and is a critical component for regulated expression of a large number of genes during type 2 immune responses in vivo. To analyze the Stat6-dependent gene expression profile in the lung and intestine after N. brasiliensis infection, we compared cDNA samples generated from total lung tissue and small intestine (jejunum) from wild-type and Stat6-deficient mice during the acute phase of the immune response on day 9 after infection by competitive hybridization on spotted oligonucleotide arrays covering about 17,000 genes of the mouse genome (Fig. 1A). 431 and 134 genes were induced more than 2.8-fold in a Stat6-dependent fashion in the lung and intestine, respectively, 11 of which were commonly induced at both sites (Fig. 1B and Table 1). In contrast, only 26 and 101 genes were repressed more than 2.8-fold in a Stat6-dependent manner in lung and intestine, respectively. Ly6E and carboanhydrase were the only two genes that were repressed in both organs. Among the Stat6-dependent genes that were induced in lung and intestine during infection were IgA, calcium activated chloride channel 3, small proline rich proteins 2 and intelectin-2, which has recently been identified as a gene induced by nematode infection in the small intestine (Table 1) (Komiya, 1998; Pemberton, 2004b). Two intelectin genes have been described in mouse and man with high homology to a Xenopus oocyte granule lectin (Lee, 2001; Pemberton, 2004a; Suzuki, 2001b). Since human intelectin has been described to bind galactofuranose sugars present in cell walls of bacteria, fungi and protozoan parasites and therefore might play a role in immune defense, its mouse homolog was chosen here for further investigation (Daffe, 1990; Latge, 1994; Suzuki, 2001a; Suzuki, 2002; Tsuji, 2001).
Figure 1.
Microarray analysis of Stat6-dependent gene expression in lungs and small intestine after N. brasiliensis infection. A) Microarray analysis was performed 9 days after N. brasiliensis infection of BALB/c (WT) or Stat6-deficient mice (Stat6-/-) as described in Materials and Methods. Each dot corresponds to a single gene on the microarray. A-values on the x-axes indicate the expression levels on a log2 scale and M-values on the y-axes indicate the difference in expression levels between WT and Stat6-/- mice on a log2 scale. Horizontal lines indicate the cut-off for genes considered significantly up- or down-regulated in a Stat6-dependent fashion (M>1.5 or <-1.5, which corresponds to more than 2.8 fold up- or down-regulation, respectively). B) Venn diagram of genes that were up- or down-regulated by Stat6 in lung and/or intestine.
Table 1.
List of genes commonly induced under control of STAT6 by N. brasiliensis infection in lung and intestine. The values shown are the fold induction in wild-type mice over STAT6-deficient mice.
| STAT6-dependent induction (fold) |
||||
|---|---|---|---|---|
| Name | Symbol | Lung | Intestine | GenBank |
| ATPase, Na+/K+, beta 1 | Atp1b1 | 3.7 | 3.7 | AK010677 |
| Chloride channel calcium activated 3 | Clca3 | 62 | 8.3 | NM_017474 |
| Dimethylaniline monooxigenase | Fmo5 | 3.4 | 3.3 | NM_010232 |
| Ig alpha heavy chain (IgA) | Igh-VJ558 | 3.1 | 4.4 | AF466769 |
| Intelectin 2 | Itln2 | 3.8 | 2.9 | NM_010584 |
| Malate decarboxylase | Mod1 | 4.8 | 6.0 | NM 008615 |
| RAS-related C3 botulinum substrate 2 | Rac2 | 2.9 | 4.8 | NM_009008 |
| RIKEN cDNA 2010110O04 gene | 8.9 | 3.0 | AK008389 | |
| Signal transd. and act. of transcription | 6Stat6 | 4.1 | 2.8 | NM 009284 |
| Small proline-rich protein 2A/B | Sprr2a | 3.6 | 4.6 | NM 011468 |
| Small proline-rich protein 2K | Sprr2k | 4.9 | 18 | NM 011477 |
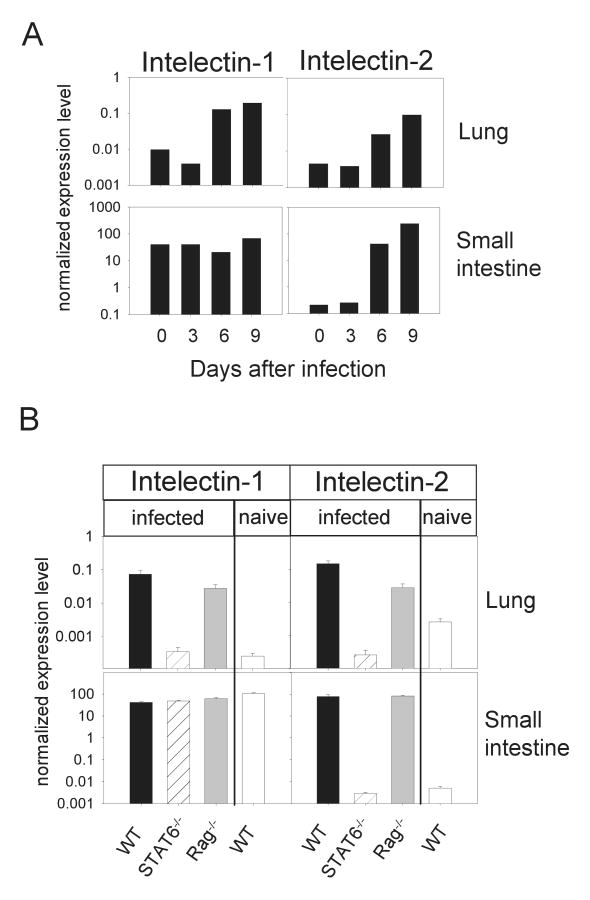
The oligonucleotide probe on the microarray could not distinguish between intelectin-1 and -2 cDNAs due to their highly homologous sequences (94% identity). Therefore, quantitative RT-PCR was performed from lung and intestine with sequence-specific primers. As shown in Fig. 2A, expression of both intelectin-1 and -2 started to increase in the lung at day 6 after infection, when the larvae have already left the lung and infiltration of IL-4/IL-13 producing effector cells occurs (Voehringer, 2004). In the small intestine intelectin-1 was expressed at constitutively high levels confirming previous results (Pemberton, 2004a), whereas intelectin-2 was induced from day 6 after infection. Intelectin expression was then compared at the peak of the immune response (day 9) in wild-type, Stat6-deficient and Rag-deficient mice (Fig.2B). Wild-type mice showed up to 300-fold higher expression levels as compared to Stat6-deficient mice in the lung after N. brasiliensis infection. This increase was largely independent of adaptive immunity, since Rag-deficient mice also showed substantial intelectin-1 and -2 expression after infection. In contrast, in the intestine intelectin-2 expression was induced about 3000-fold in a Stat6-dependent manner. As in the lung, cells of the adaptive immune system were not required to induce intelectin-2 expression in the intestine.
Figure 2.
Quantitative RT-PCR for intelectin-1 and intelectin-2. A) Expression levels of intelectin-1 and intelectin-2 in lung and small intestine was analyzed on day 0, 3, 6 and 9 after N. brasiliensis infection of BALB/c mice by quantitative RT-PCR. B) Expression levels of intelectin-1 and intelectin-2 in lung and small intestine of naïve wild-type mice (WT naive) or day 9 N. brasiliensis-infected WT, Stat6-/- or Rag-/- mice were determined by quantitative RT-PCR. Data were normalized to HPRT. Error bars indicate standard deviations.
Since L3 larvae of N. brasiliensis migrate through the lung, where they undergo a molting step to the L4 form, we overexpressed intelectins in the lungs of transgenic mice to assess potential larvicidal activity of these proteins in vivo, which can be determined by counting adult worms at their final destination, the small intestine. Two transgenic mice, SPINT-1 and SPINT-2, were generated which express either intelectin-1 or intelectin-2 under control of the surfactant protein C promoter in type 2 pneumocytes in the lung (Fig. 3). Sensitive RT-PCR analysis of both transgenic lines revealed that they expressed the transgene at high levels in the lung but not in other organs like spleen or liver (Fig. 3B and C). Neither transgenic line showed any signs of spontaneous inflammation in the lung and microarray analysis comparing lung tissue from transgenic and non-transgenic littermates did not reveal any significant differences in the gene expression profile in lung tissue aside from the respectively over-expressed transgenes (data not shown).
Figure 3.
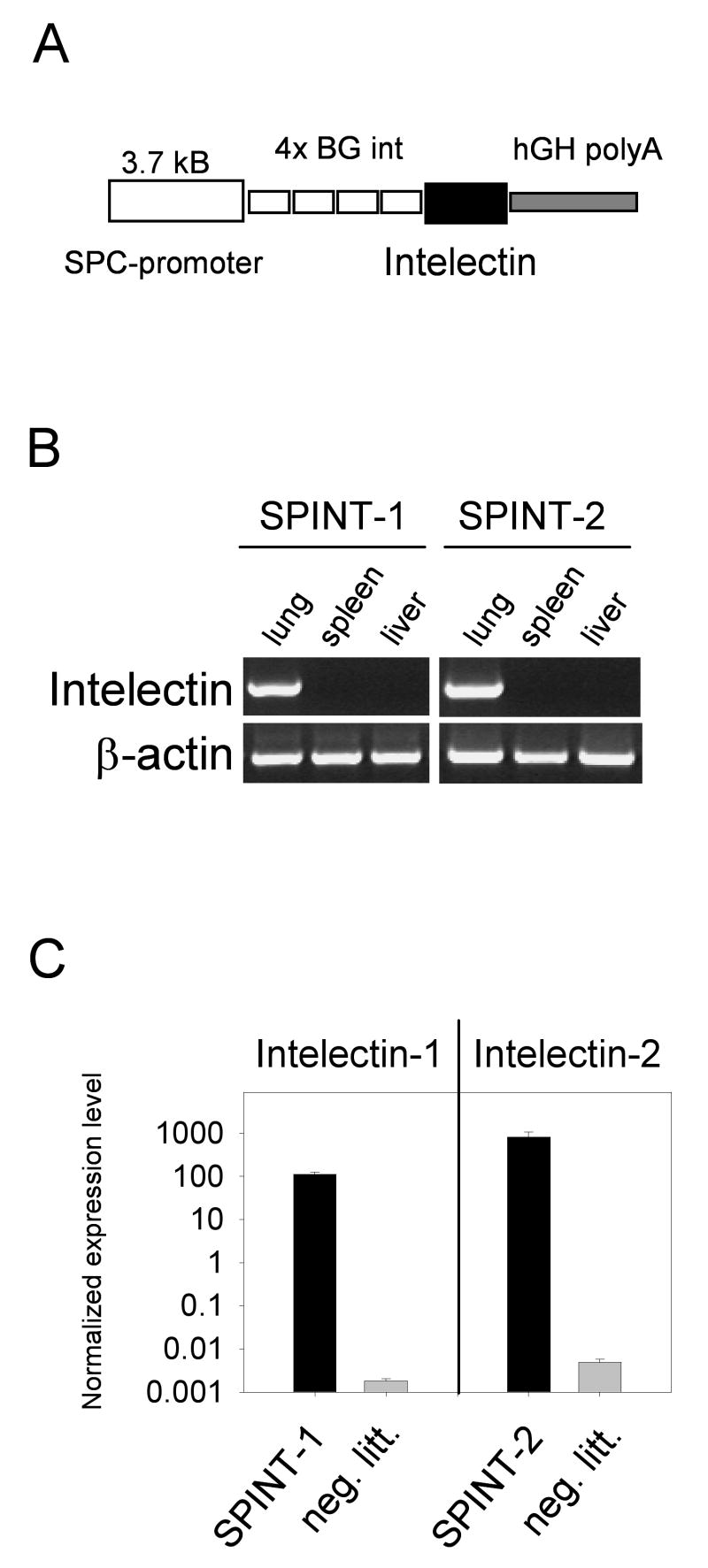
Expression of intelectin-1 and intelectin-2 in the lung of transgenic mice. A) Schematic representation of the expression vector used to generate intelectin-1 (SPINT-1) and intelectin-2 (SPINT-2) transgenic mice. B) RT-PCR from lung, spleen and liver of SPINT-1 and SPINT-2 transgenic mice with transgene-specific primers. C) Quantitative RT-PCR from lung of SPINT-1 or SPINT-2 mice and their negative littermates (neg. litt.) normalized to HPRT expression. Error bars indicate standard deviations.
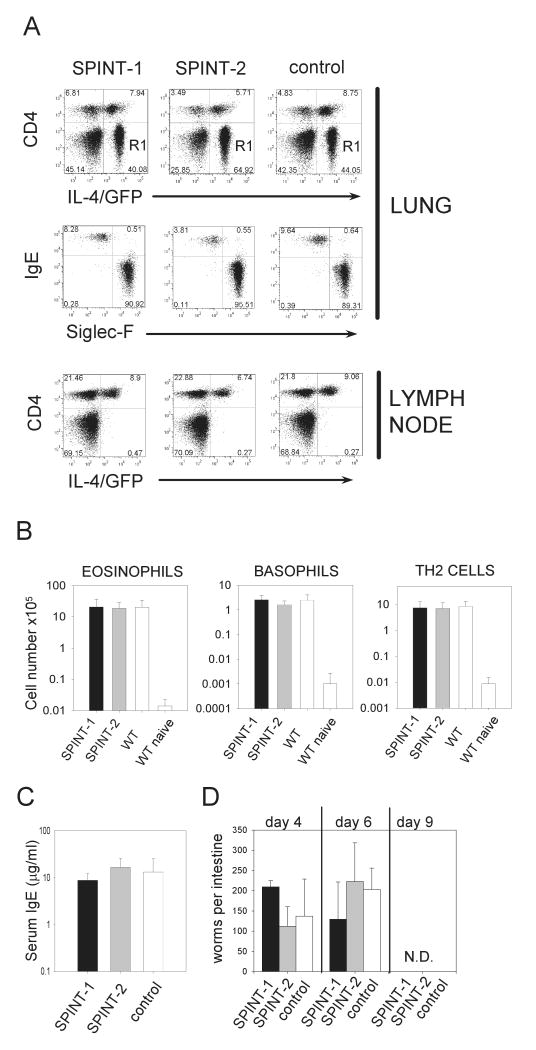
The natural ligand for mouse intelectin might be galactofuranose, as has been proposed for human intelectin (Tsuji, 2001). This sugar is not produced by mammals but is present in a variety of microbial pathogens. Therefore, intelectin might serve as a microbial pattern recognition receptor and mediate the induction of a pro-inflammatory immune response. To test this hypothesis, a pool of different galactofuranose-derivatives or a galactopyranose-disacharide were administered twice over 2 days intranasally to SPINT-1 or SPINT-2 mice. The recruitment of eosinophils and basophils, both important effector cells during asthma and helminth infections, was determined by flow cytometry. Both compounds induced a weak recruitment of eosinophils and basophils that was independent of transgene expression (Fig. 4). Next, the immune response of SPINT-1 and SPINT-2 mice after N. brasiliensis infection was analyzed. To visualize IL-4 expressing cells without prior restimulation, both transgenic lines were crossed to 4get mice, which express IL-4 and GFP as a bicistronic reporter construct from the IL-4 locus (Mohrs, 2001). Analysis of lung and paratracheal lymph nodes at the peak of the immune response in the lung 9 days after infection revealed no significant differences in Th2 cell differentiation or recruitment of Th2 cells, eosinophils or basophils to the lung (Fig. 5A and B). Furthermore, total serum IgE levels and the kinetics of worm expulsion were comparable in SPINT-1, SPINT-2 and control mice (Fig. 5C and D). Thus, constitutive intelectin expression in the lung does not promote or inhibit the type 2 immune response induced by N. brasiliensis infection and does not result in enhanced worm immunity.
Figure 4.
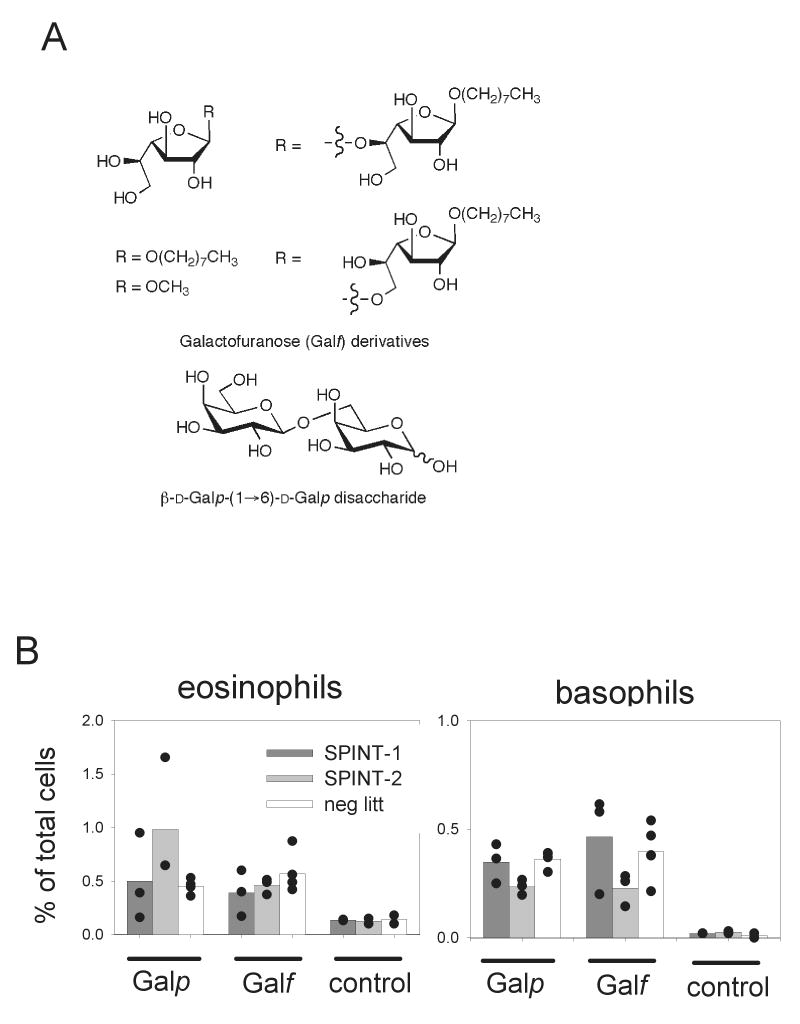
Administration of galactofuranose (Galf) or galactopyranose (Galp)containing–glycoconjugates. A) Chemical structures of the β-D-Galf glycosides and the β-D-Galp-(1→6)-D-Galp disacharide used for intranasal administration. B) Recruitment of eosinophils and basophils to the lung was determined by flow cytometry 4 days after administration of pooled Galf-glycosides or the Galp-disacharide in SPINT-1 or SPINT-2 transgenic mice or their negative littermates. Untreated transgenic or wild-type mice served as control.
Figure 5.
Analysis of the immune response of SPINT-1 and SPINT-2 transgenic mice after N. brasiliensis infection. A) Flow-cytometric analysis of lung and paratracheal lymph nodes of SPINT-1, SPINT-2 and 4get mice on day 9 after N. brasiliensis infection. SPINT mice were crossed to 4get mice to visualize IL-4-expressing cells ex vivo, as described in Materials and Methods. Dot-plots in the second row are gated on R1 (CD4-GFP+) from the dot-plots above and show the frequency of basophils (IgE+SiglecF-) and eosinophils (IgE-SiglecF+) within R1. B) Total number of eosinophils, basophils and Th2 cells in the lung on day 9 after infection. Uninfected wild-type mice were used as controls. C) Total serum IgE levels on day 9 after N. brasiliensis infection. D) The kinetics of worm expulsion was determined in SPINT-1, SPINT-2 and negative littermate control mice. Five mice per group were analyzed. Error bars indicate standard deviations.
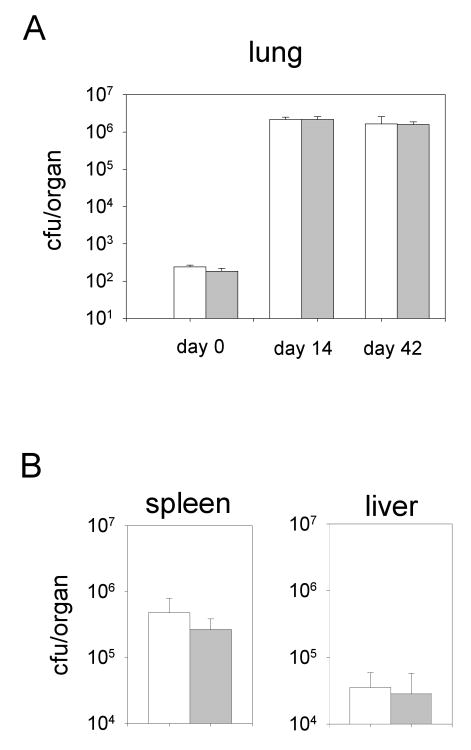
Galactofuran, a polymer of galactofuranose sugars, is a major component of mycobacterial cell walls and is essential for their growth (Pan, 2001). Intelectin might interfere with cell wall synthesis by binding to galactofuranose residues in this organism and could perhaps be used as therapeutic tool against TB infection. To test this hypothesis, SPINT-1, SPINT-2 and control mice were infected with Mycobacterium tuberculosis by aerosol inhalation and colony counts were determined 2 and 6 weeks after infection. As shown in Fig. 6, constitutively high levels of intelectin-1 or -2 in the lung did not attenuate the growth of M. tuberculosis in the lung or impede their dissemination to the spleen and liver.
Figure 6.
Infection of mice with aerosolized Mycobacterium tuberculosis.A) Intelectin transgenic mice (closed bars) or wild-type mice (open bars) were infected with M. tuberculosis as described in Materials and Methods. Colony forming units (cfu) in the lung were determined on day 0, 14 and 42 after infection. B) 42 days after infection cfu were determined in spleen and liver. 3-4 mice per group were analyzed. Error bars indicate standard deviations.
Taken together, we identified intelectin-1 and -2 as novel Stat6-dependent genes expressed in the lung and small intestine during helminth infection. However, constitutive high-level expression in the lung neither modulated the immune response against N. brasiliensis nor inhibited the growth of M. tuberculosis in the lung.
Materials and Methods
Mice
Intelectin-1 and intelectin-2 cDNA from small intestine of N. brasiliensis-infected mice were amplified by PCR using the following primer pairs: 5′INT Sal I (5′-gagggtcgaccaccatgacc-3′) and 3′INT BH I (5′-aagtcaggatccatcccg-3′) for intelectin-1 and 5′INT Sal I and 3′INT-2 BH I (5′-aagtcaggatccatccca-3′) for intelectin-2. Full-length cDNAs were sequenced and cloned into a lung-specific expression vector behind a 3.7 kB surfactant protein C promoter element and 4 exon/intron repeats from bovine growth hormone followed by the human growth hormone poly A sequence as indicated in Fig.3A (Korfhagen, 1990). Transgenic mice were generated by injection of the linearized construct into C57BL/6xDBA/2 F2 oocytes. Transgenic mice were backcrossed with 4get/BALB/c mice for 2-4 generations. 4get mice have been described (Mohrs, 2001). In brief, they express a bicstronic IL-4/GFP reporter construct which allows detection of IL-4 expressing cells without prior restimulation. BALB/c and Stat6-deficient mice (Kaplan, 1996) were purchased from The Jackson Laboratory (Bar Harbor, ME). Rag-deficient BALB/c mice were obtained from Taconic farms (Germantown, NY).
Nippostrongylus brasiliensis infection
Third-stage larvae (L3) of N. brasiliensis were recovered from the cultured feces of infected rats, washed extensively in 0.9% saline (37°C) and injected (500 organisms) into mice subcutaneously at the base of the tail. Mice were treated with antibiotic-containing water (2 g/l neomycin sulfate, 100 mg/l polymyxin B sulfate; Sigma-Aldrich, St. Louis, MO) for the first 5 days after infection. Mice were usually analyzed on day 9 after infection at the peak of the immune response, except for the analysis of early worm expulsion where mice were sacrificed on day 4 and 6 after infection to count adult worms in the small intestine.
Mycobacterium tuberculosis infection
For infections with Mycobacterium tuberculosis, bacteria were grown in 7H9 medium to mid log phase, washed once with PBST, spun at 500 rpm to pellet clumps, sonicated for 15 sec, and suspended at OD = 0.1. Aerosol exposure of the mice was carried out for 15 min, followed by a 30 min purge with air, using a custom built aerosol exposure chamber (University of Wisconsin, Madison), as described previously (MacMicking, 2003). Mice were euthanized by CO2 exposure and organs were removed for enumeration of bacterial CFUs as previously described (Stanley, 2003).
Galactofuranose derivatives
The chemical synthesis of the galactofuranose-containing mono- and disaccharides shown in Fig. 4 will be reported elsewhere (Completo, G. C. and T. L. Lowary. Manuscript in preparation). Briefly, a fully benzoylated galactofuranosyl thioglycoside was coupled with the appropriate acceptor alcohol under the promotion of N-iodosuccinimide and silver triflate (Konradsson, 1990). Subsequent deprotection of these products with sodium methoxide yielded the target molecules.
Microarray analysis
Total RNA was isolated from lung and small intestine of 3 individual BALB/c or Stat6-deficient mice on day 9 after N. brasiliensis infection using a RNA isolation kit (Fluka, Buchs, Switzerland). 30 μg total RNA was used to generate aminoallyl-dUTP-incorporated cDNA. Samples were labeled with Cy3-(for Stat6-deficient samples) and Cy5- (for BALB/c samples) reactive dyes (CyDye™, Amersham Biosciences, Peapack, NJ) and hybridized to oligonucleotide microarrays spotted on amino silane-treated glass slides (OPERON mouse genome oligoset v 2.0; QIAGEN, Hilden, Germany) according to the following protocol: http://arrays.ucsf.edu/protocols/cdna_transcription_and_coupling.pdf. Slides were scanned on an Axon 4000B scanner using Genepix 3.0 software (Axon Instruments, Inc., Molecular Devices Corporation, Union City, CA) and normalized by ‘lowess’ normalization on the pixel medians without background subtraction using Acuity 4.0 software (Axon Instruments, Inc.). “A” values indicate the total signal intensity of a given spot on the microarray and are calculated as A = 1/2*log2(R*G), where R and G give the intensity for the Cy5 and Cy3 channels, respectively. “M” values indicate the difference in gene expression on a log2 scale and are calculated as M = log2(R/G) (e.g.: M=1 indicates a 2-fold higher expression in the sample that was labeled with the Cy5 dye compared to the sample that was labeled with the Cy3 dye, and M=0 indicates equal expression in both samples). Genes with an “M” value of more than 1.5 or less than -1.5 were selected as being significantly Stat6-dependent. The results have been deposited at the GEO database (accession numbers GSM52086 and GSM52087).
Quantitative RT-PCR
Total RNA was isolated from lung or small intestine (jejunum) of naïve BALB/c mice or N. brasiliensis-infected BALB/c, Rag- or Stat6-deficient mice or from the lungs of SPINT-1/-2 transgenic mice and their non-transgenic littermates. The RNA from 2 individual mice per group was pooled and 2 μg RNA were transcribed to cDNA using the Superscript III™ reverse transcriptase kit (Invitrogen, Carlsbad, CA). cDNA sample triplicates were amplified by SYBR-green Lightcycler-PCR using a DNA Engine Opticon 2® System (MJ Research, Waltham, MA) and the following primer pairs for intelectin-1: 5′INT-3 (5′-aacctgggcatctgg-3′) and ILN1 P7R (5′-gagttccatatccatcccaatc-3′); for intelectin-2: 5′INT-3 and ILN2 P7R (5′-gagttccatatccattcgcatc-3′) and for HPRT: HPRT-1 (5′-gttggatacaggccagactttgttg-3′) and HPRT-2 (5′-gagggtaggctggcctataggct-3′). The annealing temperature was 61°C and SYBR green incorporation was read at 81°C for HPRT and 84°C for intelectin-1 and -2. The expression levels of intelectin-1 and -2 were normalized to HPRT.
Multiple tissue RT-PCR
cDNA was generated from 2 μg total RNA isolated from lung, spleen and liver of SPINT-1 and SPINT-2 transgenic mice or negative littermates and amplified by PCR with primer pairs 5′INT-3 and 3′hGH-2 (5′-actggagtggcaacttccag-3′) for intelectin-1 and -2 and Ac1 (5′-atggatgacgatatcgct-3′) and Ac2 (5′-atgaggtagtctgtcaggt-3′) for β-actin. The 3′hGH-2 primer binds only the transgenic cDNAs and therefore prevents amplification of endogenous intelectin cDNAs. PCR reactions were run with 35 cycles at 61°C annealing temperature and 60 sec extension time.
Galactofuranose/galactopyranose administration
The β-linked galactofuranose-containing glycosides shown in Fig.4 were generated by chemical synthesis as described above. The β-D-galactopyranosyl-(1→6)-D-galactopyranose disaccharide was purchased from Sigma-Aldrich. 50 μg of pooled galactofuranose-derivatives or the galactopyranose-disacharide were administered intranasally in 50 μl PBS on day 0 and day 2. Mice were analyzed on day 4 by flow cytometry.
Flow cytometry
Lungs and paratracheal lymph nodes were isolated and single cell suspensions were generated as described (Voehringer, 2004). Samples were stained with biotinylated anti-IgE (R35-72; BD Pharmingen), PerCP-Cy5.5-labeled anti-CD4 (L3T4; BD Pharmingen) and PE-labeled anti-SiglecF (E50-2440; BD Pharmingen) antibodies followed by allophycocyanin-labeled streptavidin (Molecular Probes, Eugene, OR) and analyzed on a digital LSR II flow-cytometer with FACS Diva software (BD Immunocytometry Systems, San Jose, CA) and Flowjo 5.1 software (Tree Star, Inc., Stanford, CA).
IgE ELISA
Serum IgE levels were determined by standard ELISA technique using the monoclonal antibody B1E3 for coating and the biotinylated monoclonal antibody EM95 for detection.
Discussion
Rapid pathogen recognition is facilitated by binding of pathogen associated microbial patterns (PAMPs) by receptors of the innate mammalian immune system. This initial recognition of a variety of different PAMPs, such as LPS, bacterial DNA and viral RNA can be mediated by members of the Toll-like receptor (TLR) family. However, the recognition of carbohydrates by TLRs has not been described. Certain carbohydrate structures like chitin, beta-glucan, zymosan, arabinomannan and galactofuran are not produced by mammals and might therefore serve as PAMPs. Microbial carbohydrates can be recognized by cell surface receptors of the C-type lectin receptor (CLR) family, such as the mannose receptor or dectin-1 (McGreal, 2005). PAMPs can also serve as targets for effector responses of the immune system. Soluble lectins like collectins and ficolins can opsonize bacteria and induce the lectin-dependent pathway of complement activation (Fujita, 2004; Lu, 2002). The identification of mouse intelectin expression in intestinal Paneth cells and the binding of recombinant human intelectin-1 to galactofuranose in vitro suggested that intelectins might play a role in immune defense (Nair, 2006; Tsuji, 2001). Using the helminth parasite N. brasiliensis, we confirmed previous reports using Trichinella spiralis or Trichiuris muris infection in mice to show strong induction of intelectin expression in secretory Paneth cells and goblet cells of the intestine (Datta, 2005; Pemberton, 2004a). N. brasiliensis larvae migrate through the lung and develop later to adult worms in the intestine. Therefore, this infection model allowed the simultaneous identification of intelectin-1 and -2 expression in lung and small intestine. Intelectin expression has also been reported in lung tissue using different mouse asthma models, in biopsies from human asthma patients and in malignant pleural mesothelioma tumors (Kuperman, 2005; Wali, 2005). Rag-deficient mice, which lack an adaptive immune system, showed similar induction of intelectin-2 expression in lung and small intestine as compared to wild-type mice. Thus, IL-4/IL-13 expressed from cells of the innate immune system, such as eosinophils, basophils or mast cells, is sufficient to induce Stat6-dependent intelectin expression. Importantly, Rag-deficient mice cannot expel the parasites and therefore the induction of intelectin-2 expression and constitutively high intelectin-1 levels in the small intestine does not correlate with worm expulsion in these mice. Additionally, C57BL/6 mice, which lack the intelectin-2 gene, expel N. brasiliensis with the same kinetics as BALB/c mice (Ishiwata, 2002; Pemberton, 2004a). Further, early confrontation of migration larvae with high intelectin levels in the lung of transgenic mice did not result in enhanced worm expulsion as compared to wild-type mice (Fig. 5D). Therefore it seems unlikely that intelectins are directly involved in N. brasiliensis expulsion. Intelectin-1 is expressed at high constitutive levels in the small intestine but not the lungs of wild-type, Stat6-deficient and Rag-deficient mice and could serve as pattern recognition receptor for galactofuranose-containing intestinal pathogens.
Human intelectin-1 has independently been cloned as lactoferrin receptor from samples of human fetal intestine (Suzuki, 2001b). Lactoferrin is involved in antibacterial responses by either agglutinating bacteria or binding of iron, which lowers the available iron critically required for bacterial growth (Otto, 1992; Teraguchi, 1996). Indeed, the growth advantage of M. tuberculosis in β2m-deficient mice is at least partially due to iron overload in these mice, as this can be relieved by lactoferrin treatment (Schaible, 2002). Given the two potential antimicrobial activities of intelectins against M. tuberculosis, including recognition of galactofuranose present in the cell wall of these organisms (Pan, 2001) and the ability to sequester iron-loaded lactoferrin (thereby lowering the local iron availability), we further investigated whether intelectin-transgenic mice were more resistant to M. tuberculosis infection and whether intelectins could have therapeutic potential against TB infection. However, under the conditions used here, no protective function could be observed (Fig. 6).
The requirement of Stat6 for expression of intelectin-1 in the lung but not the intestine indicates a complex regulation of this gene in different tissues or cell types which requires further investigation. Expulsion of helminth parasites is likely to involve several distinct effector mechanisms, which are centrally controlled by the transcription factor Stat6. Although intelectin-2 is one of only a few genes in the small intestine that are highly induced during helminth parasite infection, our analysis using transgene over-expressing mice suggests that this pathway does not contribute substantially to worm expulsion. Further analysis using gene-targeted mice, however, is probably warranted.
Acknowledgments
We thank Ninetta Flores, Linda Stowring and Davina Wu for technical support. This work was supported in part by NIH grants AI30663 and HL56385, HHMI , the Sandler Asthma Basic Research Center (to R.M.L.) and the Emmy Noether Program of the Deutsche Forschungsgemeinschaft (grant Vo944/2 to D.V.). The authors have no conflicting financial interests.
abbreviations
- Galf
galactofuranose
- Galp
galactopyranose
- Stat6
signal transducer and activator of transcription 6
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffe M, Brennan PJ, McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. Journal of Biological Chemistry. 1990;265:6734–6743. [PubMed] [Google Scholar]
- Datta R, deSchoolmeester ML, Hedeler C, Paton NW, Brass AM, Else KJ. Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infection and Immunity. 2005;73:4025–4033. doi: 10.1128/IAI.73.7.4025-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annual Review of Immunology. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Fujita T, Matsushita M, Endo Y. The lectin-complement pathway--its role in innate immunity and evolution. Immunological Reviews. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Nakao H, Nakamura-Uchiyama F, Nawa Y. Immune-mediated damage is not essential for the expulsion of Nippostrongylus brasiliensis adult worms from the small intestine of mice. Parasite Immunology. 2002;24:381–386. doi: 10.1046/j.1365-3024.2002.00472.x. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochemical and Biophysical Research Communications. 1998;251:759–762. doi: 10.1006/bbrc.1998.9513. [DOI] [PubMed] [Google Scholar]
- Konradsson P, Udodong UE, Fraser-Reid B. Iodonium-promoted reactions of disarmed thioglycosides. Tetrahedron Letters. 1990;31:4313–4316. [Google Scholar]
- Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Glasser SW, Wert SE, Bruno MD, Daugherty CC, McNeish JD, Stock JL, Potter SS, Whitsett JA. Cis-acting sequences from a human surfactant protein gene confer pulmonary-specific gene expression in transgenic mice. Proceedings of the National Academy of Sciences U S A. 1990;87:6122–6126. doi: 10.1073/pnas.87.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. Journal of Allergy and Clinical Immunology. 2005;116:305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Latge JP, Kobayashi H, Debeaupuis JP, Diaquin M, Sarfati J, Wieruszeski JM, Parra E, Bouchara JP, Fournet B. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infection and Immunity. 1994;62:5424–5433. doi: 10.1128/iai.62.12.5424-5433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Schnee J, Pang M, Wolfert M, Baum LG, Moremen KW, Pierce M. Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology. 2001;11:65–73. doi: 10.1093/glycob/11.1.65. [DOI] [PubMed] [Google Scholar]
- Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochimica et Biophysica Acta. 2002;1572:387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. Journal of Immunology. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. Journal of Experimental Medicine. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. Journal of Immunology. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annual Review of Immunology. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Otto BR, Verweij-van Vught AM, MacLaren DM. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Critical Reviews in Microbiology. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- Pan F, Jackson M, Ma Y, McNeil M. Cell wall core galactofuran synthesis is essential for growth of mycobacteria. Journal of Bacteriology. 2001;183:3991–3998. doi: 10.1128/JB.183.13.3991-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton AD, Knight PA, Gamble J, Colledge WH, Lee JK, Pierce M, Miller HRP. Innate BALB/c Enteric Epithelial Responses to Trichinella spiralis: Inducible Expression of a Novel Goblet Cell Lectin, Intelectin-2, and Its Natural Deletion in C57BL/10 Mice. Journal of Immunology. 2004a;173:1894–1901. doi: 10.4049/jimmunol.173.3.1894. [DOI] [PubMed] [Google Scholar]
- Pemberton AD, Knight PA, Wright SH, Miller HR. Proteomic analysis of mouse jejunal epithelium and its response to infection with the intestinal nematode, Trichinella spiralis. Proteomics. 2004b;4:1101–1108. doi: 10.1002/pmic.200300658. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. Journal of Experimental Medicine. 2002;196:1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM. Intestine in the lung. Journal of Biology. 2004;3:10. doi: 10.1186/jbiol8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proceedings of the National Academy of Sciences U S A. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. Journal of Immunology. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Mortara RA, Takahashi HK, Straus AH. Reactivity of MEST-1 (antigalactofuranose) with Trypanosoma cruzi glycosylinositol phosphorylceramides (GIPCs): immunolocalization of GIPCs in acidic vesicles of epimastigotes. Clinical and Diagnostic Laboratory Immunology. 2001a;8:1031–1035. doi: 10.1128/CDLI.8.5.1031-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Tanaka AK, Toledo MS, Takahashi HK, Straus AH. Role of beta-D-galactofuranose in Leishmania major macrophage invasion. Infection and Immunity. 2002;70:6592–6596. doi: 10.1128/IAI.70.12.6592-6596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001b;40:15771–15779. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Teraguchi S, Shin K, Fukuwatari Y, Shimamura S. Glycans of bovine lactoferrin function as receptors for the type 1 fimbrial lectin of Escherichia coli. Infection and Immunity. 1996;64:1075–1077. doi: 10.1128/iai.64.3.1075-1077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. Journal of Biological Chemistry. 2001;276:23456–23463. doi: 10.1074/jbc.M103162200. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- Wali A, Morin PJ, Hough CD, Lonardo F, Seya T, Carbone M, Pass HI. Identification of intelectin overexpression in malignant pleural mesothelioma by serial analysis of gene expression (SAGE) Lung Cancer. 2005;48:19–29. doi: 10.1016/j.lungcan.2004.10.011. [DOI] [PubMed] [Google Scholar]