Abstract
Uveitis is an inflammatory, putative Th1-mediated autoimmune disease that affects various parts of the eye and is a leading cause of visual loss. Currently available therapies are burdened with toxicities and/or lack definitive evidence of efficacy. Voclosporin, a rationally designed novel calcineurin inhibitor, exhibits a favorable safety profile, a strong correlation between pharmacokinetic and pharmacodynamic response, and a wide therapeutic window. The LUMINATE (Lux Uveitis Multicenter Investigation of a New Approach to TrEatment) clinical development program was initiated in 2007 to assess the safety and efficacy of voclosporin for the treatment, maintenance, and control of all forms of noninfectious uveitis. If LUMINATE is successful, voclosporin will become the first Food and Drug Administration-approved corticosteroid-sparing agent for this condition.
Keywords: voclosporin, calcineurin inhibitors, LX211, LUMINATE trials, ophthalmic diseases, uveitis, ISA247
Introduction
Uveitis, an inflammatory, Th1-mediated autoimmune disease affecting various parts of the eye, is a significant cause of visual loss. The condition accounts for 2.8% to 10% of all cases of blindness, including approximately 30,000 new cases of legal blindness in the United States each year (Nussenblatt 1990; Gritz and Wong 2004). A recent report estimates the incidence of uveitis at more than 50 cases per 100,000 person-years, with a prevalence of 115 per 100,000 persons (Gritz and Wong 2004).
Corticosteroids (topical, systemic, locally injected, or corticosteroid-eluting implants) are the current mainstay of therapy and the only Food and Drug Administration (FDA)-approved treatment for uveitis. Although often highly effective, chronic corticosteroid use, whether administered systemically or locally, is burdened with well-known, serious adverse events (AEs) including osteoporosis, metabolic disturbances, diabetogenicity and obesity, elevated blood pressure, and impaired wound healing. A number of currently available immunosuppressive agents (eg, antimetabolites, T-cell inhibitors, alkylating agents) are commonly employed as steroid-sparing therapies; however, randomized controlled clinical trials on the use of these agents in uveitis patients are broadly lacking (Jabs and Karamursel 2005). Moreover, many of these agents are associated with significant, serious AEs. Therefore, immunosuppressive agents with proven efficacy and an acceptable safety profile are a much needed addition to the therapeutic armamentarium for the management of this serious disease.
Voclosporin
Description/mechanism of action
Voclosporin (Lux Biosciences, Inc. Jersey City, NJ) is a rationally designed novel calcineurin inhibitor (Isotechnika, Inc., 2008) currently in phase 2/3 clinical development for noninfectious uveitis. The inventor of the compound, Isotechnika, Inc., (Edmonton, Canada), is concurrently developing the drug for plaque psoriasis and the prevention of allograft rejection (No authors listed 2007).
Voclosporin is a next-generation calcineurin inhibitor. Calcineurin inhibitors are potent immunosuppressants that reversibly inhibit T-cell proliferation and prevent release of proinflammatory cytokines by blocking the activity of the calcium-regulated serine-threonine phosphatase calcineurin, an enzyme found in cell cytoplasm (Schreiber and Crabtree 1992; Dumont 1996). Calcineurin inhibitors also block lymphokine production and release, fibroblast proliferation, and vascular endothelial growth factor expression (Dumont 1996; Ho et al 1996; Cho et al 2002). After entering the lymphocyte, calcineurin inhibitors bind intracellularly to immunophilins and form complexes that subsequently bind to and inhibit calcineurin (Schreiber and Crabtree 1992; Stalder et al 2003). This process prevents translocation to the nucleus of the cytoplasmic component of the nuclear factor of activated T cells (NFAT), which in turn impairs transcription of the genes encoding interleukin-2 (IL-2) and other lymphokines (Schreiber and Crabtree 1992; Ho et al 1996).
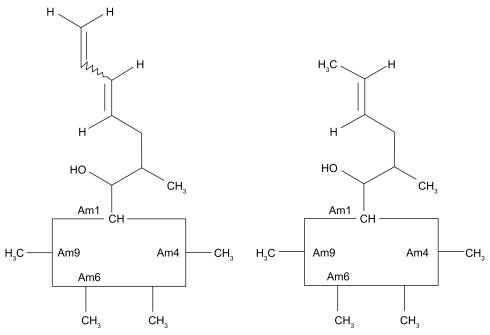
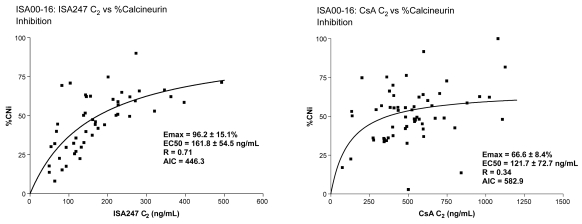
Voclosporin was developed by modification of the functional group on the amino acid residue at position 1 of the cyclosporin A (CsA) molecule (Figure 1) (Dumont 2004). The result is a more predictable pharmacokinetic profile than that of CsA (Yatscoff et al 2002; Abel et al 2003; Wasel et al 2006) and an approximately 4-fold greater inhibitory potency as measured by the in vitro calcineurin inhibition assay. (Abel et al 2004; Aspeslet et al 2004). Results of phase 1, phase 2, and phase 3 clinical trials indicate that voclosporin exhibits a well correlated pharmacokinetic/pharmacodynamic (PK/PD) relationship (Figures 2–3) (Yatscoff et al 2002; Abel et al 2003; Wasel et al 2006). The utility of systemically administered voclosporin for the prevention and treatment of experimental autoimmune uveoretinitis (EAU) in rats has been reported recently (Cunningham et al 2007).
Figure 1.
Comparison of structures of voclosporin (left) and cyclosporin A (right).
Figure 2.
Comparison of calcineurin inhibition of voclosporin versus CsA.
Abbreviations: AIC, Akaike information criterion; CsA, cyclosporin A; Emax, maximal effect.
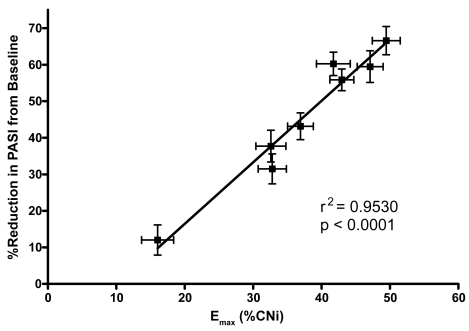
Figure 3.
Emax versus reduction in PASI in phase 3 psoriasis study.
Abbreviations: CNi, calcineurin inhibition; Emax, maximal effect; PASI, Psoriasis Area and Severity Index.
Clinical trials with voclosporin
The PK/PD profile of voclosporin has been evaluated in six phase 1 studies in healthy volunteers. Doses in single-dose studies ranged from 0.25 mg/kg to 4.5 mg/kg, and in multiple-dose studies, from 0.25 mg/kg bid to 1.5 mg/kg bid. Voclosporin exhibited good bioavailability, rapid absorption (median time to maximum [Tmax] < 2 hours), and first-order pharmacokinetics indicated by linear increases in area under the curve (AUC) and maximum concentration (Cmax) as a function of dose. Whole blood concentration profiles indicated multiphasic elimination after 24 hours. In a multiple-dose study of 0.25 mg/kg and 0.5 mg/kg bid for 10 days, the T1/2 was 6.9 to 7.8 hours on Day 1 and 30.1 to 33.2 hours on Day 13, indicating an approximate 4-fold accumulation of voclosporin whole blood levels on Day 13 (Isotechnika, Inc., 2008).
Voclosporin undergoes extensive first-pass hepatic metabolism via hydroxylation and N-demethylation reactions to active metabolites. Cytochrome P450 3A4/5 acts as the primary enzyme involved in phase I of the metabolism of voclosporin (Freitag et al 2006; Isotechnika, Inc., 2008). Voclosporin inhibits the activity of P450 3A4, but not CYP2D6 or CYP2C9. Based on animal studies, fecal excretion is the primary route of elimination. voclosporin should be taken on an empty stomach to ensure adequate drug concentration, based on results of a pharmacokinetic study that indicate a food effect on the rate and extent of drug absorption (Isotechnika, Inc., 2008).
Results of phase 1 trials indicate that the pharmacodynamic ability of voclosporin to inhibit calcineurin appears to be dose-proportional up to a maximum of 1 mg/kg bid for 10 days, after which the degree of inhibition plateaus (Isotechnika, Inc., 2008). The efficacy of voclosporin in organ transplantation and psoriasis has been reported and is indicative of the potential scope of the compound in the treatment of immune disorders, including noninfectious uveitis.
In a phase 2a, 12-week, randomized, multicenter, open-label study, the primary endpoints of a low incidence of acute rejection and stable kidney function were maintained in 132 stable, post–renal transplant patients who were switched from CsA (Neoral®) to voclosporin (Yatscoff et al 2003; Dumont 2004). Further, voclosporin produced a level of immunosuppression comparable to CsA at 33% of the blood drug concentration. Strong correlations were seen between C0 (concentration of drug in plasma at the time of an instantaneous intravenous injection of a drug that is instantaneously distributed to its volume of distribution) and both AUC and calcineurin inhibition for voclosporin, but not for CsA (Figure 2).
A 24-week, randomized, open-label, phase 2b trial in de novo renal transplant patients to compare the efficacy and safety of voclosporin and tacrolimus has recently completed enrollment. Starting doses were 0.4, 0.6, and 0.8 mg/kg bid for voclosporin and 0.05 mg/kg bid for tacrolimus, with doses titrated to target trough concentrations (Gaston et al 2006). In a recent safety update of the first 116 patients who completed ~4 months of therapy (range, 3.7 ± 2.2 to 4.5 ± 2.2 months), similar efficacy, as measured by biopsy-proven acute rejection episodes, was demonstrated between the two agents, as well as an acceptable safety profile. Excellent renal function was noted in all voclosporin dose groups (Busque et al 2007).
In a 24-week randomized trial in 451 patients with moderate to severe plaque psoriasis, a statistically significant difference for voclosporin vs placebo was seen at weeks 12 and 24 in the Psoriasis Area and Severity Index (PASI 75) response, as well as a 2-point reduction in the Static Global Assessment score (Gupta et al 2006; Langley et al 2006). Patients received placebo or voclosporin (0.2 mg/kg, 0.3 mg/kg, or 0.4 mg/kg bid) for 12 weeks, after which the placebo group was switched to voclosporin 0.3 mg/kg bid. Drug concentration was highly correlated with calcineurin inhibition (r = 0.79), and percent calcineurin inhibition was highly correlated with mean percent reduction in the efficacy endpoint (PASI; r = 0.86), illustrating the strong PK/PD correlation of voclosporin (Figure 3) (Gupta et al 2006).
Clinical safety of voclosporin
Voclosporin was well tolerated and safe in all single- and multiple-dose phase 1 trials in dosages up to 4.5 mg/kg, and in phase 2 and phase 3 trials in renal transplantation and psoriasis patients (Aspeslet et al 2001, 2004; Dumont 2004; Gaston et al 2006; Guenther et al 2006; Isotechnika, Inc., 2007a; No authors listed 2007). In phase 2 and 3 trials, the most common AEs, typically of low frequency and mild severity, were diarrhea, headache, and hypertension (Yatscoff et al 2003; Gaston et al 2006; Guenther et al 2006). These events appeared to be dose-dependent. No significant changes in levels of cholesterol, triglycerides, or other biochemical parameters, or in new-onset diabetes or infectious complications, have been reported in studies to date.
Renal function, assessed by creatinine clearance, remained stable in patients in clinical trials, with few experiencing clinically significant changes. Specifically, in the phase 3 study in psoriasis, the highest mean change in serum creatinine in any dosing group (0.2, 0.3, or 0.4 mg/kg bid) at week 12 was 5.6% above baseline, which is considered to be within normal analytic and physiologic variation (Langley et al 2006; Isotechnika, Inc., 2007b; No authors listed 2007). A total of 5 patients (4 [4%] in the high-dose and 1 [1%] in the mid-dose group) withdrew due to a clinically significant decrease in kidney function (No authors listed 2007) At 24 weeks, there were no clinically significant changes in mean serum creatinine or glomerular filtration rate (GFR), but 5 (2%) patients withdrew due to changes in renal function (30% decrease in GFR confirmed) (Papp et al 2004). After 60 weeks, mean serum creatinine remained stable, with clinically significant changes in kidney function occurring in 4% of patients (Isotechnika, Inc., 2007b).
Therapeutic window of voclosporin
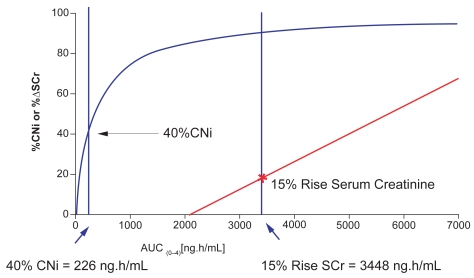
These safety data, combined with a predictable PK/PD relationship, suggest a broad therapeutic window for voclosporin. This is in stark contrast to the narrower therapeutic ranges of other calcineurin inhibitors, such as CsA and tacrolimus, which may require the use of therapeutic drug monitoring to correlate the parent substance and its metabolites with important clinical parameters (Staatz and Tett 2004; Vollenbroeker et al 2005). Voclosporin achieved a clinically relevant range of calcineurin inhibition (40% to 70%) in phase 1 and 2a trials. Within this therapeutic range, no significant changes in creatinine clearance, mean arterial blood pressure, or serum magnesium levels have been observed. An effect of voclosporin on creatinine clearance has only been observed at a drug exposure >100% of that required to achieve 70% calcineurin inhibition (2250 vs 900 ng*h/mL) (Yatscoff et al 2002). More recent data indicate that 40% calcineurin inhibition is achieved with voclosporin at an AUC of 226 ng*h/mL, but a 15% rise in serum creatinine (the lower threshold of clinical significance) occurs only at an voclosporin AUC of 3448 ng*h/mL (Figure 4) (Yatscoff et al 2002; Isotechnika, Inc., 2008).
Figure 4.
Voclosporin therapeutic window for renal toxicity.
Abbreviations: AUC, area under the curve; CNi, calcineurin inhibitor; SCr, serum creative.
LUMINATE clinical trials program
Study rationale and objective
Although a number of corticosteroid-sparing immunomodulatory agents have been utilized in the treatment of uveitis over the past 2 decades, voclosporin is the first to enter into pivotal clinical trials for FDA-approval. This landmark program is being conducted in North America, Europe, and India, and will enroll over 500 patients.
The LUMINATE program comprises three randomized, double-blind, placebo-controlled, phase 2/3 trials in patients with noninfectious, sight-threatening uveitis that is active intermediate-, anterior and intermediate-, posterior-, or pan-uveitis (Study 1, LUMINATE Active); quiescent intermediate-, anterior and intermediate-, posterior-, or panuveitis in patients requiring systemic immunosuppresssion for disease control (Study 2, LUMINATE Maintenance); or active anterior in patients requiring systemic immunosuppression for disease control (Study 3, LUMINATE Anterior) (Protocol 1, 2, 3). The study designs and procedures are highly consistent with the corticosteroid-tapering paradigm used widely in clinical practice.
Enrollment criteria
Table 1 summarizes selected inclusion and exclusion criteria for the three clinical trials. The primary enrollment criterion is the presence of noninfectious uveitis that is either uncontrolled despite systemic or periocular corticosteroid therapy, or that requires systemic or periocular corticosteroids and/or immunosuppressive agents for control (Protocol 1, 2, 3; Protocols 1–3 Amendments).
Table 1.
Selected inclusion and exclusion criteria for the LUMINATE clinical trials program (Protocol 1, p. 22–24; Protocol 2, p. 22–25; Protocol 3, p. 20–23)
| LUMINATE Active | LUMINATE Maintenance | LUMINATE Anterior | |
|---|---|---|---|
| Selected inclusion criteria | |||
| Documented history of noninfectious intermediate-, anterior and intermediate-, posterior-, or pan-uveitis | Diagnosis of noninfectious intermediate-, anterior and intermediate-, posterior-, or pan-uveitis of at least 3 months duration prior to enrollment, requiring treatment during that period to control intraocular inflammatory disease and avoid sight-threatening complications due to inflammation | Documented history of noninfectious anterior-, anterior and intermediate-, or pan-uveitis | |
| Uncontrolled uveitis, evidenced by Grade 2+ or higher vitreous haze in atleast one eye for ≥2 weeks prior to randomization | Minimum prescribed therapy upon enrollment is ≥1 of the following: | Uncontrolled uveitis, evidenced by Grade 2+ or higher anterior chamber cells in at least one eye for ≥2 weeks prior to randomization | |
| Current uveitis therapy must conform to ≥1 of the following: | Systemic prednisone or equivalent averaging ≥10 mg/day | In subjects whose diagnosis is not exclusively anterior uveitis, the predominant manifestation of their condition at the time of enrollment must be anterior segment inflammation | |
| Prednisone monotherapy at a dose of ≥10 mg/day (or equivalent) for ≥2 weeks prior to randomization | Received ≥2 intravitreal/periocular corticosteroid administrations for control of inflammatory disease within the past 8 months, but not within 6 weeks of randomization | Current uveitis therapy must conform to ≥1 of the following: | |
| Received ≥2 injections of corticosteroid (intravitreal or periocular) for control of disease within the past 8 months, but not within 2 weeks of randomization; subjects may be receiving systemic corticosteroids | Note: Subjects may be receiving topical corticosteroids for control of anterior inflammation during study | Prednisone monotherapy at a dose of ≥10 mg/day (or equivalent) for ≥2 weeks prior to randomization | |
| Subjects for whom corticosteroid therapy (oral or systemic) is medically inappropriate, or who refuse corticosteroid therapy | Subjects with clinically quiescent uveitis in both eyes at enrollment and who have been on a treatment regimen that has neither been intensified nor augmented for a minimum of 6 weeks | Received ≥2 injections of corticosteroid (intravitreal or periocular) for control of disease within the past 8 months, but not within 2 weeks of randomization; subjects may be receiving systemic corticosteroids | |
| Subjects who do not plan to undergo elective ocular surgery (eg, cataract extraction) during study | Considered by the investigator to require immunomodulatory therapy | Subjects for whom corticosteroid therapy (oral or systemic) is medically inappropriate, or who refuse corticosteroid therapy | |
| A minimum ability to count fingers at a distance of 1 meter, 30 centimeters using the study eye | Must have best corrected visual acuity (BCVA) in the worst involved eye of 20/400 or better (Early Treatment Diabetic Retinopathy Study [ETDRS] logMAR <1.34) | Subjects who do not plan to undergo elective ocular surgery (eg, cataract extraction) during study | |
| Considered by the investigator to require immunomodulatory therapy | Subjects who do not plan to undergo elective ocular surgery (eg, cataract extraction) during study | A minimum ability to count fingers at a distance of 1 meter, 30 centimeters using the study eye | |
| Must be ≥13 years of age and must weigh at least 38 kg (84 lbs) and no more than 110 kg (242 lbs) | Must be ≥13 years of age and must weigh at least 38 kg (84 lbs) and no more than 110 kg (242 lbs) | Considered by the investigator to require immunomodulatory therapy | |
| Must be ≥13 years of age and must weigh atleast 38 kg (84 lbs) and no more than 110 kg (242 lbs) | |||
| LUMINATE Active | LUMINATE Maintenance | LUMINATE Anterior | |
| Selected exclusion criteria | |||
| Uveitis of infectious etiology | ✓ | ✓ | ✓ |
| Clinically suspected or confirmed central nervous system or ocular lymphoma | ✓ | ✓ | ✓ |
| Primary diagnosis of anterior uveitis | ✓ | ✓ | |
| Uncontrolled glaucoma, evidenced by an intraocular pressure of >21 mmHg while on medical therapy, and chronic hypotony (<6 mmHg) | ✓ | ✓ | ✓ |
| Presence of an ocular toxoplasmosis scar | ✓ | ✓ | ✓ |
| Any implantable corticosteroid-eluting device (eg, Retisert™, Posurdex®, Medidur™, I-vation™ TA intravitreal implant) | ✓ | ✓ | ✓ |
| Treatment with an immune suppression regimen that includes an alkylating agent within the previous 90 days | ✓ | ✓ | ✓ |
| Treatment with a monoclonal antibody or any other biologic therapy within the previous 30 days, or with alemtuzumab within the previous 12 months | ✓ | ✓ | ✓ |
| Use of any drugs or substances known to be strong inhibitors of CYP 3A4/5 enzymes within 7 days of the first dose, or grapefruit juice and star fruit within 24 hours of the first dose | ✓ | ✓ | ✓ |
| Lens opacities or obscured ocular media upon enrollment such that reliable evaluations and grading of the posterior segment cannot be performed | ✓ | ✓ | |
| Evidence of active, uncontrolled noninfectious uveitis | ✓ | ||
| History or diagnosis of Behçet’s disease | ✓ | ||
| Local (periocular/intravitreal) administration of corticosteroids within the previous 6 weeks | ✓ | ||
Study endpoints
The primary efficacy assessments largely follow the SUN Working Group guidelines for measurement of ocular inflammation in clinical trials (Jabs et al 2005). Table 2 summarizes the primary and secondary endpoints for each study in the LUMINATE clinical trial program. The primary endpoint in LUMINATE Active is mean change from baseline in vitreous haze score, and in LUMINATE Anterior, mean change from baseline in anterior chamber cells. In LUMINATE Maintenance, the primary endpoint is inflammatory exacerbation, defined as a clinically significant deterioration from baseline of at least 2 grades in vitreous haze score and/or anterior chamber cells, and/or a deterioration in visual acuity of at least +0.3 LogMAR (Protocol 1, 2, 3; Protocol 1–3 Amendments). As secondary endpoints, all three trials examine changes in corticosteroid use, visual acuity, and quality of life.
Table 2.
Primary, secondary, and additional endpoints of the LUMINATE clinical trial program* (Protocol 1, p. 18; Protocol 2, p. 18; Protocol 3, p. 17)
| LUMINATE Active | LUMINATE Maintenance | LUMINATE Anterior |
|---|---|---|
| Primary endpoints | ||
| Mean change from baseline in graded vitreous haze after 16 weeks of therapy or at time of rescue, if earlier
Mean change from baseline in graded vitreous haze after 24 weeks of therapy or at time of rescue, if earlier |
The proportion of subjects experiencing inflammatory exacerbation during 26 weeks of treatment, as defined by a clinically significant deterioration in either eye for one or more of the following:
|
Mean change from baseline in graded AC cells after 16 weeks of therapy or at time of rescue, if earlier
Mean change from baseline in graded AC cells after 24 weeks of therapy or at time of rescue, if earlier |
| Secondary and additional endpoints | ||
| Proportion of subjects tapered to ≤5 mg/day of prednisone (or equivalent) by Week 16, and who do not require intensification or augmentation of therapy through Week 24 | Change from baseline in dose of oral corticosteroid at week 26 or at time of rescue, if earlier | Proportion of subjects tapered to ≤5 mg/day of prednisone (or equivalent) by Week 16, and who do not require intensification or augmentation of therapy through Week 24 |
| Mean change from baseline in BCVA using the ETDRS method at week 24 or subject’s last visit, if earlier | Change from baseline in BCVA at week 26 or at time of rescue, if earlier | Mean change from baseline in graded vitreous haze after 16 and 24 weeks of therapy or at time of rescue, if earlier, in the subset of subjects entering the study with a diagnosis of either panuveitis or anterior and intermediate uveitis |
| Mean change from baseline in thickness of the macula, as measured by OCT, at Week 24 or at subject’s last visit, if earlier | Change from baseline in QoL, NEI VFQ-25, EQ-5D, and SF-36, at week 26 or at time of rescue, if earlier | Mean change from baseline in BCVA using the ETDRS method at 24 weeks of therapy or at time of rescue, if earlier |
| Mean change from baseline in graded AC cells after 16 weeks of therapy or at time of rescue, if earlier, in the subset of subjects entering the study with a diagnosis of either panuveitis or anterior and intermediate uveitis | Time to occurrence of deterioration from baseline of ≥2 grades of vitreous haze during 26 weeks of therapy | Change from baseline in QoL, NEI VFQ-25, EQ-5D, and SF-36 |
| Mean change from baseline in graded AC cells after 24 weeks of therapy or at time of rescue, if earlier, in the subset of subjects entering the study with a diagnosis of either panuveitis or anterior and intermediate uveitis | Time to occurrence of deterioration from baseline in graded AC cells during 26 weeks of therapy in the subset of patients entering the study with a diagnosis of either panuveitis or anterior and intermediate uveitis | Change from baseline in thickness of macula at 24 weeks of therapy or at time of rescue, if earlier, as confirmed by OCT |
| Mean change from baseline in daily prednisone dose at 24 weeks or at time of rescue, if earlier | Time to occurrence of inflammatory exacerbation during 26 weeks of therapy | Mean change from baseline in graded AC flare at 16 and 24 weeks of therapy or at time of rescue, if earlier |
| Mean change from baseline in thickness of the macula, as measured by OCT, at Week 16 | Extension of an existing lesion and/or occurrence of new lesions requiring treatment | |
| Change from baseline in area of macular hyperfluorescence as measured by FA | ||
| Change from baseline in QoL, NEI VFQ-25, EQ-5D, and SF-36 | ||
| Change from baseline in presence and/or severity of ocular exam inflammatory abnormalities | ||
Note: All relevant measures in each study will be analyzed for the other eye.
Abbreviations: AC, anterior chamber; BCVA, best corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; EQ-5D, EuroQoL-5 domains; FA, fluorescein angiography; NEI VFQ-25, National Eye Institute Visual Functioning Questionnaire; OCT, optical coherence tomography; QoL, quality of life; SF-36, 36-Item Short Form Health Survey.
Study design/methodology
All studies are phase 2/3 prospective, multicenter, double-masked, placebo-controlled, parallel-group, randomized trials in patients ≥13 years of age. Enrollment at approximately 60 sites in North America, Europe, and India is expected to range from 100 for LUMINATE Anterior to approximately 210 and 220, respectively, for LUMINATE Active and LUMINATE Maintenance. In each study, patients will be randomized in a 2:2:2:1 ratio to 1 of 4 treatments: voclosporin at a dose of 0.2, 0.4, or 0.6 mg/kg bid, or placebo (Protocol 1, 2, 3).
Patients will be assessed at baseline and at weeks 2, 4, 8, 12, 16, 20, and 24 with an optional 24 week extension in the LUMINATE Active and Anterior studies; in the LUMINATE Maintenance study, assessments will be made at baseline and at weeks 2, 6, 10, 14, 18, 22, and 26 with an optional 24 week extension. Assessments include best corrected visual acuity, slit lamp exam and tonometry, dilated fundus exam, graded assessments of vitreous haze and AC cells, quality of life, and selected laboratory assessments that include CBC with differential, serum chemistry, lipid profile, and renal and liver function tests. Optical coherence tomography will be performed in the LUMINATE Active and Anterior studies while fluorescein angiography will be performed only in the former. Endothelial cell density (ECD) will be measured as a safety parameter in the LUMINATE Maintenance study (Protocols 01, 02, 03 – p12). All three trials include a standardized algorithm for tapering systemic and topical corticosteroids (Protocol 1, 2, 3).
Study treatment will be discontinued and rescue therapy administered if (1) patients with active noninfectious uveitis experience either a deterioration of at least 1 grade in vitreous haze or AC cells at week 4 or show no improvement from baseline by week 8, or (2) patients with quiescent noninfectious uveitis experience a study endpoint (ie, inflammatory exacerbation) (Protocol 1, 2, 3).
Rationale for dose selection
Preclinical and clinical data suggest that approximately 50% calcineurin inhibition (achieved with voclosporin at a dosage of 0.4 mg/kg bid) leads to a meaningful clinical effect (Aspeslet et al 2004; Gupta et al 2006). This was demonstrated in a recently conducted randomized double-blind placebo-controlled study of patients with plaque psoriasis in which voclosporin (0.4 mg/kg bid) produced 50% calcineurin inhibition and proved to be both effective and well tolerated. A dosage of 0.2 mg/kg in that study was associated with 30% calcineurin inhibition and found to be subtherapeutic (Gupta et al 2006; Langley et al 2006).
PK/PD modeling of calcineurin inhibition suggests that bracketing the 0.4 mg/kg bid dosage with dosages of 0.2 mg/kg bid and 0.6 mg/kg bid would offer a sufficiently wide interval for dose ranging, resulting in approximately 30% to 70% calcineurin inhibition (Protocol 1, 2, 3). This dose ranging will allow investigation of any correlation between the amount of drug administered and the impact on various parameters of uveitis (eg, inflammation, macular thickening) and, ultimately, on clinical efficacy.
Additional protocol features
Optical coherence tomography
The potential effect on retinal macular thickness by voclosporin will be evaluated by means of optical coherence tomography (OCT) in the LUMINATE Active and Anterior studies. Baseline measurement in addition to measurements over the course of the trials will be undertaken.
Fluorescein angiography
Macular hyperfluorescence will be evaluated in the LUMINATE Active study as assessed by fluorescein angiography.
Quality of life
Assessments will be performed in the LUMINATE program to examine the potential effect of treatment of uveitis with voclosporin on quality of life (QoL). Among the instruments to be used are the National Eye Institute Visual Functioning Questionnaire, the Euro QoL-5, and the Short Form Health Survey.
Conclusions: Potential impact of LUMINATE findings
The LUMINATE program possesses several unique design features that will make it a landmark among clinical trials of uveitis therapies. It is the first pivotal-stage program undertaken for marketing approval of a corticosteroid-sparing agent for the treatment, control, and maintenance of all forms of noninfectious uveitis. It is also the first program to examine the correlation between dose and effects on various parameters of uveitis, including inflammation, vision, macular thickness, and angiographic changes.
If the LUMINATE program is successful, voclosporin will become the first steroid-sparing immunosuppressive drug approved by the FDA for the treatment of uveitis.
These findings could have significant implications for other ophthalmic diseases that are characterized by inflammatory mechanisms (eg, dry eye syndrome, age-related macular degeneration, and diabetic retinopathy) and vascular leakage (eg, age-related macular degeneration and diabetic macular edema).
In summary, if voclosporin proves to be an effective and safer treatment for noninfectious uveitis, inflammation will be controlled, visual acuity will be maintained, and the need to take corticosteroids will be reduced or eliminated. Voclosporin could thus represent a new standard of care offering hope for patients with this devastating condition.
Footnotes
Disclosures
Eddy Anglade and Sidney Weiss are founders and shareholders of Lux Biosciences, Inc; Launa Aspeslet is a shareholder of Isotechnika, Inc.
References
- ISA 247: trans-ISA 247, trans-R 1524, ISA(TX)247, ISAtx 247, ISATx247, LX 211, LX211, R 1524, R-1524. Drugs R D. 2007;8:103–12. doi: 10.2165/00126839-200708020-00005. [No authors listed] [DOI] [PubMed] [Google Scholar]
- Abel MD, Aspeslet LJ, Foster RT, et al. Proceedings of the International Association of Therapeutic Drug Monitoring. Basel; Switzerland: 2003. ISA247 A new generation calcineurin inhibitor [abstract] [Google Scholar]
- Abel M, Muller F, Freitag D, et al. 3D structure of ISA247 correlates with improved cyclophilin binding and calcineurin inhibition [abstract]. Proceedings of the 3rd International Congress on Immunosuppression; San Diego, CA, USA. 2004. p. 150489. [Google Scholar]
- Aspeslet L, Freitag D, Trepanier D, et al. ISA(TX)247: a novel calcineurin inhibitor. Transplant Proc. 2001;33:1048–51. doi: 10.1016/s0041-1345(00)02325-3. [DOI] [PubMed] [Google Scholar]
- Aspeslet L, Freitag D, Huizinga RB, et al. Pharmacokinetics-pharmacodynamics and safety of Trans-ISA247: A novel immunosuppressant [abstract]. Proceedings of the 3rd International Congress on Immunosuppression; San Diego, CA, USA. 2004. p. 150367. [Google Scholar]
- Busque S, Laftavi M, Gaston R, et al. ISA247: Preliminary results of a phase IIb multicentre, de novo renal transplant trial [abstract] Proceedings of the American Transplant Congress May 6, 2007. 2007:49. [Google Scholar]
- Cho Ml, Cho CS, Min SY, et al. Cyclosporine inhibition of vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Arthritis Rheum. 2002;46:1202–9. doi: 10.1002/art.10215. [DOI] [PubMed] [Google Scholar]
- Cunningham MA, Li Z, Chan CC, et al. Subcutaneous injections of LX211 prevent and reverse experimental autoimmune uveoretinitis in rats [poster]. Proceedings of the Association for Research in Vision and Ophthalmology Annual Meeting; May 6th–10th; Fort Lauderdale, FL, USA. 2007. p. B730. [Google Scholar]
- Dumont FJ. Cyclosporine A and tacrolimus (FK-506) immunosuppression through immunophilin-dependent inhibition of calcineurin function. In: Lieberman R, Mukherjee A, editors. Principles of Drug Development in Transplantation and Autoimmunity. Vol. 1. New York: Chapman and Hall; 1996. pp. 175–205. [Google Scholar]
- Dumont FJ. ISAtx-247 (Isotechnika/Roche) Curr Opin Investig Drugs. 2004;5:542–50. [PubMed] [Google Scholar]
- Freitag D, Mayo P, Aspeslet L, et al. The novel immunosuppressant ISA247 demonstrates a different metabolic profile than cyclosporine A in vitro and in vivo [abstract]. Proceedings of the World Transplant Congress; July 22nd–27th; Boston, MA, USA. 2006. p. 2434. [Google Scholar]
- Gaston R, Busque S, Cantarovich M, et al. for the PROMISE Study Investigators. ISA247: A novel calcineurin inhibitor (CNI). A promising safety profile with enhanced efficacy [poster]. Proceedings of the American Society of Nephrology,; San Diego, CA, USA. 2006. [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Guenther L, Barber K, Vender R, et al. ISA247 Remains a safe and efficacious agent after 48 weeks of continuous therapy: interim results of the SPIRIT Extension Study [abstract]. Proceedings of the American 15th Congress of the European Academy of Dermatology and Venereology; October 4th–8th; Rhodes, Greece. 2006. p. 950670. [Google Scholar]
- Gupta A, Tomi Z, Kunynetz R, et al. Pharmacokinetics and pharmacodynamics of ISA247 in a Phase III, randomized, multicentre, double-blind, placebo-controlled study [abstract]. Canadian Dermatology Association 81st Annual Conference; June 27th–July 2nd; Winnipeg, Canada. 2006. [Google Scholar]
- Ho S, Clipstone N, Timmermann L, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80(3 Pt 2):S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Isotechnika, Inc. Data on file. Isotechnika, Inc.: Edmonton, Canada; 2008. [Google Scholar]
- Isotechnika, Inc. . Phase I study summaries. [Accessed July 8, 2007];2007a URL: http://www.isotechnika.com/in_development/isa247/how/
- Isotechnika, Inc. [Accessed July 8, 2007];Phase 3 Canadian Psoriasis Study. 2007b URL: http://www.isotechnika.com/in_development/isa247/psoriasis.
- Jabs A, Karamursel E. Immunosuppression for posterior uveitis. Retina. 2005;25:1–18. doi: 10.1097/00006982-200501000-00001. [DOI] [PubMed] [Google Scholar]
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- Langley R, Bissonnette R, Searles G, et al. 24 Week results of a Phase III randomized, double-blind, multicentre, placebo-controlled study of ISA247 in plaque psoriasis [abstract]. Canadian Dermatology Association 81st Annual Conference; June 27th–July 2nd; Winnipeg, Canada. 2006. [Google Scholar]
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- Papp K, Langley R, Bissonnette R, et al. A Phase III, randomized, multicenter, double-blind, placebo-controlled study of ISA247 in plaque psoriasis patients [abstract] J Am Acad Dermatol. 2004;54(Suppl 1):P33. doi: 10.1016/j.jaad.2005.10.061. [DOI] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK-506. Gr Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- Stalder M, Birsan T, Hubble RW, et al. In vivo evaluation of the novel calcineurin inhibitor ISATX247 in non-human primates. J Heart Lung Transplant. 2003;22:1343–52. doi: 10.1016/s1053-2498(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Vollenbroeker B, Koch JH, Fobker M, et al. Determination of cyclosporine and its metabolites in blood via HPLC-MS and correlation to clinically important parameters. Transplant Proc. 2005;37:1741–4. doi: 10.1016/j.transproceed.2005.03.149. [DOI] [PubMed] [Google Scholar]
- Wasel N, Gupta A, Tomi Z, et al. Pharmacokinetics and pharmacodynamics of ISA247 in a Phase III randomized, multicenter, double-blind, placebo-controlled study [abstract] J Am Acad Dermatol. 2006;54(Suppl 1):P36. [Google Scholar]
- Yatscoff RW, Broski AP, Abel MD, et al. Phase 2 trial results of ISATX247, a novel calcineurin inhibitor with a therapeutic window [abstract]. Proceedings of the IXI International Congress of The Transplantation Society; Miami, FL, USA. 2002. p. 0471. [Google Scholar]
- Yatscoff RW, Abel MD, Aspeslet LJ, et al. Phase 2 randomized, multicenter, open-label study of ISA247 and Neoral in post-renal transplant patients [abstract]. Proceedings of the American Transplant Congress; Washington DC, USA. 2003. p. 1215. [Google Scholar]