Abstract
Purpose
To evaluate the safety of Systane® Lubricant Eye Drops in relieving the symptoms of dry eye following laser-assisted in situ keratomileusis (LASIK) surgery.
Methods
This was a randomized, double-masked, single-center, placebo-controlled, contralateral eye study of 30 patients undergoing LASIK surgery. The mean age of patients was 42.4 ± 10.7 years, and the mean spherical equivalent was −3.29 (range, +1.75 to −7.38). Patients’ right and left eyes were randomized to receive either Systane® or placebo – a preserved, thimerosal-free saline solution – beginning from the day of surgery and ending 30 days following surgery. Outcome measures included tear film break up time (TFBUT), visual acuity, degree of corneal and conjunctival staining, and treatment-related adverse events.
Results
Preoperatively, placebo-treated eyes had statistically significantly higher sum corneal staining score than Systane®-treated eyes (p = 0.0464); however, the difference was clinically insignificant (p = 0.27). Two weeks post operatively, the average TFBUT in the Systane®-treated eyes was 1.23 seconds longer than that of the placebo-treated eyes (p = 0.028). All other evaluated variables were comparable between the two treatments. No adverse events were reported in the study.
Conclusion
Systane® Lubricant Eye Drops are safe for use following LASIK surgery to relieve the discomfort symptoms of dry eye associated with the procedure.
Keywords: Systane®, safety, dry eye, LASIK
Introduction
Laser-assisted in situ keratomileusis (LASIK) is an effective procedure used to correct refractive errors. It affords patients a predictably fast and painless recovery of vision. To date, one of the major complications of LASIK surgery is persistent or temporary dry eye-like symptoms or the exacerbation of pre-existing dry eye conditions (Toda et al 2001). In healthy eyes, the tear film supports and maintains the ocular surface. When tear production is needed, sensation from the ocular surface stimulates the brain, which sends secretomotor nerve impulses to the lacrimal glands to produce tears. In dry eyes, however, an abnormal or decreased tear film causes irritation of the ocular surface, resulting in the release of cytokines that disrupt the neural arc. This disruption leads to interruption of secretomotor nerve impulses, neurogenic inflammation of the lacrimal gland, activation of T-cells, and secretion of cytokines into tears that further inflame the ocular surface and reduce normal tear production (Salib et al 2006). It is believed that severing the corneal nerves during LASIK surgery may interrupt the neural feedback loop to the lacrimal glands as well (Stern et al 1998; Patel et al 2001; Donnenfeld et al 2004).
Dry eye is a complex disease of inadequate lubrication of the ocular surface. It has a multifactorial etiology governed by various underlying conditions and environmental factors. Although there are many contributing factors, ocular dryness is predominantly due to either aqueous deficiency or to excessive evaporation of the tear film. Patients may complain of symptoms of dry eye in the presence or absence of signs. Moreover, dry eye may be diagnosed based only on the signs observed by a healthcare professional or by an administered questionnaire such as the Ocular Surface Disease Index (OSDI). Dry eye is a highly prevalent disease and has the potential to cause functional impairment and a reduced quality of life (Foulks 2007). The tear film contains a mixture of lipid, aqueous, and mucin layers in an interactive hydrated mucin gel pattern. Lipids are present not only on the surface of the tear film, but also throughout the gel in association with proteins (Foulks 2005). It is generally accepted that the tear film in dry eye patients is unstable and incapable of maintaining the protective qualities that are necessary for its structure and function. This instability causes patients to experience the discomfort symptoms associated with dry eye, such as burning, stinging, grittiness, foreign body sensation, tearing, ocular fatigue, and dryness (Terry 2001).
The mainstay therapy of dry eye is tear film correction and improvement, which is achieved with topically administered lubricant eye drops. Relief from these drops essentially is transient due to low retention time on the ocular surface. Systane® Lubricant Eye Drops (Alcon Laboratories, Inc., Fort Worth, TX, USA) contains polyethylene glycol 400 (0.4%) and propylene glycol (0.3%) demulcents with the polymer hydroxypropyl guar (HP-Guar) as a gelling agent, and is preserved with POLYQUAD® 0.001% (polidronium chloride). Systane® is also available as a unit-dose, preservative-free preparation. In the bottle, Systane® consists of a loosely cross-linked meshwork created by covalent interactions between borate and HP-Guar. When exposed to the higher pH (pH 7.5) of the ocular surface and tears (Yamada et al 1997), the HP-Guar forms a reversible cross-link with borate, culminating in a gel-matrix with sheer thinning viscoelastic and bioadhesive properties. The gel-matrix is designed to promote retention of the active demulcents, thereby protecting the ocular surface (Hartstein et al 2005; Gifford et al 2006).
The purpose of this study was to evaluate the safety of Systane® as compared with marketed saline (placebo) for the relief of dry eye symptoms following LASIK refractive surgery.
Patients and methods
Study population
Thirty (30) adults, regardless of gender or race, undergoing bilateral LASIK surgery, were enrolled in the study. All subjects signed an informed consent form before the beginning of the study. An institutional review board approved the study, and all testing was conducted in accordance with the board’s guidelines.
Inclusion and exclusion criteria
Study subjects were taken from a standard group of typical candidates for LASIK surgery, including patients over 18 years of age and post-menopausal women, with a healthy ocular status and a preoperative refractive anisometropia of less than one diopter (1 D) between eyes. Prior to being approached for the study, all patients had expressed a desire for bilateral LASIK surgery, had a pre-operative evaluation (screening visit; Visit 1) indicating healthy ocular status, and had an established date for surgery. Other considerations for inclusion included the ability and willingness to appear at all in-office visits and to follow instructions. Women of childbearing potential were included in the study if they met all the following conditions: were not breast-feeding, had negative urine pregnancy test at the screening visit, agreed to undertake urine pregnancy test at the end of the study, were not planning to become pregnant during the course of the study, and agreed to use adequate birth control methods for the duration of the study. Subjects who did not wear soft contact lenses or rigid gas permeable lenses 3 days and 3 weeks, respectively, prior to screening visit, and were willing to discontinue contact lens wear for the duration of the study were also included. Subjects were also included if they were willing and able to discontinue the use of all topical ocular medications and abstain from systemic medications known to cause ocular dryness for at least 2 weeks prior to LASIK surgery and throughout the duration of the study.
Patients were excluded from the study if they had had intraocular surgery, ocular trauma, active rosacea in either eye 3 months prior to the screening visit, or had used RESTASIS® eye drops 30 days prior to the screening visit. Evidence of active intraocular inflammation, chronic or recurrent uveitis or other inflammatory eye disease in either eye, end stage salivary or lacrimal gland dysfunction, hepatitis-C or HIV infection, or graft versus host disease were also considered exclusion criteria. Other exclusion criteria included: dry eye due to Stevens-Johnson syndrome, Riley-Day syndrome, sarcoidosis, leukemia, severe meibomian gland dysfunction requiring treatment, active chemical burns, or nerve paresis; history of radiation therapy to the head above the angle of the jaw; and eyelid surface abnormalities that affect eyelid function.
Study medications and dose regimen
Test article: Systane® Lubricant Eye Drops.
Placebo: Sensitive Eyes® saline solution (Bausch and Lomb, Rochester, NY, USA).
Patients self-administered medication – 1 to 2 drops in each eye 2 times daily (bid) – beginning at the day of surgery (Day 0) and ending 30 days following LASIK surgery (Day 30). Patients were allowed to use the study medications every 30 minutes as needed immediately following LASIK surgery until bedtime the evening of surgery, and as rescue medication if necessary during the course of the study. Since the study was contralateral, patients were instructed to use the rescue medication only in the eye requiring additional medication, using the medication assigned for that eye. Furthermore, patients were required to document how much and how often rescue medication was used.
Study visits
The study consisted of 5 visits carried out in an approximately 5-week period. Patients underwent eligibility screening and ocular surface evaluation on Visit 1 (Day −7 ± 2). Visit 2 (Day 0) was the day of surgery on which the ocular surface was re-evaluated prior to surgery. Follow-up visits were Visit 3 (Day 1), Visit 4 (Day 14 ± 3), and Visit 5 (Day 30 ± 7). Patients exited the study on Visit 5.
Each study visit included medical and medication history; overall dry eye and discomfort questions; dry eye post-LASIK questionnaire; uncorrected and best corrected visual acuity (VA) measurements (on Visit 3, only uncorrected VA was measured); and type, time, and frequency of rescue medication used (except on Visit 1). A thorough, slit-lamp biomicroscope examination was conducted on all study visits to examine and record any abnormalities in the cornea, conjunctiva, eyelids/eyelashes, anterior chamber, iris, and lens. Measurement of tear film break up time (TFBUT), un-anesthetized Schirmer test, slit-lamp examination, sodium fluorescein corneal staining, and lissamine green conjunctival staining were conducted on all study visits except on Visit 3.
Study design
This was a prospective, randomized, double-masked, placebo-controlled, single-center, contralateral eye study. At the screening visit (Visit 1), subjects’ demographic information was recorded along with current/prior use of medications, lubricant eye drops, and ocular pathologies. Medications considered necessary to maintaining subjects’ health were used at the discretion of the investigator. Eyes of subjects satisfying the inclusion criteria were randomized to receive Systane® in one eye and placebo in the contralateral eye following LASIK surgery. To ensure proper masking of patients, medical personnel, and the examining investigators regarding the study medications, the test article and placebo were supplied as a pre-numbered kit containing two 15-mL bottles with sterile closures and masked labeling. To decrease the risk of cross-placement of drops, the masked medication labels were marked with a large “R” for the right eye, and “L” for the left eye.
To evaluate tear clearance rate and break up time, 5 μL of 2% preservative-free sodium fluorescein (Alcon Laboratories, Inc.) were instilled into the inferior conjunctival cul-de-sac of each eye to measure the time taken from the blink until the first dark spots or streaks appeared within the fluorescein enhanced tear film. The test was repeated 3 times, and the score was the average of the 3 consecutive measurements. To ensure the accuracy of the TFBUT tests, the tests were assessed by the same examiner at all visits using the same slit-lamp. Schirmer tests were carried out according to the Atlas of Primary Eyecare Procedures (Casser et al 1997).
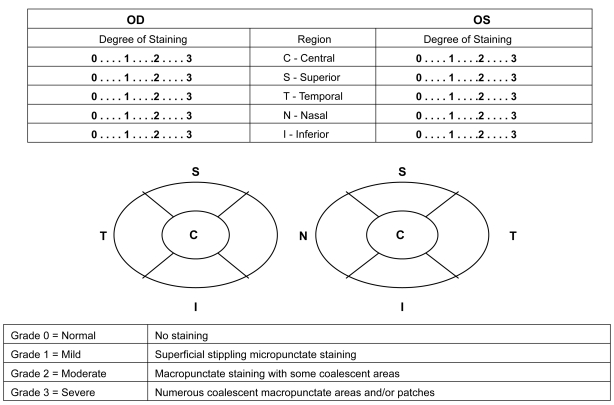
Corneal staining was conducted 3 to 4 minutes after the TFBUT measurements. The type (severity) of staining was assessed for each of the 5 regions of the cornea (central, inferior, temporal, superior, and nasal) (Figure 1). The type of staining was graded using the following grading scale: 0 = normal–no staining, 1 = mild–superficial stippling micro-punctate staining, 2 = moderate–macropunctate staining with some coalescent areas, 3 = severe–numerous coalescent macropunctate areas and/or patches. The scores of the 5 regions were summed to obtain the total area of staining score, with a minimum staining score of 0 and a maximum score of 15 for each eye.
Figure 1.
Corneal regions and staining grades.
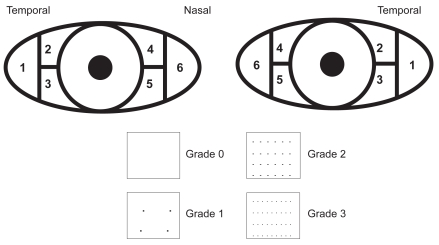
To stain the conjunctiva, an Eppendorf ® pipette was used to instill 5 μL of 1% preservative-free lissamine green drops in each eye. The severity of staining was assessed for the 6 regions of the conjunctiva, and the degree of staining was recorded separately for the 3 portions of the temporal conjunctiva and the 3 portions of the nasal conjunctiva. The severity of conjunctival staining was graded using a scale ranging from 0 (best) to 3 (worst), with a minimum staining score of 0 and a maximum score of 18 for each eye (Figure 2).
Figure 2.
Conjunctival regions and staining grades.
Uncorrected and best-corrected VA (UCVA and BCVA) testing preceded any examination requiring administration of eye drops to anesthetize or dilate the eye, or any examination requiring contact with the eye. ETDRS chart was used and the VA was calculated as a logMAR score.
Surgical procedure
All patients underwent bilateral simultaneous LASIK surgery performed by an experienced LASIK surgeon (DD). All flaps were created with the 60 kHz IntraLase femtosecond laser (Advanced Medical Optics, Irvine, CA, USA) with a planned flap diameter of 8.5 mm and an intended mean flap thickness between 100 and 110 μm. The Allegretto Wavelight excimer laser was used in 25 patients. The remaining 5 patients received wavefront-guided treatment using the Alcon LADAR4000 (Alcon Laboratories, Inc.) because of significant preoperative higher order aberrations. Standard postoperative medications including a fourth-generation fluoroquinolone anti-inflammatory eye drops (1 drop 4 times daily) and fluorometholone (FML) steroid eye drops (1 drop 4 times daily) were prescribed to all patients. Oral pain medications were prescribed at the surgeon’s discretion.
Statistical analysis
Descriptive statistics were performed for all variables: average, standard deviation (SD), mean, and minimum/maximum value for continuous variables. Pearson’s Chi-square was chosen for categorical outcomes, and paired t-test was chosen for between-treatment comparisons of numeric outcomes with a bilateral significance level of 5%.
Results
Demographics
Thirty (30) patients were enrolled and successfully completed all aspects of the study. The mean age of subjects was 42.4 ± 10.7 years (range, 19 to 60); 16 patients (53%) were female.
Tear film break up time (TFBUT)
TFBUT values were statistically comparable between the Systane®-treated and placebo-treated eyes throughout the study except on Visit 4 (Day 14) where the mean TFBUT in the Systane®-treated eyes was 1.23 seconds longer than that of the placebo-treated eyes (p = 0.028) (Table 1).
Table 1.
Comparison of tear film break-up time (TFBUT) between Systane®-treated eyes and placebo-treated eyes
| Variable | # of eyes | Systane® |
Placebo
|
p valuea | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |||
| Visit 1 (screening) | 30 | 7.42 (3.68) | 2.67–14.00 | 7.80 (3.26) | 3.00–12.67 | 0.2757 |
| Visit 2 (Day 0) | 30 | 6.94 (3.66) | 2.33–21.00 | 7.63 (3.58) | 2.33–19.33 | 0.0703 |
| Visit 3 (Day 1) | 30 | 5.17 (2.04) | 1.33–9.00 | 5.57 (2.67) | 1.00–12.33 | 0.3535 |
| Visit 4 (Day 14 ± 3) | 30 | 6.40 (3.10) | 2.33–14.00 | 5.17 (2.37) | 2.00–11.67 | 0.0281 |
| Visit 5 (Day 30 ± 7) | 30 | 6.33 (3.18) | 2.00–15.00 | 6.28 (2.81) | 2.00–13.33 | 0.8662 |
p values of the paired t-test for comparisons between the two treatments using contralateral eyes as controls.
Schirmer tests
Before the initiation of the treatment, the mean Schirmer scores averaged 19.9 and 20.6 for Systane®- and placebo-treated eyes, respectively. There were no significant differences in Schirmer scores between the two treatment groups following treatment (average 22.0 and 22.3, respectively).
Ocular surface evaluation
There were no significant differences measured by sodium fluoroscein or lissamine green staining between eyes treated with either Systane® or placebo except on Visit 1 where placebo-treated eyes had statistically higher sum corneal staining score than Systane®-treated eyes (p = 0.046); however, the difference was clinically insignificant (p = 0.27).
Safety
No eye in either treatment group showed an increase in corneal, conjunctival, lens, anterior chamber, or iris slit-lamp findings. Intraocular pressure measurements at Visits 1 and 5 showed no significant differences between treatment groups or as compared to baseline values. Likewise, there were no treatment-related adverse events or serious adverse events in this study. These results demonstrate that Systane® used 2 times daily after LASIK surgery was safe and well tolerated by refractive surgery patients.
Discussion
In this study, the safety of Systane® Lubricant Eye drops following LASIK surgery was assessed in 30 patients undergoing bilateral surgery. Systane® was administered into one randomly assigned eye, and the contralateral eye served as control, receiving Sensitive Eyes® preserved saline solution. The results show that Systane® was safe to use following LASIK surgery, including the day of surgery, to relieve the postoperative dry eye symptoms induced by LASIK surgery. It is important to note that the Systane® formulation used in this study was the Polyquad-preserved formulation not the preservative-free formulation. There were no differences between Systane®- and placebo-treated eyes in vital staining, UCVA, BCVA, and TFBUT. However, 14 days post operatively, the mean TFBUT in the Systane®-treated eyes was 1.23 seconds longer than that of the placebo-treated eyes. These data add more evidence and weight to the earlier conclusions drawn by Christensen et al regarding the overall safety profile of Systane® (Christensen et al 2004).
The ocular surface microenvironment is made up of surface epithelial cells, the glycocalyx, and the tear film. A breakdown in any one of these components can lead to disruption of the tear film and damage to epithelial cells, which may result in exacerbation of dry eye signs and symptoms (Ousler et al 2002). While the damaged cells are being replaced, the ocular surface remains unprotected. Although the cause of dry eye after LASIK has not been completely clarified, severing the corneal nerves during LASIK surgery during the creation of the flap, as well as laser ablation, have been suspected to play an important role (Linna et al 1998; Donnenfeld et al 2004); therefore, intervention to help protect the ocular surface environment during the healing process potentially would be beneficial.
While new therapeutic strategies based on the pathophysiology of dry eye are currently being developed, lubricant eye drops continue to be the mainstay care of dry eye. The major goal of these products is to restore the ocular surface integrity in an attempt to reduce the symptoms associated with dry eye. Lubricant eye drops provide a physical protective barrier that works in conjunction with blinking to help spread the tear film over the ocular surface (Versura et al 2008). A protected ocular surface, in fact, will exist as long as the tear film is stable and when the TFBUT matches or exceeds the inter-blink interval (IBI). Conversely, when the TFBUT is less than the IBI, the corneal surface will be unprotected for a period of time between blinks, inducing ocular dryness (Ouslar et al 2007). Systane® works by binding to the hydrophobic exposed areas of the epithelial cells and attaching a protective HP-Guar tear-gel matrix that helps restore the ocular surface and increases TFBUT (Korb et al 2002; Korb et al 2005).
In summary, LASIK surgery induces tear-deficient dry eye and exacerbates preexisting dry eye by disturbing corneal innervations. Candidates for surgery should be screened properly so that preexisting dry eye is detected, and all candidates should be warned against the possible development of LASIK-induced dry eye. Administration of Systane® starting from the day of surgery and insertion of punctual plugs are recommended to reduce discomfort during the symptomatic postoperative period.
This study had two limitations. Systane®-treated eyes may have benefited from a preoperative treatment period and perhaps from a longer than 1 month postoperative treatment period. An extended postoperative treatment period may have evidenced more differences between the two treatment groups and provided additional power to the safety data. Likewise, additional significant differences between the treatment groups may have been found with a larger patient population.
Conclusion
Systane® Lubricant Eye drops are safe for use following LASIK surgery to relieve the dry eye discomfort symptoms induced or exacerbated by this procedure.
Acknowledgments
The study was funded by a grant from Alcon Laboratories, Inc., Fort Worth, Texas, USA.
The authors thank Heba Costandy, MD, MS for medical writing contributions and Jenny Song, MD, MS for statistical analysis.
Footnotes
Disclosures
Drs. Durrie and Stahl are consultants of Alcon Research, Ltd., Fort Worth, Texas, USA. None of the authors has any commercial or proprietary interest in any of the products mentioned in the manuscript.
References
- Casser L, Fingeret M, Woodcome HT. Atlas of primary eye care Procedures. Stanford, CT: Appleton and Lange; 1997. pp. 124–5. [Google Scholar]
- Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-Guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004;28(1):55–62. doi: 10.1076/ceyr.28.1.55.23495. [DOI] [PubMed] [Google Scholar]
- Donnenfeld ED, Ehrenhaus M, Solomon K, et al. Effect of hinge width on corneal sensation and dry eye after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30(4):790–7. doi: 10.1016/j.jcrs.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Foulks GN. Determinants of tear film stability. The Castroviejo Lecture.American Academy of Ophthalmology Annual Meeting; October 15–18; Chicago. 2005. [Google Scholar]
- Foulks GN. Clinical evaluation of the efficacy of PEG/PG lubricant eye drops with gelling agent (HP-Guar) for the relief of the signs and symptoms of dry eye disease: A review. Drugs Today. 2007;43(12):887–96. doi: 10.1358/dot.2007.43.12.1162080. [DOI] [PubMed] [Google Scholar]
- Gifford P, Evans BJ, Morris J. A clinical evaluation of Systane. Contact Lens Ant Eye. 2006;29:31–40. doi: 10.1016/j.clae.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hartstein I, Khwarg S, Przydryga J. An open-label evaluation of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult population. Curr Med Res Opin. 2005;21(2):255–60. doi: 10.1185/030079905x26252. [DOI] [PubMed] [Google Scholar]
- Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearer. US-based study. CLAO J. 2002;28(4):211–6. doi: 10.1097/01.ICL.0000029344.37847.5A. [DOI] [PubMed] [Google Scholar]
- Korb DR, Herman JP, Greiner JV, et al. Lid wiper epitheliopathy and dry eye symptoms. US-based study. Eye Contact Lens. 2005;31(1):2–8. doi: 10.1097/01.icl.0000140910.03095.fa. [DOI] [PubMed] [Google Scholar]
- Linna TU, Pérez-Santonja JJ, Tervo KM, et al. Recovery of corneal nerve morphology following laser in situ keratomileusis. Exp Eye Res. 1998;66:755–63. doi: 10.1006/exer.1998.0469. [DOI] [PubMed] [Google Scholar]
- Ousler GW, Emory TB, Welch D, et al. Factors that influence the inter-blink interval (IBI) as measured by the ocular protection index (OPI). Poster. The Association of Research in Vision and Ophthalmology 2002 [Google Scholar]
- Ousler GW, Michaelson C, Christensen MT. An evaluation of tear film break-up time extension and ocular protection index scores among three marketed lubricant eye drops. US-based study. Cornea. 2007;26(8):949–52. doi: 10.1097/ICO.0b013e3180de1c38. [DOI] [PubMed] [Google Scholar]
- Patel S, Pérez-Santonja JJ, Alió JL, et al. Corneal sensitivity and some properties of the tear film after laser in situ keratomileusis. J Refract Surg. 2001;17:17–24. doi: 10.3928/1081-597X-20010101-02. [DOI] [PubMed] [Google Scholar]
- Salib GM, McDonald MB, Smolek M. Safety and efficacy of cyclosporine 0.05% drops versus unpreserved artificial tears in dry-eye patients having laser in situ keratomileusis. J Cataract Refract Surg. 2006;32:772–8. doi: 10.1016/j.jcrs.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–9. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Terry MA. Dry eye in the elderly. Drugs Aging. 2001;18(2):101–7. doi: 10.2165/00002512-200118020-00003. [DOI] [PubMed] [Google Scholar]
- Toda I, Asano-Kato N, Komai-Hori Y, et al. Dry eye after laser in situ keratomileusis. Am J Ophthalmol. 2001;132:1–7. doi: 10.1016/s0002-9394(01)00959-x. [DOI] [PubMed] [Google Scholar]
- Versura P, Profazio V, Campos EC. One month use of Systane® improves ocular surface parameters in subjects with moderate symptoms of ocular dryness. Clin Ophthalmoly. 2008;2(3):629–35. doi: 10.2147/opth.s3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Mochizuki H, Kawai M, et al. Fluorophotometric measurement of pH of human tears in vivo. Curr Eye Res. 1997;16:482–6. doi: 10.1076/ceyr.16.5.482.7050. [DOI] [PubMed] [Google Scholar]