Abstract
Device satisfaction and preference are important patient-reported outcomes to consider when choosing inhaled therapy. A subset of adults (n = 153) with moderate or severe asthma participating in a randomized parallel-group, double-dummy trial that compared the efficacy and safety of 12 weeks’ treatment with budesonide delivered via Respimat® Soft Mist™ Inhaler (SMI) (200 or 400 μg bd) or Turbuhaler® dry powder inhaler (400 μg bd), completed a questionnaire on patient device preference and satisfaction (PASAPQ) as part of a psychometric validation. As the study used a double-dummy design to maintain blinding, patients used and assessed both devices, rating their satisfaction with, preference for, and willingness to continue using each device. The mean age of patients was 41 years, 69% were female and the mean duration of disease was 16 years. Total PASAPQ satisfaction scores were 85.5 and 76.9 for Respimat® SMI and Turbuhaler® respectively (p < 0.0001); 112 patients (74%) preferred Respimat® SMI and 26 (17%) preferred Turbuhaler®. Fourteen subjects (9%) indicated no preference for either inhaler. Willingness to continue using Respimat® SMI was higher than that for Turbuhaler® (mean scores: 80/100 and 62/100, respectively). Respimat® SMI was preferred to Turbuhaler® by adult asthma patients who used both devices in a clinical trial setting.
Keywords: asthma, Respimat® Soft Mist™ Inhaler, Turbuhaler®
Introduction
A patient’s satisfaction with and preference for an inhaler device are important outcomes to consider when choosing inhaled therapy for patients with asthma and chronic obstructive pulmonary disease (COPD). Patient satisfaction is important in determining whether patients persist with medication,1 and has been shown to be influenced by such things as the process of taking or using the product and its subsequent outcomes.2 Among healthcare professionals, both the ease of use and patient preference for an inhaler device are regarded as amongst the most important considerations when selecting an inhaler for patients.3,4
Using inhaler devices to treat obstructive lung diseases is by no means a straightforward process for patients, who must learn how to prepare and operate them before being able to take the medication as prescribed. Furthermore, it is also clear that not all patients can use all inhalers equally well. They clearly vary in their preferences for different inhalers5,6 and frequently do not see devices as being interchangeable. Although a large range of inhaler devices is now available, established devices such as the pressurized metered-dose inhaler (pMDI) and the dry powder inhaler (DPI) are still the most frequently used. The pMDI is robust and can be simple to use, although many patients have trouble using it correctly, particularly in co-ordinating the actuation of the device with inhalation,7,8 a disadvantage that prompted the development of breath-actuated pMDIs. With DPIs, the co-ordination challenge can be avoided, but a minimum inspiratory flow is required which may be limiting for some patients, particularly young children,9 and some multidose designs need to be stored in a dry place to prevent moisture degrading the powder. One popular multidose design is the Turbuhaler®, which has been shown to deliver a high proportion of the inhaled dose in the form of fine particles.10
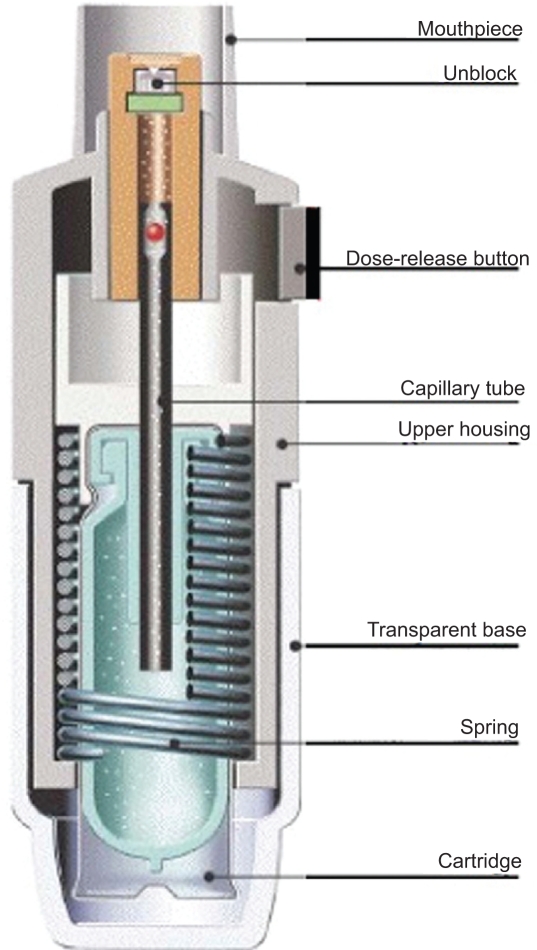
Respimat® Soft Mist™ Inhaler (SMI) (Figure 1) is a new type of propellant-free inhaler that generates a fine aerosol cloud which is emitted more slowly and lasts 4 to 10 times longer than the aerosol from pressurized metered-dose inhalers (pMDIs).11 This device has been shown to deliver a higher proportion of the emitted dose to the lungs than a pMDI,12–14 and this allows patients to take a lower nominal dose of bronchodilator (relative to pMDI) without affecting efficacy or safety, in both asthma and COPD.15–17 Lung deposition from Respimat® SMI was also found to be greater than from Turbuhaler® in asthma patients.14 In a controlled clinical trial, COPD and asthma patients found Respimat® SMI easy to use and preferred it to a hydrofluoroalkane (HFA)-propelled MDI.6
Figure 1.
Schematic diagram of Respimat® Soft Mist™ Inhaler. Courtesy of Boehringer Ingelheim GmbH.
The objective of the current study was to compare the level of patient satisfaction with Respimat® SMI and Turbuhaler®. This analysis was performed on a subset of patients who had participated in a multicenter clinical trial (Boehringer-Ingelheim study #1047.16 – data on file). The vast majority of patients studied were familiar with and using the pMDI, only a minority having had prior experience with the Turbuhaler® and none having used Respimat® SMI before. In the original clinical trial, all subjects were instructed in the proper use of the Respimat® SMI, Turbuhaler®, and pMDI devices. Performance and convenience of the devices were assessed by means of a questionnaire specifically designed to measure patient satisfaction with inhaler devices (Patient Satisfaction and Preference Questionnaire; PASAPQ).18 In the original trial, peak expiratory flow was the primary clinical endpoint and no clinically significant difference was observed between test groups at end of study.
Methods
Study design
The study presented in this article was undertaken in a subset of adults with clinically stable moderate or severe asthma who had taken part in a randomized, parallel-group, placebo-controlled, double-blind, multicenter clinical trial. The subset chosen for the current analysis consisted of 153 English-speaking and German-speaking patients (from study centers in Canada, Germany and South Africa), selected because the PASAPQ questionnaire was only available in those languages. The aim of the primary trial was to compare the efficacy and safety of 12 weeks’ treatment with budesonide delivered either via Respimat® SMI or Turbuhaler®. The efficacy and safety results of the clinical trial are reported separately (Boehringer-Ingelheim, study #1047.16 – data on file).
As the Respimat® SMI and Turbuhaler® are different in appearance, a double-dummy design was used; thus, patients used both devices for the full duration of the trial. All patients were randomly allocated to study treatment with budesonide for 12 weeks according to one of the three regimens shown in Table 1. For all regimens, patients took 2 inhalations twice daily from both of the devices, resulting in total daily budesonide dosages of either 400 or 800 μg. The order in which patients used the two inhalers on each day of the study was determined by a separate random allocation step.
Table 1.
Treatment arms in the clinical study showing the double-dummy design
| Treatment arm | Respimat®SMI | Turbuhaler® |
|---|---|---|
| 1 | Budesonide 100 μg per puff | Placebo |
| 2 | Budesonide 200 μg per puff | Placebo |
| 3 | Placebo | Budesonide 200 μg per puff |
Patients
To be enrolled into the screening phase of the clinical study, men and women aged 18–65 years had to have a diagnosis of moderate or severe asthma of at least 6 months’ duration, be a non-smoker or ex-smoker (stopped at least 1 year before screening and with a history of no more than 10 pack-years), and be receiving either a) high-dose inhaled corticosteroids (ICS) plus short-acting beta2-agonist as needed, with or without other asthma medications such as long-acting beta2-agonists, or b) low-dose ICS plus either inhaled long-acting beta2-agonists or oral xanthines.
During a run-in period of 1–3 weeks, ICS dosage was standardized to beclomethasone dipropionate 400 μg/day delivered via pMDI, plus salbutamol via pMDI for rescue use as needed.
To enter the study itself (randomized treatment phase), patients had to fulfill a second set of criteria based on spirometry and record card data. Forced expiratory volume in 1 second (FEV1) had to be 50% to 81% of predicted and either FEV1 or peak expiratory flow (PEF) had to be at least 10% lower than the screening visit value. In addition, at least one of the following diary card criteria had to be met (for at least 2 out of 7 days, based on the most recent 7 consecutive days of the run-in period): morning PEF ≤ 80% predicted; diurnal PEF variability ≥ 20%; ≥ 6 inhalations/day of beta2-agonist, and asthma symptom scores of ≥2 (day) or ≥1 (night).
Assessments
At the end of the 12-week treatment period, all patients rated the two devices using the Patient Satisfaction and Preference Questionnaire (PASAPQ). The PASAPQ is a multi-item measure of satisfaction and preference with inhaler devices that is designed to be easy to understand and administer to asthma and chronic obstructive pulmonary disease (COPD) patients. The items were developed using literature search, focus groups and expert opinion, and the resultant questionnaire has been confirmed to have validity, reliability and responsiveness in psychometric analyses.18
The PASAPQ is self-administered and contains 13 satisfaction items grouped into two domains, performance and convenience, that together constitute the total score (Table 2). These items are measured on a Likert-type response scale from 1 (very dissatisfied) to 7 (very satisfied). The three other items are an overall satisfaction question, also measured on the 1 to 7 scale, a question on preference (selection between devices, as well as a “no preference” option) and a question on willingness to continue using the device in the future, measured on scale of 0 (not willing) to 100 (definitely willing). As this study provided data for the psychometric analyses, 46 additional questions were included for these purposes,18 but these did not contribute to the total or domain PASAPQ scores reported in this paper.
Table 2.
Items in Patient Satisfaction and Preference Questionnaire (PASAPQ)
| Device attribute | Question | Scoring |
|---|---|---|
| Performance | Overall feeling of inhaling
Inhaled dose goes to lungs Amount of medication left Works reliably Ease of inhaling a dose Using the inhaler Speed medicine comes out |
Each contributes equally to total PASAPQ score |
| Convenience | Instructions for use
Size of inhaler Durability of inhaler Ease of cleaning inhaler Ease of holding during use Convenience of carrying |
|
| Other | Overall satisfaction
Preference Willingness to continue |
Stand alone questions (each scored independently) |
Domain and total scores are first summed and then transformed to scores on a scale from 0 (least) to 100 (most) for patients who complete at least half of the questions. If the patient completes at least half of the items in a domain, values for missing items are imputed using the mean of the completed items in that domain (the “half-scale rule”).19 The total score can be calculated only when both domain scores have computable scores and is calculated as the sum of the 13 items after substitution for missing items at the domain level has taken place. In other research,18 estimates of the minimal important difference (MID) for the PASAPQ had demonstrated that a score difference between test inhalers of 8 to 10 points on the domain and total scores represented a medium difference in effect, and a difference of 3 to 6 points represented a small difference in effect, depending on which domain was measured. For the current study, therefore, a score difference of >10 points was considered to represent an important difference, and the number of patients who scored one inhaler higher than the other by >10 points was recorded for each domain separately and for the total PASAPQ score.
For half of the patients, the questionnaires had Respimat® SMI listed first and for the other half, the Turbuhaler® device was listed first, the order of device listing being randomly allocated.
Statistical analysis
In addition to descriptive statistics, the mean PASAPQ satisfaction scores and willingness-to-continue ratings (the latter expressed as least squares means) were tested for statistically significant differences between the two devices using a SAS general linear model with a nested design. Comparisons of individual items were tested using a paired t-test. Difference in preference was analyzed using a chi-square test. The 5% level of significance was used (two-tailed test).
Results
Patient demographics and PASAPQ response rates
The mean age of patients in the subset was 41.0 years (standard deviation [SD], 11.5 years), 106 (69.3%) were women and the mean duration of asthma was 16.1 years (SD, 12.5 years). Before entering the trial, 30 patients had previously received inhaled treatment via Turbuhaler® and no patients had used Respimat® SMI. In the original data set, 306 subjects responded to the PASAPQ and 299 completed the full questionnaire (7 subjects left at least one item missing but had computable scores). There were no unusable questionnaires. For the purposes of the comparison between the Respimat® SMI and Turbuhaler®, PASAPQ satisfaction scores were calculated for all 153 patients in the relevant subset, of whom 152 responded to the preference question.
Satisfaction with device performance and convenience
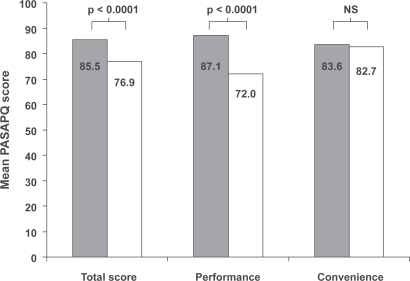
For the total PASAPQ score and the performance domain score, mean scores for Respimat® SMI were significantly higher than those for Turbuhaler® (p < 0.0001, general linear models analysis). The mean scores for the convenience domain also showed a numerical superiority for Respimat® SMI, but the difference between devices was not statistically significant (Figure 2). Analysis by country also showed total PASAPQ score and performance domain score to be significantly higher for Respimat® SMI in each case (data not shown).
Figure 2.
Mean patient device preference and satisfaction (PASAPQ) scores for each device (all countries combined), with range of scores transformed to 0 to 100 points; p values for difference between devices from general linear models analysis. Grey bars, Respimat® SMI; white bars, Turbuhaler®.
Abbreviations: NS, not significant.
Comparison of scores by item showed that in the performance domain, mean scores for Respimat® SMI for all questions were significantly higher than those for Turbuhaler® (p < 0.0001, paired t-test) with mean differences between device scores ranging from 0.6 to 1.4. In the convenience domain, the mean differences between device scores were smaller (range, −0.2 to 0.2) and generally not of statistical significance, with the difference favoring Respimat® SMI for four items and Turbuhaler® for two (Table 3).
Table 3.
Satisfaction with inhaler attributes in performance and convenience domains
| Domain and inhaler attribute |
Respimat®SMI |
Turbuhaler® |
Difference, Respimat®−Turbuhaler® |
||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | p value1 | |
| Performance domain | |||||||
| Overall feeling of inhaling the medicine | 153 | 6.0 (1.3) | 153 | 5.2 (1.6) | 153 | 0.8 (2.0) | <0.0001 |
| Feeling that the inhaled dose goes to the lungs | 153 | 6.3 (1.2) | 153 | 4.9 (1.8) | 153 | 1.4 (2.0) | <0.0001 |
| Can tell amount of medication left in container | 153 | 6.0 (1.4) | 153 | 4.7 (1.8) | 153 | 1.3 (1.9) | <0.0001 |
| Inhaler works reliably | 153 | 6.4 (0.9) | 153 | 5.5 (1.6) | 153 | 1.0 (1.7) | <0.0001 |
| Ease of inhaling a dose | 153 | 6.3 (1.1) | 152 | 5.7 (1.5) | 152 | 0.7 (1.7) | <0.0001 |
| Using the inhaler | 151 | 6.3 (1.1) | 151 | 5.7 (1.5) | 151 | 0.6 (1.6) | <0.0001 |
| Speed of medication coming out of inhaler | 151 | 6.3 (1.1) | 151 | 5.6 (1.5) | 151 | 0.7 (1.8) | <0.0001 |
| Convenience domain | |||||||
| Instructions for using the inhaler | 152 | 6.1 (1.1) | 152 | 6.0 (1.2) | 152 | 0.1 (0.9) | 0.0893 |
| Size of the inhaler | 153 | 5.7 (1.4) | 153 | 5.9 (1.2) | 153 | −0.2 (0.9) | 0.0045 |
| Durability of the inhaler | 153 | 6.3 (1.0) | 153 | 6.1 (1.2) | 153 | 0.2 (0.9) | 0.0096 |
| Ease of cleaning the inhaler | 153 | 5.9 (1.1) | 153 | 5.8 (1.2) | 153 | 0.1 (0.8) | 0.0881 |
| Ease of holding inhaler during use | 151 | 6.3 (1.0) | 151 | 6.1 (1.0) | 151 | 0.2 (1.0) | 0.0064 |
| Overall convenience of carrying inhaler | 151 | 5.8 (1.3) | 151 | 5.9 (1.2) | 151 | −0.1 (0.9) | 0.2462 |
| Overall satisfaction with inhaler | 151 | 6.2 (1.1) | 151 | 5.6 (1.6) | 151 | 0.6 (1.7) | <0.0001 |
By paired t-test.
Abbreviations: SD, standard deviation; SMI, soft mist inhaler.
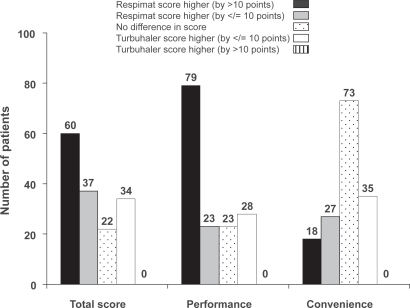
For the total PASAPQ satisfaction score, 60 patients (39.2%) gave a higher score for Respimat® SMI than for Turbuhaler® that also exceeded the pre-defined threshold of 10 points representing an important difference. For the performance and convenience domains, the corresponding patient numbers were 79 (51.6%) and 18 (11.8%). This pattern of results was similar across the three countries (data not shown). No patients gave a score for Turbuhaler® that exceeded the Respimat® SMI score by more than 10 points (Figure 3).
Figure 3.
Distribution of score differences between inhalers, showing the number of patients for whom differences met the pre-defined criterion for an important difference (>10 points).
Preference and willingness to continue
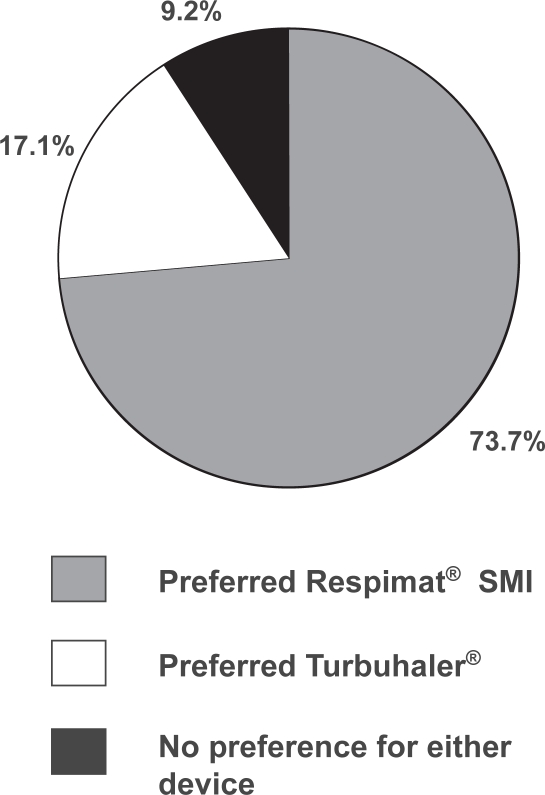
Respimat® SMI was preferred by 112 of 152 patients (73.7%) and Turbuhaler® preferred by 26 (17.1%), while 14 patients (9.2%) expressed no preference for either inhaler (Figure 4). When these proportions were restated with the subset of patients who indicated a preference for one device as the denominator, 81.2% preferred Respimat® SMI and 18.8% preferred Turbuhaler®, a difference that was statistically significant (p < 0.0001). In the small group of patients who had used Turbuhaler® previously, preference for Respimat® SMI was still markedly higher than for Turbuhaler®, with 20% of patients expressing no preference (Table 4).
Figure 4.
Proportion of patients (n = 152) indicating overall preference for Respimat® SMI and Turbuhaler® (or no preference).
Table 4.
Analysis of preference for device according to previous use of Turbuhaler®
|
Number preferring each device (n, %) |
||||
|---|---|---|---|---|
| Respimat®SMI preferred | Turbuhaler®preferred | No preference | Total | |
| Previous use of Turbuhaler® | 20 (66.7%) | 4 (13.3%) | 6 (20%) | 30 (100%) |
| No previous use of Turbuhaler® | 92 (75.4%) | 22 (18.0%) | 8 (6.6%) | 122 (100%) |
The least squares mean score on the willingness-to-continue question for Respimat® SMI was 79.9, significantly higher than the mean score for Turbuhaler® (61.8; p < 0.0001).
Discussion
In a group of patients with moderate or severe asthma who had just completed a clinical trial, satisfaction with device performance was significantly higher with Respimat® SMI than with Turbuhaler® as measured by the PASAPQ. Patient satisfaction with the convenience of the two devices was similar. When patients were asked which device they preferred, just over 80% of those who expressed a definite preference preferred Respimat® SMI.
Our findings are consistent with those of a randomized crossover study in which 224 COPD and asthma patients who were regular users of pMDIs received combination bronchodilator therapy (ipratropium plus fenoterol) via Respimat® SMI and pMDI for 7 weeks each.6 In that trial, as in our analysis, both total PASAPQ satisfaction score and performance domain score were significantly higher for Respimat® SMI, patient preference significantly favored Respimat® SMI (72%, versus 17% for pMDI) and patients were significantly more willing to continue using Respimat SMI after the study than pMDI.6
The aerosol produced by Respimat® SMI is generated mechanically by a spring when the user actuates the inhaler by pressing the dose-release button. Co-ordination of actuation with the patient’s inspiratory breath is an easier task than with the standard pMDI, because the aerosol cloud from Respimat® SMI is emitted more slowly and
so is longer-lasting (1.5 seconds, compared with 0.15 to 0.36 seconds for typical pMDIs).11 No co-ordination of actuation with inhalation is necessary with the Turbuhaler®, but as with other DPIs, generation of an aerosol from the device relies on the energy of the patient’s own inspiratory effort. This would not normally pose a difficulty to an adult asthma patient with stable disease, even if he or she was experiencing bronchoconstriction.20 Nevertheless, a comparison of scores for individual items in our study suggested that the difference in aerosol production method between the test devices was apparent to patients. This comparison also confirmed that for all aspects of device performance, patients were significantly more satisfied with Respimat® SMI than Turbuhaler®. The largest score differences were for the “feeling that the inhaled dose goes to the lungs” and the ability to tell how much medication remains in the inhaler. Other attributes that scored significantly better for Respimat® SMI included the overall feeling of inhaling, the ease of inhaling a dose and the speed at which medication comes out of the inhaler. There was much less difference between the two devices in the level of satisfaction with convenience attributes such as size, portability and handling.
The use of patient-reported outcomes (PROs) for assessing the effectiveness of products for regulatory approval is a developing phenomenon.21 Although device satisfaction and preference is a valid patient-reported outcome, it is less studied than other PROs like quality of life.22 In a recent review of 30 published inhaler preference studies, only two studies were found to have used robust instruments for measuring preference and satisfaction.22 The two instruments in question, the Patient Device Experience Assessment (PDEA)23 and the Patient Satisfaction and Preference Questionnaire (PASAPQ),18 were developed by experts in psychometric testing and subjected to field testing. Of these, only the PASAPQ has a published validation18 which also included a determination of the minimum important difference between devices, allowing an assessment of the clinical significance of differences observed in testing. For this reason we chose to use the PASAPQ as the preference assessment tool for the current study. Of 97 patients (63% of the sample) who gave higher total PASAPQ satisfaction scores to Respimat® SMI than Turbuhaler®, the score difference met the pre-defined criterion for the minimum important difference (>10 points) in 60 patients, nearly 40% of all those in the study. The score difference did not meet this criterion in any patient who gave Turbuhaler® a higher total satisfaction score than Respimat® SMI. In our analysis, the mean willingness-to-continue score after the 12-week study period was nearly 80 out of 100 for Respimat® SMI, but just over 60 out of 100 for Turbuhaler®.
The time-honored paradigm that inhaler preference will lead to improved medication adherence and consequently to better clinical outcomes seems plausible, but has been difficult to prove.21 Treatment satisfaction has been shown to be associated with the probability that patients would persist with a prescribed medication1 and at least in theory, patients might be able to achieve better inhaler technique with a device they find more satisfying to use, thus improving the effectiveness of the inhaled drug regimen. Recent evidence supporting the preference–adherence–outcome paradigm is beginning to emerge, as it does appear that in real life conditions, choice of inhaler device can have an impact on treatment adherence and disease control, and that the patient/device interface is crucial, for both asthma24–25 and COPD.26–28
The design of our study reduced the possible sources of bias that could affect the patients’ assessments of satisfaction with the two devices. The same active ingredient was delivered by both devices, as recommended in a review of device preference and satisfaction studies by Anderson.22 Deposition of budesonide in asthma patients was shown to be significantly higher with Respimat® SMI than Turbuhaler® when both were used with optimal technique (51.6 and 28.5% of ex-valve dose respectively),14 but if this, or the use of two different doses in Respimat® SMI had resulted in a discernible efficacy difference over the period of our study, patients could not have attributed the difference to a single device because of the double-dummy design that was employed. In addition, in the original clinical trial, no significant difference in the primary outcome measure of peak expiratory flow was detected between groups. For some patients, the novelty of a new type of inhaler might translate into short-term expressed preference over a familiar device such as the pMDI. However, because the majority of patients in our study were naïve to both Respimat® SMI and Turbuhaler®, this would probably not have affected the observed preference in favor of the Respimat® SMI. The use of the PASAPQ also helped to reduce bias, since the questions in it were designed to be specific to attributes of the inhaler rather than the treatment.
In summary, adults with asthma who used Respimat® SMI and Turbuhaler® devices in a clinical trial setting reported significantly greater satisfaction with the performance of Respimat® SMI, and the level of patient satisfaction with convenience of these two devices was very similar. This translated into a higher preference for Respimat® SMI, and a greater willingness to continue using it, compared with the Turbuhaler®.
Acknowledgments
The authors would like to acknowledge the help of Chris Kozma, West Columbia, SC, USA for his original work on the PASAPQ, and Roger Nutter, Chester, UK for assistance in drafting the paper.
Respimat® and Soft Mist™ are trade marks of the Boehringer Ingelheim group of companies. Turbuhaler® is a trade mark of the AstraZeneca group of companies. Research conducted by Reese Associates Consulting and by Ms Slaton was funded by Boehringer Ingelheim.
References
- 1.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7:204–215. doi: 10.1111/j.1524-4733.2004.72252.x. [DOI] [PubMed] [Google Scholar]
- 3.Tjwa MKT. Budesonide inhaled via Turbuhaler: a more effective treatment for asthma than beclomethasone dipropionate via Rotahaler. Ann Allergy Asthma Immunol. 1995;75:7–111. [PubMed] [Google Scholar]
- 4.Fletcher M, Hardy AJ, Karbal B, et al. Effects of asthma training on inhaler device selection and utilization in general practice [abstract] Eur Respir J. 2004;24(Suppl 48):57s. [Google Scholar]
- 5.Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. Respir Med. 2000;84:496–500. doi: 10.1053/rmed.1999.0767. [DOI] [PubMed] [Google Scholar]
- 6.Schürmann W, Schmidtmann S, Moroni P, Massey D, Qidan M. Respimat Soft Mist Inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfaction. Treat Respir Med. 2005;4:53–61. doi: 10.2165/00151829-200504010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Epstein SW, Manning CP, Ashley MJ, Corey PN. Survey of the clinical use of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813–816. [PMC free article] [PubMed] [Google Scholar]
- 8.Armitage JM, Williams SJ. Inhaler technique in the elderly. Age Aging. 1988;17:275–278. doi: 10.1093/ageing/17.4.275. [DOI] [PubMed] [Google Scholar]
- 9.Kamps AW, van Ewijk B, Roorda RJ, Brand PL. Poor inhalation technique, even after inhalation instructions, in children with asthma. Pediatr Pulmonol. 2000;29:39–42. doi: 10.1002/(sici)1099-0496(200001)29:1<39::aid-ppul7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Borgström L. The importance of the device in asthma therapy. Respir Med. 2001;95(Suppl B):S26–S29. doi: 10.1053/rmed.2001.1143. [DOI] [PubMed] [Google Scholar]
- 11.Hochrainer D, Hölz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat® Soft Mist Inhaler™ and pressurized metered dose inhalers. J Aerosol Med. 2005;18:273–282. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- 12.Newman SP, Steed KP, Reader SJ, Hooper G, Zierenberg B. Efficient delivery to the lungs of flunisolide aerosol from a new portable handheld multidose nebulizer. J Pharm Sci. 1996;85(9):960–964. doi: 10.1021/js950522q. [DOI] [PubMed] [Google Scholar]
- 13.Newman SP, Brown J, Steed KP, Reader SJ, Kladders H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of Respimat with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113:957–963. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- 14.Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat® Soft Mist™ Inhaler compared to deposition by metered dose inhaler or by Turbuhaler® dry powder inhaler. J Aerosol Med. 2005;18:264–272. doi: 10.1089/jam.2005.18.264. [DOI] [PubMed] [Google Scholar]
- 15.Vincken W, Bantje T, Middle MV, Gerken F, Moonen D. Long-term efficacy and safety of ipratropium bromide plus fenoterol via Respimat Soft Mist Inhaler (SMI) versus a pressurised metered-dose inhaler in asthma. Clin Drug Investig. 2004;24(1):17–28. doi: 10.2165/00044011-200424010-00003. [DOI] [PubMed] [Google Scholar]
- 16.von Berg A, Jeena PM, Soemantri PA, et al. Efficacy and safety of ipratropium bromide plus fenoterol inhaled via Respimat® Soft Mist™ inhaler vs a conventional metered dose inhaler plus spacer in children with asthma. Pediatr Pulmonol. 2004;37:264–272. doi: 10.1002/ppul.10428. [DOI] [PubMed] [Google Scholar]
- 17.Kilfeather SA, Ponitz HH, Beck E, et al. Improved delivery of ipratropium bromide/fenoterol from Respimat® Soft Mist™ Inhaler in patients with COPD. Respir Med. 2004;98:387–397. doi: 10.1016/j.rmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Kozma CM, Slaton TL, Monz BU, Hodder R, Reese PR. Development and validation of a patient satisfaction and preference questionnaire for inhalation devices. Treat Respir Med. 2005;4:41–52. doi: 10.2165/00151829-200504010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Harris W, Gandek B, Rogers B, Reese P. MAP-R for Windows Multitrait/multi-item analysis program: revised user’s guide. Boston MA, USA: Health Assessment Lab; 1997. [Google Scholar]
- 20.Borgström L. On the use of dry powder inhalers in situations perceived as constrained. J Aerosol Med. 2001;14(3):281–287. doi: 10.1089/089426801316970231. [DOI] [PubMed] [Google Scholar]
- 21.Hodder R. Design and interpretation of device preference trials: marketing tools or scientific instruments? In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory drug delivery X. River Grove, Illinois: Davis Healthcare International; 2006. pp. 1–17. [Google Scholar]
- 22.Anderson P. Patient preference for and satisfaction with inhaler devices. Eur Respir Rev. 2005;14(96):109–116. [Google Scholar]
- 23.Welch MJ, Nelson HS, Shapiro G, et al. Comparison of patient preference and ease of teaching inhaler technique for Pulmicort Turbuhaler versus pressurized metered-dose inhalers. J Aerosol Med. 2004;17:129–139. doi: 10.1089/0894268041457174. [DOI] [PubMed] [Google Scholar]
- 24.Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246–251. doi: 10.1183/09031936.02.00218402. [DOI] [PubMed] [Google Scholar]
- 25.Price D, Thomas M, Mitchell G, Niziol C, Featherstone R. Improvement of asthma control with a breath-actuated pressurised metered dose inhaler (BAI): a prescribing claims study of 5556 patients using a traditional pressurised metered dose inhaler (MDI) or a breath-actuated device. Respir Med. 2003;97:12–19. doi: 10.1053/rmed.2002.1426. [DOI] [PubMed] [Google Scholar]
- 26.Corden Z, Bosley C, Rees P, et al. Home nebulized therapy for patients with COPD: patient compliance with treatment and its relation to quality of life. Chest. 1997;112:1278–1282. doi: 10.1378/chest.112.5.1278. [DOI] [PubMed] [Google Scholar]
- 27.Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63:831–838. doi: 10.1136/thx.2007.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R, Vines DL, Sikkema-Ortiz J, Gardner DD, Wilkins RL. Medication adherence issues in patients treated for COPD. Int J COPD. 2008;3:371–384. doi: 10.2147/copd.s3036. [DOI] [PMC free article] [PubMed] [Google Scholar]