Abstract
Background
Aquaporin-1 (AQP1) functions as an osmotic water channel and a gated cation channel. Activation of the AQP1 ion conductance by intracellular cGMP was hypothesized to involve the carboxyl (C-) terminus, based on amino acid sequence alignments with cyclic-nucleotide-gated channels and cGMP-selective phosphodiesterases.
Results
Voltage clamp analyses of human AQP1 channels expressed in Xenopus oocytes demonstrated that the nitric oxide donor, sodium nitroprusside (SNP; 3–14 mM) activated the ionic conductance response in a dose-dependent manner. Block of soluble guanylate cyclase prevented the response. Enzyme immunoassays confirmed a linear dose-dependent relationship between SNP and the resulting intracellular cGMP levels (up to 1700 fmol cGMP /oocyte at 14 mM SNP). Results here are the first to show that the efficacy of ion channel activation is decreased by mutations of AQP1 at conserved residues in the C-terminal domain (aspartate D237 and lysine K243).
Conclusions
These data support the idea that the limited amino acid sequence similarities found between three diverse classes of cGMP-binding proteins are significant to the function of AQP1 as a cGMP-gated ion channel, and provide direct evidence for the involvement of the AQP1 C-terminal domain in cGMP-mediated ion channel activation.
Background
AQP1 proteins assemble as tetramers of membrane-spanning subunits, each composed of six transmembrane domains and intracellular amino and carboxyl termini [1]. This general structural motif is shared by cyclic nucleotide-gated (CNG) channels and voltage-gated potassium channels [2]. AQP1 provides for osmotic water flux in tissues including eye, brain (choroid plexus), kidney and the vascular system [3-8], and also it functions as a gated cation channel that is activated by intracellular signaling in Xenopus oocytes [9,10]. The forskolin-sensitive pathway originally suggested for AQP1 channel activation [10] proved to be indirect and thus variable [11]. It has since been shown that the direct mechanism of AQP1 ion channel activation requires binding of intracellular cGMP [9], an observation confirmed by Pohl and colleagues with AQP1 reconstituted in lipid bilayers [12]. The cGMP-dependent activation of AQP1 ion channels was hypothesized to involve the carboxyl (C)-terminus [9]. Truncation of the C-terminal domain prevented ion channel activation of AQP1 by cGMP [12], suggesting functional significance for this region. Limited sequence similarities at key residues have been observed between the C-termini of AQP1 and CNG channels [9,13,14] and the substrate selectivity domain of cGMP-selective phosphodiesterases [15,16]. In CNG channels, studies of cGMP binding and channel activation support the identification of a putative cyclic-nucleotide-binding domain (CNBD) in the C-terminus [13,14,17]. Three residues not previously linked to cGMP-mediated signaling are identical between AQP1, CNG channels and PDEs [16]. The relevance of these residues for AQP1 ion channel activation was unknown, and is the focus of work presented here.
The molecular structure of AQP1 investigated by high resolution imaging suggests the presence of four individual pathways for transmembrane water movement (one in each subunit) that are structurally incompatible with ion conduction [1,18,19]. It is proposed that a gated pathway for cations might be in the central pore of aquaporin ion channels [11,18]. In the oocyte expression system, only a small proportion of the population of aquaporin water channels appear active as ion channels, as determined by comparing the total water permeability and the total ion conductance values. For example, AQP1 expressed in Xenopus oocytes exhibited water-to-ion channel ratios ranging from 1:30,000 to 1:180,000, depending on the batch of oocytes (Boassa and Yool, unpublished observations). AQP1-expressing oocytes injected with 1 ng cRNA showed a water permeability (Pf) value of approximately 95 × 10-4 cm/s, and an ionic conductance of approximately 50 μS after activation by cGMP [9,20]. Similarly, AQP6 expressed in oocytes injected with 5–10 ng cRNA showed a Pf value of approximately 93 × 10-4 cm/s and an ionic conductance of approximately 35 μS after activation by HgCl2 [21], suggesting a comparably low overall ratio of total water permeability to ionic conductance (reasonably assuming the unitary conductances within an order of magnitude to those of AQP1). Low levels of ion channel activity might reflect an incomplete environment in non-native expression systems, or alternatively might suggest that not many AQP ion channels are needed to exert a physiological effect.
The fact that several members of the MIP family have been found to show ion channel activity suggests that this function is physiologically relevant, rather than a rare accident of protein misfolding as has been suggested for AQP1 [12]. Ion channel function has been shown for mammalian AQP0 (lens MIP) [22-24], AQP1 [9,12], AQP6 [21], soybean nodulin 26 [25], and Drosophila Big Brain [26]. Physiological roles of ion channel function remain to be demonstrated for any of these channels expressed in native tissues. Possible regulatory mechanisms that might govern aquaporin ion channel availability in native tissues remain to be identified.
Model-based calculations indicate that even a relatively small proportion of AQP1 channels (1:56000) having an ion conductance stimulated by cGMP could contribute a physiologically relevant increase in renal Na+ reabsorption [11]. Furthermore, the ability of a small number of ion channels to potently influence membrane potential is well established, in that relatively tiny net ion fluxes drive large changes in membrane potential [27]. Ion channels need not be abundant to have physiologically significant functions. In addition, the existence of a majority of non-active channels has precedent. For example, the long opening events (P0 ≥ 0.5) described for epithelial Na channels (ENaC) using patch clamp have been found to involve a minority of the channels at the oocyte cell surface; if channel activity is calculated instead considering the whole ENaC population, the mean open probability is estimated at 0.014 to 0.004 [28].
Although water passes through individual pores in each subunit of the tetramer, the water permeability of AQP1 requires tetrameric assembly of the channel in the plasma membrane [29,30]. In contrast to the ion conductance, water flux is suggested to be independent of signaling mediated through the C-terminus. Proteolytic removal of the AQP1 C-terminus after proteins were incorporated into red blood cell membranes did not abolish osmotic water permeability [31]. Because water flux depends on correct assembly and membrane localization, the expression of osmotic water permeability is a useful tool to rule out gross disruptions of protein folding or targeting in AQP1 mutant channels.
C-terminal amino acids that are conserved between AQP1, CNG channels and cGMP-selective phosphodiesterases (PDE) were analyzed by site-directed mutagenesis in the present study. Mutations of two key amino acids (D237 and K243) were found to decrease the amplitude of the AQP1 ionic conductance to sodium nitroprusside (SNP). Several mutations at other C-terminal residues had no effect on the cGMP-dependent conductance response. These data support the hypothesis that the C-terminal domain mediates cGMP-induced activation of ion channel function in AQP1.
Results
Confirmation of wild type and mutant AQP1 channel expression by osmotic swelling
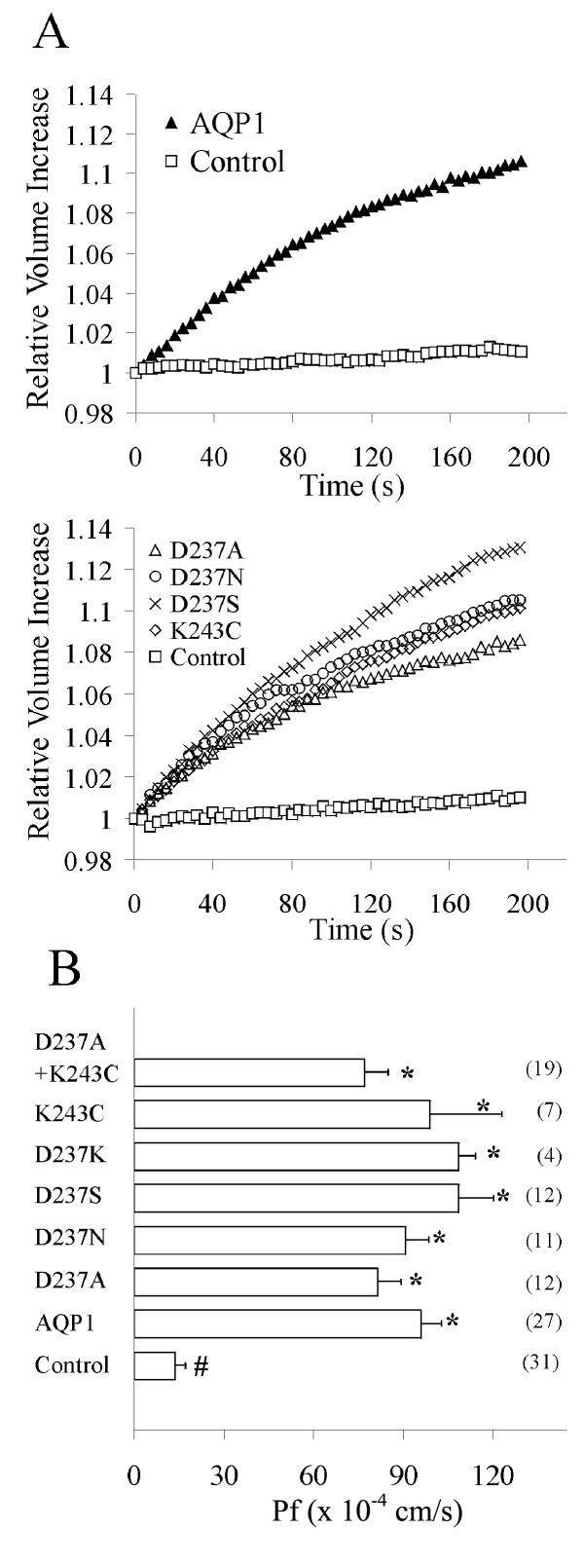
Expression of AQP1 wild type and mutant channels in the oocyte plasma membrane was confirmed by osmotic swelling assays (Fig. 1). Xenopus oocytes were injected with 50 nl of sterile water containing no cRNA (control), or with 1 ng of human AQP1 wild type or site-directed mutant cRNAs, at least two days prior to analysis. Relative volume increases were measured by osmotic swelling assays (as described in the Methods). Figure 1A shows data for relative volume increases in representative examples of control oocytes and oocytes expressing AQP1 wild type and mutant channels. In all experiments, control and AQP1-expressing oocytes were compared within the same batch of oocytes; replicate experiments were done in separate batches of oocytes. Expression of the wild type and mutant channels enabled rapid osmotically-driven increases in relative volume in 50% hypotonic saline. Control oocytes did not show any appreciable increase in relative volume over several minutes in the same condition. Figure 1B summarizes the osmotic water permeability (Pf) values for wild type and mutant channels, compiled from a total of 123 oocytes from 11 batches. Mutant channels showed osmotic water permeability values equivalent to those of wild type (significantly greater than control, but not significantly different than wild type AQP1), ruling out non-specific effects of the mutations on translation, folding and targeting of the subunits.
Figure 1.
Osmotically induced swelling in oocytes expressing AQP1 wild type and C-terminal mutant channels. A. Relative volume increases were determined by videomicroscopy as a function of time after the transfer of the oocytes from 200 mOsM into 100 mOsM saline at time 0. The change in cross-sectional area of the oocyte was converted to volume to calculate relative volume increase and standardized to the initial volume at time zero. Data are shown for representative individual oocytes. Panels show data for control and AQP1 wild type- or mutant-expressing oocytes from within the same batches of oocytes. B. Summary of the osmotic water permeability (Pf) values for wild type and mutant channels. Data for Pf (cm/s × 10-4) are mean ± SEM; n values are shown in parentheses (right). Unpaired two-tailed Student's t tests were used to analyze significance. An asterisk (*) indicates a value that is significantly different (p < 0.0005) from control; the pound sign (#) indicates a value that is significantly different (p < 0.009) from wild type AQP1.
Dose-dependent activation of ionic current in AQP1 channels by a nitric oxide donor
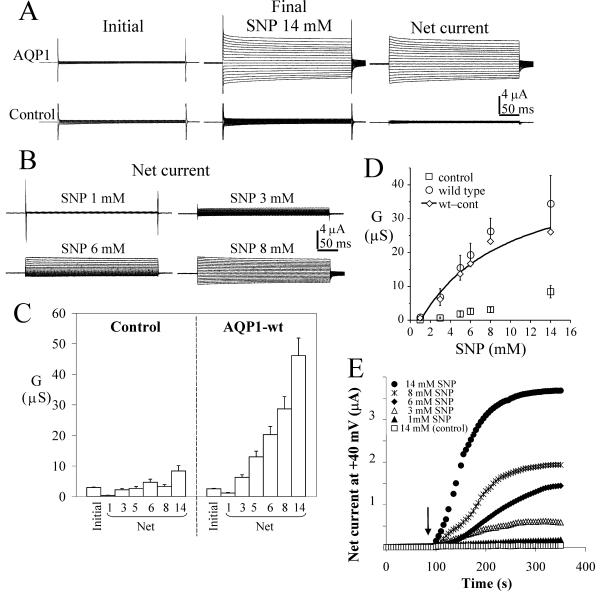
Direct injection of cGMP or extracellular application of a membrane-permeant cGMP analog has been used previously to activate the AQP1 ion conductance, although relatively slowly [9]. Extracellular SNP, a nitric oxide donor, is a more rapid and simple method for inducing the AQP1 ion current, as illustrated in Fig. 2 for the wild type AQP1 channel. Oocytes express soluble guanylate cyclase that can be stimulated by nitric oxide to produce cGMP [32]. SNP caused a dose-dependent activation of the ionic conductance in AQP1-expressing oocytes, with significantly less effect on the native channels of control oocytes (Fig. 2A). In each experiment, data for control, AQP1 wild type- and AQP1 mutant-expressing oocytes were compared within the same batch of oocytes. Oocytes were tested either with a single dose or with a series of increasing concentrations of SNP; no differences were observed in the final amplitudes of the induced responses. In most cases at lower doses (3–6 mM SNP) the AQP1 conductance showed an outward rectification (Fig. 2B), but it was linear in current-voltage relationship at higher doses. This effect is generally similar to that seen for cGMP-gated channels from rod photoreceptors, which rectify at sub-saturating concentrations of cGMP and, at saturating levels of cGMP, show a linear current-voltage relationship [33-35]. In Figure 2C, the "initial conductance" represents the value for oocytes recorded prior to SNP, and the "final conductance" is the response at 4 min after SNP application. The "net conductance" responses after SNP were calculated by subtracting the initial from the final traces for each oocyte. Control oocytes showed a small but reproducible conductance response to 14 mM SNP, and variable conductances at higher SNP concentrations that prevented assessment of doses exceeding 14 mM. For AQP1 channels, the component of the response that is attributed specifically to a cGMP-dependent conductance is the difference in net conductance values between AQP1 and control oocytes (Fig. 2D). Data for the difference between mean net wild type and control conductances (wt-cont) were fit with the equation:
Figure 2.
Wild type AQP1 channels show a dose-dependent ionic conductance response to the nitric oxide donor, sodium nitroprusside. A. Comparison of representative currents before and after 14 mM SNP in AQP1-expressing and control oocytes. Voltage steps were +60 mV to -110 mV in -10 mV intervals, from -40 mV holding potential. The net current is (final)-(initial). B. Dose-dependence of net current responses of wild type AQP1-expressing oocytes, with the same voltage protocol as in (A). C. Summary of data for conductance responses (mean ± SEM, n = 9–34 oocytes per group) at different doses of SNP (indicated in mM on horizontal axis). 'Initial' data are the conductance levels of the oocytes recorded prior to SNP application, and represent total conductance rather than net values. The net conductance responses were calculated by subtracting the matched initial values individually from the SNP response for each oocyte.D. Mean values ± SEM (n = 7 oocytes per group within the same batch of oocytes) are shown for net conductance responses to SNP for control (square) and AQP1-expressing oocytes (circle). The difference between mean net AQP1 and control conductances (diamond) was fit to estimate an EC50 value of 7.8 mM SNP; see text for details. E. Dose-dependent rate of activation of ionic current by SNP in representative AQP1-expressing oocytes, measured at 5 sec intervals with voltage steps to +40 mV from a holding potential of -40 mV. The arrow indicates the time of application of SNP in the recording chamber bath. Data prior to the application are superimposed.
G(wt-con) = C + {[SNP] Gmax / [SNP]+EC50}
yielding an estimated EC50 of 7.8 mM (correlation coefficient R = 0.98). This value should be considered as an approximation at best, since saturating doses of SNP were not achieved (our studies were limited by endogenous oocyte currents at SNP concentrations greater than 14 mM).
The ion conductance response initiated by SNP typically showed a latency of 5–10 s, and approached a dose-dependent plateau of current amplitude after ~4 min in most batches of oocytes (Fig. 2E). This plateau reflected a steady state between the proportion of channels activated and not activated at a given concentration of SNP, which was presumed to correspond directly to the intracellular level of cGMP as a balance between synthesis and degradation. However, to confirm this, it was necessary to correlate the dose of SNP with the steady state cGMP concentration achieved, and to ensure that the SNP effect was in a linear dose-response range for cGMP production.
SNP stimulates cGMP production in Xenopus oocytes
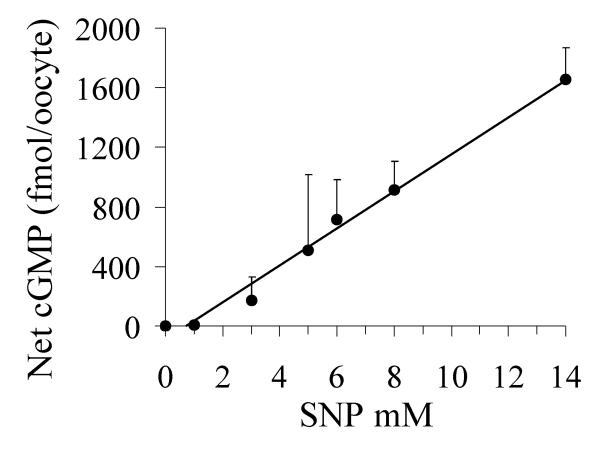
In order to relate the magnitude of the ionic conductance responses induced by SNP to increases in oocyte cGMP levels, we used an enzyme-based immunoassay to measure cGMP concentrations under the same conditions used in the electrophysiological experiments (Fig. 3). The mean basal cGMP content in the unstimulated state was 199 ± 137 fmol/oocyte (mean ± SEM, for 4 replicate samples of 10 oocytes per sample, with each sample from a different batch of oocytes). The large standard error indicates that some variability exists in endogenous levels of cGMP between batches of oocytes derived from different female donor frogs; a similar phenomenon has been noted for basal cAMP levels in oocytes [36]. In our experiments, oocytes were incubated with different doses of SNP for 4 minutes, matching the conditions of the electrophysiological assays (above). The level of cytosolic cGMP in the oocyte reflects the balance between nitric oxide stimulation of guanylate cyclase and the levels of phosphodiesterase activities. Phosphodiesterase inhibitors were not used in the electrophysiological or biochemical assays.
Figure 3.
Intracellular cGMP produced in oocytes in response to SNP. Oocytes were tested in conditions comparable to those used for electrophysiological analyses, being assayed after 4 minutes of treatment with various concentrations of SNP in K+ recording saline. The amount of cGMP per oocyte was measured by enzyme immunoassay. Each point represents the mean of duplicate or triplicate measurements from four different experiments (separate batches of oocytes). Values shown are mean ± SEM.
A linear concentration relationship was found between the dose of SNP and the resulting net increase in intracellular cGMP. The net increase in cGMP above basal levels did not show saturation within the range of SNP doses tested. Thus, the maximal ionic conductance responses seen in voltage clamp recordings are unlikely to result from an inherent limit on total oocyte cGMP production, but instead appear to reflect a maximal effect of the steady state cGMP level on AQP1 ion channel activity. However, we cannot rule out possible localized degradation of a membrane-compartment associated pool of cGMP in limiting the response, similar to that described for cAMP in HEK cells [37].
An equilibrium dissociation constant (KD) value of 0.2 μM cGMP was estimated previously from competition binding studies with cGMP for AQP1 channels expressed in SF9 cells [9]. The concentration required for the half maximal response (EC50) value for AQP1 is expected to be higher than the KD value, since receptor occupancy is not identical to response efficacy [38]. A dose of ~8 mM SNP (estimated EC50 value) corresponds to ~900 fmol cGMP per oocyte (Fig. 3), or approximately 1.8 μM cGMP (assuming a cytoplasmic volume of 0.5 μl), and thus falls into a reasonable range for the observed dose-dependent activation.
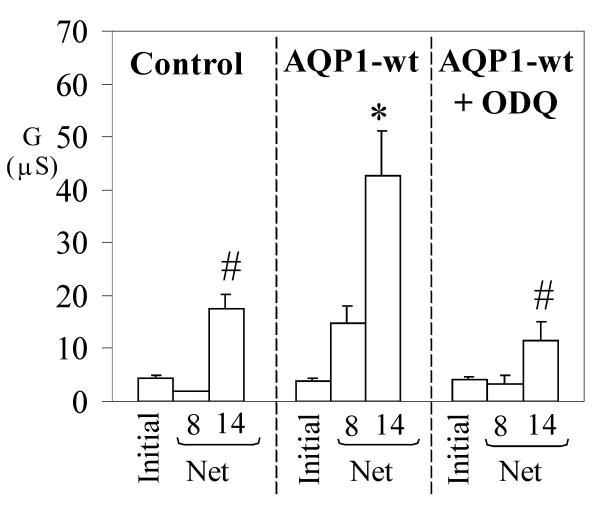
Ion channels can be affected by nitric oxide through chemical modification, such as S-nitrosylation [39]. To test for a direct role of cGMP in the responses we observed, AQP1-expressing oocytes were pre-incubated 3 hours with ODQ (1H-[1,2,4]oxadiazolo [4,3-a]quinoxalin-1-one), an inhibitor of soluble guanylate cyclase. If AQP1 channel activation were due to nitric oxide modification, then the conductance should persist in ODQ. However, the ODQ inhibitor effectively prevented an AQP1 conductance response to SNP (Fig. 4), confirming that the activation of the conductance is dependent on guanylate cyclase activity and cGMP production, and cannot be attributed to a possible chemical modification of AQP1 residues by nitric oxide.
Figure 4.
Inhibition of soluble guanylate cyclase prevents the AQP1 conductance response to SNP. Wild type AQP1-expressing and control oocytes were incubated three hours in ND96 saline with or without an inhibitor of soluble guanylate cyclase (1H-[1,2,4]oxadiazolo [4,3-a]quinoxalin-1-one, ODQ, 10 μM) prior to electrophysiological analysis of the response to SNP (doses indicated in mM on horizontal axis). Data are mean ± SEM; n = 3 or 4 oocytes per group. An asterisk (*) indicates values that are significantly different (p < 0.04) from control; the pound sign (#) indicates values that are significantly different (p < 0.04) from wild type AQP1 (unpaired two-tailed Student's t test).
Conserved amino acids in the carboxyl terminal domain contribute to the cGMP-induced activation
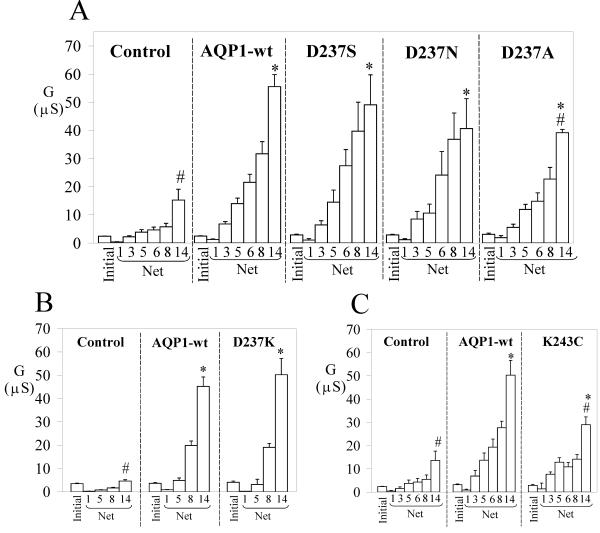
Based on similarities in amino acid sequence between the AQP1 C-terminus, and subdomains of CNG channels and cGMP-PDE [16], we generated a set of mutations in the carboxyl terminal domain of AQP1. Aspartate (D) at position 237 was substituted with serine (S), asparagine (N), lysine (K) or alanine (A). Lysine at position 243 was substituted with cysteine (C). These mutant channels showed osmotically induced water permeabilities at levels equivalent to that of AQP1 wild type (Fig. 1, Table 2). In contrast, the ionic conductance responses showed distinct differences depending on the mutation (Figs. 5, 6). Data compiled in histograms summarize the initial and net SNP-induced conductance data for mutant channels, with corresponding wild type and control responses assessed from within the same batches of oocytes (Figs. 5, 6). The "initial conductance" represents the value for oocytes recorded prior to SNP, and the "final conductance" is the response at 4 min after SNP application. The "net conductance" responses after SNP were calculated by subtracting the initial from the final traces for each oocyte. Oocytes expressing AQP1 channels with single mutations D237S, D237N (Fig. 5A) and D237K (Fig. 5B) showed dose-dependent ionic conductance responses to SNP that were comparable to that of wild type AQP1; however, the conductance response of D237A-expressing oocytes was decreased by a small but significant amount (Fig. 5A). Oocytes expressing the mutant channel K243C also showed a significantly lower conductance response as compared with wild type AQP1 (Fig. 5C).
Table 2.
Summary of mean osmotic water permeability and ionic conductance values for Aquaporin-1 wild type and mutant channels.
| Prep'n | Amino acid | Fluxes ± SEM (n) | ||
| 237 | 243 | Pf (cm/s × 10-4) | Net G/net G wt at 14 mM SNP | |
| Wild type | D | K | 95.93 ± 7.01 (27) | 1.04 ± 0.05 (28) |
| Mutants | S | K | 108.57 ± 11.62 (12) | 0.88 ± 0.19 (7) |
| N | K | 91 ± 7.57 (11) | 0.73 ± 0.19 (5) | |
| K | K | 82.88 ± 25.91 (4) | 1.11 ± 0.16 (4) | |
| A | K | 81.48 ± 7.88 (12) | 0.71 ± 0.02 (8)* | |
| D | C | 98.91 ± 24.04 (7) | 0.58 ± 0.07 (5)* | |
| A | C | 77.1 ± 8.02 (19) | 0.31 ± 0.05 (18)* | |
Osmotic water permeability (Pf) and ionic conductance (G) data are shown as mean ± SEM; n values (# oocytes) are shown in parentheses. Net ionic conductance values at 14 mM sodium nitroprusside (SNP) were normalized to the mean net conductance value of the wild type channel from the same batch of oocytes at 14 mM SNP. Net conductance values were calculated by subtracting the matched initial values recorded prior to SNP application individually from the SNP response for each oocyte. An asterisk (*) indicates values significantly different from wild type (p < 0.04; unpaired two-tailed Student's t test).
Figure 5.
Summary of the effects of AQP1 C-terminal mutations on the net conductance responses evoked by SNP. Data are grouped to compare control, AQP1 wild type and mutant responses from within the same batches of oocytes. The mutants D237S, D237N (A) and D237K (B) showed dose-dependent responses to SNP (mM) comparable to that of wild type AQP1. However, the mutations D237A (A) and K243C (C) showed decreased conductance responses at all doses of SNP as compared with wild type. 'Initial' data are the conductance levels of the oocytes recorded prior to SNP application, and represent total conductance rather than net values. The net conductance responses were calculated by subtracting the matched initial values individually from the SNP response for each oocyte. Data are mean ± SEM; n = 3–28 oocytes per group. An asterisk (*) indicates values that are significantly different (p < 0.02) from control; the pound sign (#) indicates values that are significantly different (p < 0.04) from wild type AQP1 (unpaired two-tailed Student's t test).
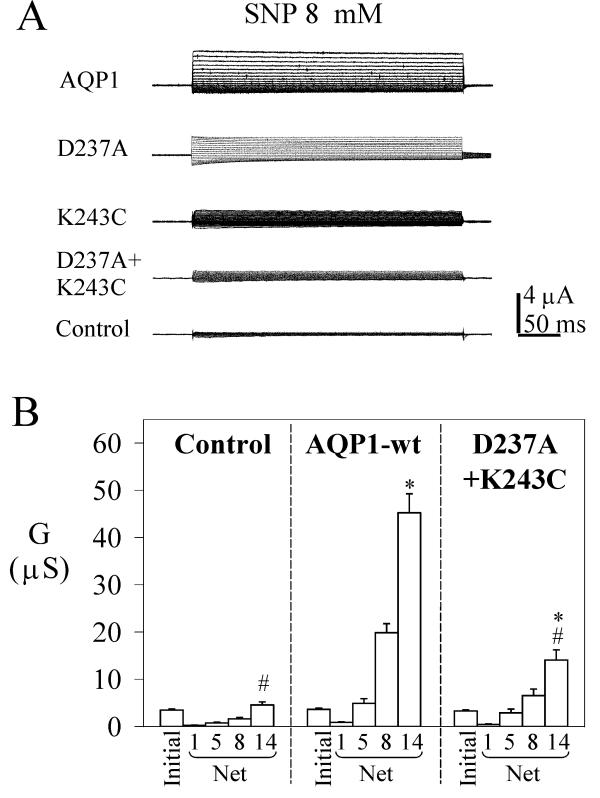
Figure 6.
Double mutation D237A + K243C in the AQP1 carboxyl terminal domain shows a decreased conductance response to SNP as compared with wild type. A. Net current responses of control oocytes, and oocytes expressing AQP1 wild type and mutant channels were measured for voltage steps from +60 mV to -110 mV in -10 mV intervals, from -40 mV holding potential, after 4 minutes in 8 mM SNP. B. Summary of conductances (mean ± SEM, n = 9–18 oocytes per group) measured at different doses of SNP (mM). 'Initial' data are the conductance levels of the oocytes recorded prior to SNP application. The net conductance responses were calculated by subtracting the matched initial values individually from the SNP response for each oocyte. An asterisk (*) indicates values that are significantly different (p < 0.006) from control; the pound sign (#) indicates values that are significantly different (p < 0.0001) from wild type AQP1 (unpaired two-tailed Student's t test).
Based on the two effective single mutations, we generated an AQP1 mutant containing the double mutation D237A and K243C (Fig. 6). Although the double mutant channel was not significantly different from wild type AQP1 in water permeability (Fig. 1, Table 2), this mutant channel showed a dramatic decrease in ion conductance amplitude at 14 mM SNP, to a level that was significantly less than wild type but significantly greater than control. Two general possibilities could explain a reduced conductance response for mutated channels. First, the decreased amplitude could reflect a decrease in the number of channels that are expressed correctly in the membrane as compared to wild type. However, the possibility that the double mutant channel was poorly expressed was contradicted by our observation that the osmotic water permeabilities of oocytes expressing wild type and double mutant AQP1 constructs were comparable, arguing against a substantial defect in channel expression. Second, a decreased amplitude could reflect a decrease in affinity for cGMP or a decrease in maximal response amplitude in the mutant channels. Additional studies are needed to distinguish between the two possibilities.
Interestingly, several other substitutions of D237 with uncharged amino acids were tolerated without grossly affecting the macroscopic properties of cGMP-dependent AQP1 channel activation, suggesting that this residue might serve a general property such as positioning of the C-terminal domain, rather than providing for direct electrostatic interactions. The persistence of residual cGMP-dependent ion channel activity in the double mutant and the tolerance of the region for other mutations at D237 indicate that binding and activation by cGMP are not absolutely disrupted by mutations of D237 and K243, suggesting that other residues contribute to the cGMP-dependent activation. Nonetheless, based on our results, a significant decrease in the response of the double mutant is clear, and suggests the structure of AQP1 C-terminal domain is important for cGMP-mediated activation.
Mutations also were generated at three other positions that are similar between AQP1 C-terminal and the corresponding region of CNG channels (data not shown). These were R263G/E/Q, and the double mutant S236H + L238R; neither construct had an appreciable effect on SNP-induced activation of the AQP1 ion conductance (Boassa, Steidl, and Yool, unpublished data). Thus, not all the observed similarities in sequence necessarily reflect positions critical for cGMP-induced channel activation, although they might serve in other regulatory interactions not identified in this study.
In order to assess the possibility that the reduced current in the double mutant was due to a rapid selective loss of channels from the membrane after SNP application, we compared the osmotic water permeability values in oocytes expressing D237A + K243C, with and without treatment with 14 mM SNP. The water permeability Pf values (given here as units cm/s × 10-4) for the double mutant without SNP treatment were 100 ± 12 (mean ± SEM, n = 11), and 79 ± 7 (n = 21) after SNP. For AQP1 wild type, the Pf values were 83 ± 10 (n = 6) without SNP treatment, and 96 ± 7 (n = 27) after SNP. No treatment group showed statistically significant differences from any of the other groups.
We next considered the possibility that the effect of cysteine substitution at K243 might alter AQP1 ion channel function by allowing the formation of disulfide bridges with other cysteine residues (Fig. 7). If bridge formation were inhibiting activation in the double mutant channel, we would expect treatment with a cysteine-reducing agent to enhance the amplitude of the conductance response. We tested this possibility by pre-incubating control, AQP1 wild type, and double mutant D237A + K243C-expressing oocytes in the reducing agent dithiothreitol (DTT, 10 mM, in ND96 saline) for one hour prior to testing for activation with SNP. Data presented in Fig. 7 indicate that wild type AQP1-expressing oocytes showed conductance responses to SNP that were not significantly different with or without DTT preincubation.
Figure 7.
Effects of preincubation with the reducing agent dithiothreitol (DTT) on SNP-induced ionic conductances. Oocytes expressing AQP1 wild type and double mutant D237A + K243C channels, and control oocytes were preincubated in ND96 saline with and without 10 mM DTT for 1 hour prior to electrophysiological recording of the response to SNP. Initial data are prior to SNP application; net conductances are (final)-(initial) for the SNP doses (mM) as indicated. An asterisk (*) indicates values that are significantly different (p < 0.006) from control; the pound sign (#) indicates values that are significantly different (p < 0.0001) from wild type AQP1 (unpaired two-tailed Student's t test) for comparisons within the same treatment group (with or without DTT). For other statistical comparisons, see details in text.
However, double mutant D237A + K243C-expressing oocytes appeared to show a significantly lower conductance after DTT treatment as compared to no DTT exposure (p < 0.03). This result contradicted the alternative hypothesis, and suggested that disulfide bridge formation does not account for the reduced ionic conductance response. In agreement with results of Fig. 6, the responses of the double mutant-expressing oocytes shown in Fig. 7 were significantly greater than that of control oocytes (p < 0.02), and significantly less than that of wild type (p < 0.007) within the same treatment groups (with and without DTT). Interestingly, the small native conductance activated by SNP in control oocytes was significantly reduced by DTT pre-treatment (p < 0.01), as compared with SNP-induced control oocytes without DTT. This suggests that the unidentified SNP-sensitive oocyte channels might have cysteine disulfide bonds that influence levels of channel activity, apparently unlike wild type AQP1. Based on this observation, it is possible that the perceived reduction in the conductance response of D237A + K243C after DTT might simply reflect the reduced contribution of the native oocyte channels to the overall final conductance, made more noticeable by the smaller signal-to-noise ratio for D237A + K243C as compared with wild type. If the net control conductances are subtracted from the net mutant conductances within each treatment group, the conductances of D237A + K243C channels with and without DTT are comparable.
In summary, our data show that the C-terminal domain of AQP1 is essential for cGMP-mediated activation of the ionic conductance, and that amino acids D237 and K243 influence the efficacy of cGMP-induced gating.
Discussion
Results here show that the cGMP-mediated activation of ion conductance in AQP1 channels depends on the amino acid sequence of the C-terminal domain, and that the efficacy of activation is altered by the mutation of two key residues. These AQP1 residues D237 and K243 are found in CNG channels and cGMP-selective phosphodiesterases [16]. The double mutation of AQP1 D237A and K243C significantly decreased the efficacy of cGMP as compared with AQP1 wild type. Several mechanisms could contribute to the lowered efficacy of macroscopic activation, including a decreased affinity for cGMP, alterations in coupling of cGMP binding to channel opening, decreased open probability or single channel conductance, the loss of modulating protein/signaling interactions, and others; these are interesting questions to pursue in future studies. Our analyses support the idea that the reduced conductance of the AQP1 channels with C-terminal mutations is not simply due to a loss of channel expression in the membrane, or to the formation of cysteine disulfide bridges involving the K243C residue.
Although the AQP1 C-terminus is small in comparison to the full CNBD of CNG channels, our data indicate this domain is important in cGMP-induced activation. The tolerance of this region for several of the various mutations tested in our study suggests that D237 and K243 do not alone account for cGMP-dependent activation. Other residues in the C-terminus, as well as other regions of the AQP1 channel could contribute to regulating channel activation. Protein-protein interactions also might regulate aquaporin ion channels. For example, AQP1 is expressed in the inner ear [40], where it is linked by PDZ-mediated protein interactions to ephrin tyrosine-kinase receptors and anion transporters that might direct the production of endolymph fluid [41]. Results presented here provide a foundation for further work exploring the role of the C-terminal sequence in the mechanism of activation of AQP1 ion channels.
The gated ion channel function of AQP1 might contribute to many regulatory mechanisms, including development, volume regulation, and transmembrane fluid transport. AQP1 is expressed in some mammalian tissues in which elevation of cGMP levels could subserve transmembrane signaling. However, at present, there are no data demonstrating physiological roles for any of the aquaporin ion channels. The presence of ion channel function in three of the mammalian aquaporins (AQP0, 1 and 6) lends credence to the hypothesis that the ion channel capacity is a useful feature whose functional role still is not completely understood. Other aquaporin channels might yet be identified once appropriate triggering signals are identified. It will be of interest to extend the studies of various aquaporin ion channels beyond the oocyte expression system, to characterize their roles in cells that endogenously express these channels and their native interacting proteins.
Methods
Oocyte preparation
Oocytes at stages V and VI were harvested from female Xenopus laevis and prepared as described previously [9]. Oocytes were injected the day after isolation with either 50 nl of water or 50 nl of water containing human AQP1 wild type, D237N, D237S, D237A, K243C, or double-mutant D237A-K243C cRNA. Oocytes were incubated at 18°C in ND96 culture medium (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 2.5 mM Na-pyruvate, 5 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin, pH 7.6) for 2–5 days to allow protein expression before recording. cDNA for wild type human AQP1 was provided by P. Agre, Johns Hopkins, Baltimore MD [42]. In all experiments, control and AQP1 wild type- and mutant-expressing oocytes were compared within the same batch of oocytes; replicate experiments were done in separate batches of oocytes.
Swelling assay
Osmotic swelling was analyzed from volume changes recorded by a high performance CCD video camera (Cohu, San Diego, CA). At time zero, oocytes were transferred from Na+ saline (~200 mOsM) into 50% hypotonic Na+ saline (~100 mOsM) to induce swelling. The Na+ saline contained (in mM) NaCl 100; MgCl2 5; HEPES 5 (pH 7.3). The hypotonic saline contained (in mM) NaCl 50; MgCl2 5; HEPES 5 (pH 7.3). Images were captured every 4 s for 3 minutes and analyzed using Scion Image Software (Scion Corporation, Frederick, Maryland).
The measured maximal cross sectional area of the oocyte was used to calculate spherical volume. The relative volume increase was determined as the proportional increase in volume, standardized to the initial volume at time zero. The relative volume increase responses plotted as a function of time of exposure to hypotonic saline were fit with a model-independent second order polynomial, and the initial rates of swelling [(d(V/V0)/dt] were calculated from the linear component of the fit. Osmotic water permeability (Pf) values for each oocyte are defined as: Pf = [V0 × d(V/V0)/dt]/ [S × Vw × (osmin - osmout)], with initial oocyte volume V0 = 9 × 10 -4 cm3, initial oocyte surface area S = 0.045 cm2, molar ratio of water Vw = 18 cm3/mol, osmolarity inside the oocyte osmin = 200 mOsM, and osmolarity outside the oocyte in hypotonic saline osmout = 100 mOsM. Pf values (cm/s × 10-4) are given as mean ± SEM.
Site-directed mutagenesis and in vitro RNA synthesis
Site-directed mutagenesis was done with the Quikchange kit (Stratagene, La Jolla, CA) using PfuTurbo DNA polymerase. Complementary pairs of synthetic oligonucleotide primers containing the desired mutation (listed in Table 1) were used to amplify the full-length plasmid with the AQP1 cDNA insert. As per the manufacturer's protocol, the PCR product was treated with Dpn I endonuclease to digest the methylated wild type DNA template, and transformed into Epicurian Coli XL1-Blue supercompetent cells.
Table 1.
List of primers used for the site-directed mutations of selected amino acids in the carboxyl terminal domain of Aquaporin-1.
| Mutation | Primer | Position | Sequence (mutation underlined) |
| D237S | Sense | bp 726-759 | 5'-GGCCCCACGCAGCAGTAGCCTCACAGACCGCGTG-3' |
| D237S | Antisense | bp 759-726 | 5'-CACGCGGTCTGTGAGGCTACTGCTGCGTGGGGCC-3' |
| D237N | Sense | bp 725-759 | 5'-TGGCCCCACGCAGCAGTAACCTCACAGACCGCGTG-3' |
| D237N | Antisense | bp 759-725 | 5'-CACGCGGTCTGTGAGGTTACTGCTGCGTGGGGCCA-3' |
| D237K | Sense | bp 727-759 | 5'-GCCCCACGCAGCAGTAAGCTCACAGACCGCGTG-3' |
| D237K | Antisense | bp 759-727 | 5'-CACGCGGTCTGTGAGCTTACTGCTGCGTGGGGC-3' |
| D237A | Sense | bp 726-760 | 5'-GGCCCCACGCAGCAGTGCCCTCACAGACCGCGTGA-3' |
| D237A | Antisense | bp 760-726 | 5'-TCACGCGGTCTGTGAGGGCACTGCTGCGTGGGGCC-3' |
| K243C | Sense | bp 744-778 | 5'-CCTCACAGACCGCGTGTGCGTGTGGACCAGCGGCC-3' |
| K243C | Antisense | bp 778-744 | 5'-GGCCGCTGGTCCACACGCACACGCGGTCTGTGAGG-3' |
| D237A + K243C | Sense | bp 744-778 | 5'-CCTCACAGACCGCGTGTGCGTGTGGACCAGCGGCC-3' |
| D237A + K243C | Antisense | bp 778-744 | 5'-GGCCGCTGGTCCACACGCACACGCGGTCTGTGAGG-3' |
Successful incorporation of the mutations was confirmed by DNA sequence analyses, using forward and reverse primers to check the entire AQP1 sequence, and confirm the absence of random mutations. Capped cRNA transcripts were synthesized in vitro with T3 RNA polymerase enzyme, using BamHI-linearized cDNA as a template. The RNA concentration was determined by UV absorbance spectrophotometry to standardize the amounts of RNA that were injected into the oocytes, and the correct size and quality was checked by agarose gel electrophoresis.
Electrophysiological recordings
Recordings were made using the two-electrode voltage clamp technique. Borosilicate electrodes were filled with 3 M KCl (1–3 MOhm). Recordings were done in K+ bath saline containing 40 mM KCl, 60 mM K gluconate, 5 mM MgCl2, 5 mM HEPES, and 2 mM EGTA, pH 7.3, at room temperature. Conductance responses were measured before, during and after the application of each SNP dose with a GeneClamp amplifier and pClamp software (Axon Instruments, Foster City, CA). Data were filtered at 2 kHz, digitized at 10 kHz, and stored to hard disk for off-line analysis. Net conductance values are reported as mean ± SEM. Statistical comparisons are described in the figure legends or text.
Sodium nitroprusside (SNP; Sigma Co., St. Louis MO) was prepared fresh daily as a stock solution (200–600 mM) in recording saline and kept on ice, protected from light. The final SNP concentration was obtained by adding a calculated amount of the stock solution to the recording chamber. Oocytes were tested either with single doses, or with a series of increasing concentrations of SNP; the results were comparable with either method of application. All oocytes were preincubated prior to recording for 1–3 hours in the presence of 10 μM H7 (1-(5-Isoquinolinesulfonyl)-2-methylpiperazine dihydrochloride; Sigma Co., St. Louis MO), a kinase antagonist that minimizes possible cross talk with other endogenous signaling pathways in oocytes [9,11].
Biochemical measures of cGMP
cGMP was assayed using the Biotrak enzyme immunoassay (EIA) system (Amersham Pharmacia, Piscataway NJ). Oocytes (10 per group) were placed in 24-well plates in K+ recording saline. SNP at different doses was added for 4 minutes to match the conditions of the electrophysiology experiments. Reactions were stopped by removal of the SNP saline, and addition of the kit assay buffer. After cooling on ice, cells were lysed and centrifuged at 1,000 g for 15 minutes. The supernatant was removed and centrifuged at 10,000 g for 20 minutes; pellets of membranes and cellular debris were discarded. The supernatant, which consisted of the oocyte cytosolic fraction, was then used immediately for the EIA assay.
Acknowledgments
Acknowledgements
We thank Dr. Jeffrey Karpen for helpful discussions, Drs. Paul McDonagh and Zoe Cohen for assistance with cGMP assays, and Amy Marble and Dr. Kathryn Bolles for technical support. This work was supported by NIH R01 GM59986 (AJY) and a predoctoral award from the American Heart Association Desert Mountain Affiliate (DB).
Contributor Information
Daniela Boassa, Email: dboassa@email.arizona.edu.
Andrea J Yool, Email: ayool@u.arizona.edu.
References
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Structural elements involved in specific K+ channel functions. Annual Review of Physiology. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- King LS, Agre P. Pathophysiology of the aquaporin water channels. Annual Review of Physiology. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Smith B, Christensen E, Agre P. Distribution of the Aquaporin CHIP in Secretory and Resorptive Epithelia and Capillary Endothelia. PNAS. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E, Winterfield J, Goings G, Bastawrous A, Upshaw-Earley J. Water channel proteins in rat cardiac myocyte caveolae: osmolarity-dependent reversible internalization. American Journal of Physiology. 1998;274:H1988–2000. doi: 10.1152/ajpheart.1998.274.6.H1988. [DOI] [PubMed] [Google Scholar]
- Stamer W, Snyder R, Smith B, Agre P, Regan J. Localization of aquaporin CHIP in the human eye: implications in the pathogenesis of glaucoma and other disorders of ocular fluid balance. Invest Ophthalmol Vis Sci. 1994;35:3867–3872. [PubMed] [Google Scholar]
- van Os CH, Kamsteeg EJ, Marr N, Deen PM. Phsyiological relevance of aquaporins: luxury or necessity? Pflugers Archiv – European Journal of Physiology. 2000;440:513–520. doi: 10.1007/s004240000317. [DOI] [PubMed] [Google Scholar]
- Venero JL, Vizuete ML, Machado A, Cano J. Aquaporins in the central nervous system. Progress in Neurobiology. 2001;63:321–336. doi: 10.1016/S0301-0082(00)00035-6. [DOI] [PubMed] [Google Scholar]
- Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ. Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Molecular Pharmacology. 2000;57:576–588. doi: 10.1124/mol.57.3.576. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Stamer WD, Regan JW. Forskolin Stimulation of Water and Cation Permeability in Aquaporin1 Water Channels. Science. 1996;273:1216–1218. doi: 10.1126/science.273.5279.1216. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Weinstein AM. New roles for old holes: ion channel function in aquaporin-1. News in Physiological Sciences. 2002;17:68–72. doi: 10.1152/nips.01372.2001. [DOI] [PubMed] [Google Scholar]
- Saparov SM, Kozono D, Rothe U, Agre P, Pohl P. Water and ion permeation of aquaporin-1 in planar lipid bilayers. Major differences in structural determinants and stoichiometry. Journal of Biological Chemistry. 2001;276:31515–31520. doi: 10.1074/jbc.M104267200. [DOI] [PubMed] [Google Scholar]
- Goulding EH, Tibbs GR, Siegelbaum SA. Molecular mechanism of cyclic-nucleotide-gated channel activation. Nature. 1994;372:369–374. doi: 10.1038/372369a0. [DOI] [PubMed] [Google Scholar]
- Varnum MD, Black KD, Zagotta WN. Molecular mechanism for ligand discrimination of cyclic nucleotide-gated channels. Neuron. 1995;15:619–625. doi: 10.1016/0896-6273(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Turko IV, Francis SH, Corbin JD. Hydropathic analysis and mutagenesis of the catalytic domain of the cGMP-binding cGMP-specific phosphodiesterase (PDE5). cGMP versus cAMP substrate selectivity. Biochemistry. 1998;37:4200–4205. doi: 10.1021/bi972448r. [DOI] [PubMed] [Google Scholar]
- Boassa D, Yool AJ. A fascinating tail: cGMP activation of aquaporin-1 ion channels. Trends in Pharmacological Sciences. 2002;23:558–562. doi: 10.1016/S0165-6147(02)02112-0. [DOI] [PubMed] [Google Scholar]
- Matulef K, Flynn GE, Zagotta WN. Molecular rearrangements in the ligand-binding domain of cyclic nucleotide-gated channels. Neuron. 1999;24:443–452. doi: 10.1016/s0896-6273(00)80857-0. [DOI] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a Glycerol-Conducting Channel and the Basis for Its Selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Brooks HL, Regan JW, Yool AJ. Inhibition of aquaporin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol Pharmacol. 2000;57:1021–1026. [PubMed] [Google Scholar]
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402:184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Hall JE, Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc Natl Acad Sci U S A. 1985;82:8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring GR, Zampighi G, Horwitz J, Bok D, Hall JE. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Gen Physiol. 1990;96:631–664. doi: 10.1085/jgp.96.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring GR, Lagos N, Zampighi GA, Hall JE. Phosphorylation modulates the voltage dependence of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Membr Biol. 1992;126:75–88. doi: 10.1007/BF00233462. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Shomer NH, Louis CF, Roberts DM. Nodulin 26, a nodule-specific symbiosome membrane protein from soybean, is an ion channel. J Biol Chem. 1994;269:17858–17862. [PubMed] [Google Scholar]
- Yanochko GM, Yool AJ. Regulated cationic channel function in Xenopus oocytes expressing Drosophila big brain. J Neurosci. 2002;22:2530–2540. doi: 10.1523/JNEUROSCI.22-07-02530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channnels of Excitable Membranes. 3. Sinauer Associates; 2001. [Google Scholar]
- Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- Zeidel ML, Nielsen S, Smith BL, Ambudkar SV, Maunsbach AB, Agre P. Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomes. Biochemistry. 1994;33:1606–1615. doi: 10.1021/bi00172a042. [DOI] [PubMed] [Google Scholar]
- Moon C, Fraser SP, Djamgoz MB. Protein kinase and phosphatase modulation of quail brain GABA(A) and non-NMDA receptors co-expressed in Xenopus oocytes. Cell Signal. 2000;12:105–112. doi: 10.1016/S0898-6568(99)00073-X. [DOI] [PubMed] [Google Scholar]
- Bader CR, Macleish PR, Schwartz EA. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen JW, Zimmerman AL, Stryer L, Baylor DA. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988;85:1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf K, Koopmann R, Eismann E, Kaupp UB. Gating by cyclic GMP and voltage in the alpha subunit of the cyclic GMP-gated channel from rod photoreceptors. J Gen Physiol. 1999;114:477–490. doi: 10.1085/jgp.114.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli MF, Smith LD. Cyclic AMP levels during the maturation of Xenopus oocytes. Dev Biol. 1985;108:254–258. doi: 10.1016/0012-1606(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BT. The competitive and noncompetitive antagonism of receptor-mediated drug actions in the presence of spare receptors. J Pharmacol Toxicol Methods. 1993;29:85–91. doi: 10.1016/1056-8719(93)90055-J. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. doi: 10.1016/S0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Beitz E, Kumagami H, Krippeit-Drews P, Ruppersberg JP, Schultz JE. Expression pattern of aquaporin water channels in the inner ear of the rat. The molecular basis for a water regulation system in the endolymphatic sac. Hear Res. 1999;132:76–84. doi: 10.1016/S0378-5955(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]