Abstract
OBJECTIVES
This study aimed to identify clinical features associated with premature mortality in a large contemporary cohort of adults with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The Finnish Diabetic Nephropathy (FinnDiane) study is a national multicenter prospective follow-up study of 4,201 adults with type 1 diabetes from 21 university and central hospitals, 33 district hospitals, and 26 primary health care centers across Finland.
RESULTS
During a median 7 years of follow-up, there were 291 deaths (7%), 3.6-fold (95% CI 3.2–4.0) more than that observed in the age- and sex-matched general population. Excess mortality was only observed in individuals with chronic kidney disease. Individuals with normoalbuminuria showed no excess mortality beyond the general population (standardized mortality ratio [SMR] 0.8, 95% CI 0.5–1.1), independent of the duration of diabetes. The presence of microalbuminuria, macroalbuminuria, and end-stage kidney disease was associated with 2.8, 9.2, and 18.3 times higher SMR, respectively. The increase in mortality across each stage of albuminuria was equivalent to the risk conferred by preexisting macrovascular disease. In addition, the glomerular filtration rate was independently associated with mortality, such that individuals with impaired kidney function, as well as those demonstrating hyperfiltration, had an increased risk of death.
CONCLUSIONS
An independent graded association was observed between the presence and severity of kidney disease and mortality in a large contemporary cohort of individuals with type 1 diabetes. These findings highlight the clinical and public health importance of chronic kidney disease and its prevention in the management of type 1 diabetes.
Despite modern therapeutics, type 1 diabetes continues to be associated with premature death. For example, mortality in individuals with type 1 diabetes from Finland is 3–4 times higher than in the general population (1). However, not all individuals with type 1 diabetes share this risk. In order to determine the current prognosis of any individual with type 1 diabetes and direct preventive interventions, it is important to identify those individuals at increased risk of death.
Chronic kidney disease is a common complication of type 1 diabetes, affecting up to 30% of patients (2). Previous studies have identified chronic kidney disease as a risk factor for mortality in type 1 diabetes (3–6). However, many of these observations were made when current management paradigms, including intensified metabolic control and blockade of the renin angiotensin system, were not widely applied. In this article, we examine the incidence and predictors of all-cause mortality in a nationally representative cohort of adults with type 1 diabetes, showing that the presence and severity of chronic kidney disease remains the major determinant of excess mortality associated with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The Finnish Diabetic Nephropathy (FinnDiane) study is an ongoing nationwide prospective multicenter study established to identify risk factors for type 1 diabetes and its complications. The FinnDiane registry has been described in detail in previous publications (7,8). In brief, between January 1997 and April 2006, 4,201 adults with type 1 diabetes were recruited from 21 university and central hospitals, 33 district hospitals, and 26 primary health care centers across Finland. The mean response rate was 78%. Type 1 diabetes was defined by insulin dependence within 1 year of diagnosis, the absence of circulating C-peptide (<0.3 nmol/l), and an age of onset of <35 years. Every participant gave written informed consent. The study was approved by ethics committees of participating centers and conducted in accordance with the Helsinki Declaration.
Characteristics of the subjects.
Data on smoking habits, alcohol intake, educational level and social class were obtained using a patient questionnaire. Details on clinical status, including age at diagnosis, insulin therapy, and other regular medications, together with presence, severity, and management of diabetic complications, including retinopathy and macrovascular disease, were obtained from medical records by the attending physician using a standardized questionnaire. The presence of proliferative retinopathy was arbitrarily defined by the previous use of laser treatment. The presence of preexisting macrovascular disease was defined in individuals with a clinical history of myocardial infarction, unstable angina requiring hospitalization, coronary revascularization, stroke, carotid surgery, peripheral revascularization, and amputation for critical limb ischemia.
Fasting blood samples were obtained for the measurement of A1C, lipids, and creatinine. The glomerular filtration rate (GFR) was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation (9). Baseline urinary albumin excretion (UAE) was stratified according to International Diabetes Federation guidelines (10), such that microalbuminuria was defined by a UAE of 20–200 μg/min, macroalbuminuria by a UAE >200 μg/min, and normoalbuminuria by a UAE <20 μg/min in two of three consecutive urine collections. The presence of chronic kidney disease was defined, according to DOQI (Dialysis Outcomes Quality Initiative) guidelines, by the presence of either microalbuminuria, macroalbuminuria, an estimated GFR <60 ml/min per 1.73 m2, or end-stage kidney disease (9).
Blood pressure measurements were performed twice in the sitting position, with a 5-min interval between testing. Hypertension was defined by antihypertensive medication use or a blood pressure greater than target levels of 130/85 mmHg over two readings in an untreated individual.
Ascertainment of outcomes.
The primary outcome was death from any cause through to the 1st of April 2007. Deaths were identified via a search of the Finnish National Death Registry and center databases. All deaths were confirmed with death certificate data. Expected mortality was estimated from general population life tables (Tilastokeskus, Helsinki, Finland) using person-year methods. Age- and sex-adjusted standardized mortality ratios (SMRs) were estimated by dividing the observed number of deaths by the expected number of deaths in each age and sex category.
Statistical analysis.
To evaluate the independent predictors of mortality in individuals with type 1 diabetes, we used Cox proportional-hazards models in both the total cohort and selected subgroups. All variables known to be associated with mortality were included in the final models, along with any variables associated with mortality in univariate analyses with a P value <0.01. In each case, model selection from candidate variables was accomplished by minimization of the Akaike and Bayesian information criteria (11). Baseline UAE was entered as a categorical variable (normoalbuminuria, microalbuminuria, and macroalbuminuria). The potential for multiple colinearity was tested using the variance inflation factor and condition number, where a variance inflation factor <10 and condition number <30 are desirable (12). Covariate functional form (including assessment of nonlinear effects) was judged by residual-by-time analysis, fractional polynomials (13), and (cubic) regression splines (14). Overall, Cox model fit was assessed by 1) approximation of cumulative Cox-Snell residuals to (-log) Kaplan-Meier estimates, residual plots, and specific testing of the proportional hazards assumption (15) and 2) Harrell's C statistic (16) and “added-variable” goodness-of-fit tests (17). Cox model performance was judged by the explained variation using 5,000 bootstrap repetitions of the whole dataset, adjusting for covariates (18). Analyses were conducted with the use of Stata statistical software (version 10.0, 2007; StataCorp, College Station, TX) and SPSS software (version 13.0; SPSS, Chicago, IL).
RESULTS
Cohort characteristics.
FinnDiane recruited 4,201 adults with type 1 diabetes, equivalent to 16% of patients with type 1 diabetes in Finland (Table 1). At baseline, 504 individuals (12%) had UAE in the microalbuminuric range, 578 individuals (14%) had UAE in the macroalbuminuric range, and 293 individuals (7%) were receiving renal replacement therapy, including 72 individuals on dialysis and 221 individuals with a functioning kidney transplant. A total of 2,296 individuals (55%) had UAE in the normoalbuminuric range. A further 13% of study participants were unclassified (n = 530) because of an inadequate number of urine collections (<3). The clinical characteristics of these individuals were intermediate between those with normoalbuminuria and those with microalbuminuria (data not shown). In individuals not receiving renal replacement therapy, 14% (n = 462) had an estimated GFR <60 ml/min per 1.73 m2. The characteristics of these patients are shown in Table 2. When the number of individuals with an estimated GFR <60 ml/min per 1.73 m2 were added to micro- or macroalbuminuria and those receiving renal replacement therapy, 33% of study participants had chronic kidney disease. In addition, over half (57%) had established retinopathy, 63% of whom had received laser therapy for proliferative changes. In addition, 10% of the cohort had established macrovascular disease.
TABLE 1.
Baseline characteristics of FinnDiane study participants, stratified according to UAE*
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | ESKD | |
|---|---|---|---|---|
| n | 2,296 | 504 | 578 | 293 |
| Age (years) | 36 ± 12 | 38 ± 12* | 41 ± 10* | 44 ± 8* |
| Male sex | 1,088 (47) | 296 (59)† | 338 (58)† | 178 (61)† |
| Duration of diabetes (years) | 20 ± 12 | 26 ± 11* | 29 ± 8* | 32 ± 8* |
| Insulin dose (IU/kg) | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.4 |
| Insulin injections/day | 4.8 ± 1.0 | 4.8 ± 1.0 | 4.5 ± 1.0* | 4.2 ± 1.0* |
| A1C (%) | 8.2 ± 1.4 | 8.8 ± 1.5* | 9.0 ± 1.5* | 8.6 ± 1.6 |
| Estimated glucose disposal rate (mg · kg−1 · min−1)‡ | 7.3 ± 2.2 | 5.1 ± 2.0* | 4.0 ± 1.6* | 4.0 ± 1.6* |
| Hypertension | 1,344 (59) | 444 (88) | 557 (96) | 288 (97) |
| Systolic blood pressure (mmHg) | 129 ± 15 | 136 ± 16* | 145 ± 20* | 153 ± 25* |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 80 ± 10* | 83 ± 10* | 86 ± 13* |
| Medication use | ||||
| ACE inhibition | 204 (9) | 271 (54)† | 425 (75)† | 61 (21) |
| Angiotensin receptor blocker | 44 (2) | 42 (8)† | 75 (13)† | 31 (5)† |
| Calcium channel blocker | 60 (3) | 40 (8)† | 208 (36)† | 163 (56)† |
| β-Blocker | 90 (4) | 46 (9)† | 190 (33)† | 188 (64)† |
| Other antihypertensive agents | 64 (3) | 48 (10)† | 282 (49)† | 170 (58)† |
| Lipid-lowering therapy | 135 (6) | 49 (10)† | 137 (23)† | 76 (26)† |
| Total cholesterol (mmol/l) | 4.8 ± 0.9 | 5.0 ± 0.9 | 5.4 ± 1.1 | 5.5 ± 1.2 |
| LDL cholesterol (mmol/l) | 3.0 ± 0.8 | 3.1 ± 0.8 | 3.5 ± 0.9 | 3.5 ± 1.1* |
| HDL cholesterol (mmol/l) | 1.1 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 |
| Triglycerides (mmol/l) | 1.1 ± 0.7 | 1.3 ± 0.9 | 1.7 ± 1.2 | 1.7 ± 0.9* |
| Any retinopathy | 889 (39) | 382 (76)† | 547 (95)† | 290 (99)† |
| Retinopathy requiring laser therapy | 340 (15) | 241 (48)† | 460 (80)† | 288 (97)† |
| Current smoker | 491 (21) | 153 (30) | 164 (29) | 49 (16) |
| Established macrovascular disease | 88 (4) | 37 (7)† | 105 (18)† | 138 (47)† |
| Estimated GFR <60 ml/min per 1.73 m2 | 73 (3) | 45 (9)† | 354 (61)† | N/A |
Data are the means ± SD or n (% deaths). To convert values for cholesterol to milligrams per deciliter, divide by 0.2586.
*P value versus patients with normoalbuminuria <0.05, calculated by Student's t test;
†P value versus patients with normoalbuminuria <0.05, calculated by χ2;
‡a measure of insulin sensitivity using the formula proposed by Williams et al. (43). ESKD, end-stage kidney disease.
TABLE 2.
Baseline characteristics of FinnDiane study participants stratified according to estimated GFR*
| >120 | 60–120 | <60 | ESKD | |
|---|---|---|---|---|
| n | 272 | 2,699 | 462 | 293 |
| Age (years) | 28 ± 10* | 37 ± 12 | 44 ± 10* | 44 ± 8* |
| Male sex | 194 (71)† | 1,320 (49) | 214 (46) | 178 (61)† |
| Duration of diabetes (years) | 17 ± 11 | 20 ± 12 | 30 ± 9* | 32 ± 8* |
| Insulin dose (IU/kg) | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.4 |
| Insulin injections/day | 4.9 ± 1.0 | 4.8 ± 1.0 | 4.5 ± 1.0* | 4.2 ± 1.0* |
| A1C (%) | 8.6 ± 1.6 | 8.4 ± 1.5 | 8.8 ± 1.5* | 8.6 ± 1.6 |
| Estimated glucose disposal rate (mg · kg−1 · min−1)‡ | 7.9 ± 2.0 | 7.3 ± 2.2 | 4.6 ± 2.0* | 4.0 ± 1.6* |
| Hypertension | 176 (65) | 1,888 (70) | 444 (96)† | 288 (97)† |
| Systolic blood pressure (mmHg) | 128 ± 14 | 132 ± 16 | 145 ± 20* | 153 ± 25* |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 79 ± 9 | 81 ± 10* | 86 ± 13* |
| Medication use | ||||
| ACE inhibition | 30 (11)† | 611 (23) | 294 (64)† | 61 (21) |
| Angiotensin receptor blocker | 3 (1)† | 118 (4) | 55 (12)† | 31 (5)† |
| Calcium channel blocker | 2 (1)† | 134 (5) | 177 (39)† | 163 (56)† |
| β-Blocker | 1 (0)† | 150 (6) | 185 (40)† | 188 (64)† |
| Other antihypertensive agents | 1 (0)† | 154 (6) | 219 (47)† | 170 (58)† |
| Lipid-lowering therapy | 11 (4)† | 206 (8) | 123 (27)† | 76 (26)† |
| Total cholesterol (mmol/l) | 4.9 ± 0.9 | 4.9 ± 0.9 | 5.3 ± 1.0* | 5.5 ± 1.2* |
| LDL cholesterol (mmol/l) | 3.0 ± 0.8 | 3.0 ± 0.8 | 3.5 ± 1.0* | 3.5 ± 1.1* |
| HDL cholesterol (mmol/l) | 1.4 ± 0.4* | 1.1 ± 0.4 | 1.3 ± 0.4* | 1.3 ± 0.4* |
| Triglycerides (mmol/l) | 1.2 ± 0.7 | 1.2 ± 0.8 | 1.7 ± 1.2* | 1.7 ± 0.9* |
| Any retinopathy | 76 (29)† | 1,313 (50) | 420 (93)† | 290 (99)† |
| Retinopathy requiring laser therapy | 27 (10)† | 683 (25) | 360 (78)† | 288 (97)† |
| Current smoker | 98 (37)† | 614 (24) | 95 (22) | 49 (16)† |
| Established macrovascular disease | 31 (11)† | 123 (5) | 116 (25)† | 138 (47)† |
| Macroalbuminuria | 2 (3)† | 210 (8) | 338 (73)† | N/A |
| Microalbuminuria | 2 (5)† | 405 (15) | 37 (8)† | N/A |
Data are n (% deaths) or means ± SD. To convert values for cholesterol to milligrams per deciliter, divide by 0.02586.
*P < 0.05 vs. patients with an estimated GFR 60–120, calculated by Student's t test;
†P < 0.05 vs. patients with an estimated GFR 60–120, calculated by χ2;
‡ a measure of insulin sensitivity using the formula proposed by Williams et al. (43). ESKD, end-stage kidney disease.
Overall mortality in individuals with type 1 diabetes.
Median follow-up was 7 years, totaling 26,863 patient-years of surveillance, during which time there were 291 deaths (7%, 1.1 per 100 person-years). This mortality rate was 3.6 times higher (95% CI 3.2–4.0) than in the age- and sex-matched general population. Excess mortality associated with type 1 diabetes was more pronounced among women (adjusted SMR 4.8, 95% CI 4.0–5.8) than among men (3.0, 2.6–3.5; sex difference P < 0.001), eliminating the sex difference in mortality, as previously described (19). The distribution of the listed primary cause of death is shown in Table 3.
TABLE 3.
Primary cause of death of FinnDiane study participants stratified according to UAE and estimated GFR
| n | Primary cause of death |
All cause[n (% cohort)] | ||||
|---|---|---|---|---|---|---|
| Cardiovascular | Cancer | Infection | Other causes | |||
| UAE | ||||||
| Normoalbuminuria | 2,296 | 14 (47) | 2 (7) | 2 (7) | 12 (40) | 30 (1.3) |
| Microalbuminuria | 504 | 15 (56)* | 1 (4) | 3 (11)* | 7 (30)* | 26 (5.2)* |
| Macroalbuminuria | 578 | 40 (45)* | 3 (3)* | 18 (20)* | 28 (31)* | 89 (15.4)* |
| ESKD | 293 | 78 (58)* | 7 (5)* | 24 (18)* | 26 (19)* | 135 (46.1)* |
| Estimated GFR | ||||||
| >120 | 272 | 5 (42) | 1 (8) | 1 (8) | 5 (42) | 12 (4.4)† |
| 60–120 | 2,699 | 28 (46) | 3 (5) | 8 (13) | 22 (36) | 61 (2.3) |
| <60 | 462 | 42 (51)† | 3 (4)† | 15 (18)† | 22 (27)† | 82 (17.7)† |
| ESKD | 293 | 78 (58)† | 7 (5)† | 24 (18)† | 26 (19)† | 135 (46.1)† |
Data are n (% deaths), unless otherwise noted.
*P value <0.05 vs. patients with normoalbuminuria <0.05, calculated by χ2;
†P value <0.05 vs. patients with an estimated GFR 60–120 ml/min per 1.73 m2, as calculated by χ2. ESKD, end-stage kidney disease.
Chronic kidney disease and mortality in type 1 diabetes.
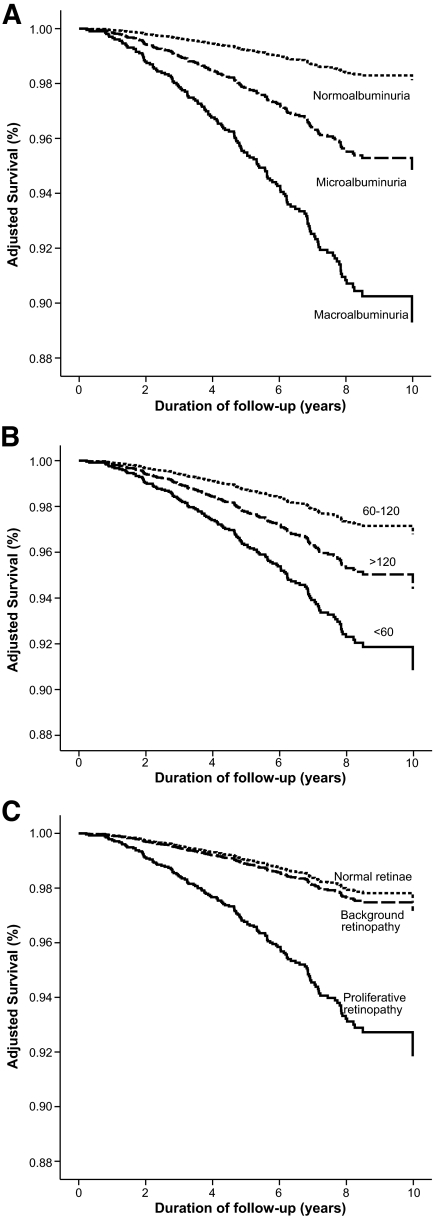
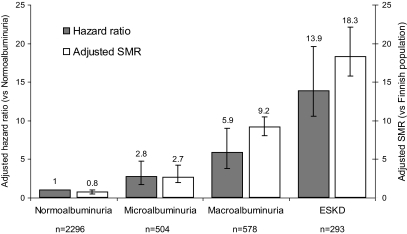
The presence and severity of chronic kidney disease was the major predictor of all-cause mortality in the FinnDiane cohort (Fig. 1A and B, Fig. 2, and the supplemental table, which is available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full//db08-1543/DC1). Indeed, excess mortality, beyond that observed in the age-and sex-matched general population, was only demonstrated in individuals with chronic kidney disease (Fig. 2). Mortality in the FinnDiane participants without chronic kidney disease (66% of the total cohort) was not significantly different compared to that observed in the general population (adjusted SMR 0.8, 95% CI 0.5–1.1), regardless of the duration of their diabetes.
FIG. 1.
Survival plots showing Cox-adjusted survival of individuals with type 1 diabetes from the FinnDiane study, stratified for the presence and severity of albuminuria (A), estimated GFR (B), and the presence and severity of retinopathy (C) at baseline. All figures are adjusted for age; sex; duration of diabetes; body habitus; the presence and extent of macro- and microvascular complications; glycemic, lipid, and blood pressure control; and drug management. The latter figure (C) is not adjusted for the presence and severity of nephropathy, as discussed in the text.
FIG. 2.
Risk of mortality in individuals with type 1 diabetes from the FinnDiane study associated each level of albuminuria and end-stage kidney disease (ESKD). Adjusted hazard ratios with 95% CIs are standardized against individuals with UAE in the normoalbuminuric range (arbitrary value of 1.0). Adjusted SMRs with 95% CIs are provided standardized against the age- and sex-matched Finnish general population (arbitrary value of 1.0).
The impact of each stage of diabetic kidney disease on mortality across the total cohort was equivalent to the presence of preexisting macrovascular disease (adjusted hazard ratio 2.5, 95% CI 1.7–3.7). In addition, the survival in individuals with both macrovascular disease and chronic kidney disease was significantly reduced compared with individuals with either condition alone, and not significantly different from that observed in individuals receiving renal replacement therapy. Whereas dyslipidemia, insulin sensitivity, and hypertension were strongly linked to chronic kidney disease in this cohort (Table 1), the association between chronic kidney disease and mortality was independent with respect to the achieved control and the use and intensity of therapies, including statins and blockade of the renin-angiotensin system (RAS), although such associations may be confounded by indication.
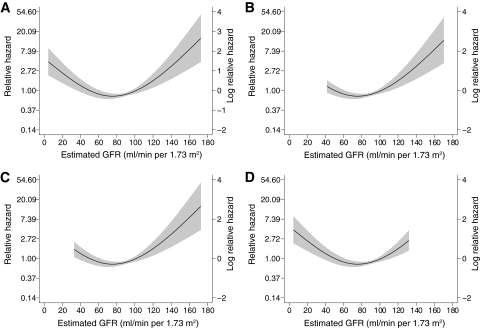
The estimated GFR was also associated with all-cause mortality (P < 0.001), independent of the level of UAE, macrovascular disease, and other baseline factors. The relationship between estimated GFR and mortality exhibited nonlinearity and was characterized using a regression spline (Fig. 3A). Mortality was increased in individuals with an estimated GFR <60 ml/min per 1.73 m2 (adjusted hazard ratio 1.7, 95% CI 1.1–2.6) (Fig. 1B). In addition, individuals with an estimated GFR >120 ml/min per 1.73 m2 also displayed increased mortality, independent of the presence or severity of albuminuria (Fig. 1B).
FIG. 3.
Relationship of estimated GFR to the hazard ratio for mortality in individuals with type 1 diabetes without end-stage kidney disease from the total FinnDiane cohort (A) and in individuals with normoalbuminuria (B), microalbuminuria (C), and macroalbuminuria (D). Dotted line shows point estimate for cubic regression spline adjusted for other predictive variables. Gray area denotes 95% CI.
A history of proliferative retinal disease (defined by the previous requirement for laser therapy) was also associated with increased mortality in the FinnDiane cohort (Fig. 1C). However, this association was eliminated after adjusting for baseline albuminuria. Because proliferative retinopathy and markers of diabetic kidney disease are strongly associated in this cohort (Table 1), and indeed may be considered manifestations of the same microangiopathy, it may not be appropriate from either a physiological or a statistical point of view to include retinopathy and chronic kidney disease in the same multiple regression analyses. In the absence of proliferative changes, background retinopathy was not associated with mortality in this cohort (Fig. 1C).
Mortality in individuals with end-stage kidney disease and in type 1 diabetes.
Of all the individuals with end-stage kidney disease, 46% died during the follow-up period (135 of 293). This mortality was 18.3 times that observed in the general population (95% CI 15.8–22.1). Mortality was highest in individuals on dialysis (68%) compared with those with a functioning kidney transplant (39%, P < 0.001). The independent predictors of mortality in individuals on dialysis (49 of 72) were the years on dialysis (adjusted hazard ratio, per year, 1.4, 95% CI 1.2–1.7) and the pulse pressure (per 10 mmHg, 1.3, 1.0–1.5). Glycemic control in individuals on dialysis was not associated with all-cause mortality. In individuals with a functioning renal transplant (86 of 221), the duration of diabetes (decades, adjusted hazard ratio 2.6, 95% CI 1.8–3.7) and A1C (1.3, 1.1–1.5) were predictors of all-cause mortality. In addition, individuals with impaired graft function characterized by an estimated GFR <60 ml/min per 1.73 m2 were over twice as likely to have died during follow-up than those with preserved graft function (adjusted hazard ratio 2.4, 95% CI 1.3–4.1).
Mortality in individuals with macroalbuminuria.
In individuals with macroalbuminuria, 15% (89 of 578) died during the follow-up period. This mortality rate was over nine times that observed in the age- and sex-matched general population (adjusted SMR 9.2, 95% CI 8.1–10.5). The major independent predictors of all-cause mortality in individuals with macroalbuminuria were duration of diabetes (decades, adjusted HR 1.6, 95% CI 1.2–2.1) and the presence of preexisting macrovascular disease (2.1, 1.2–3.4) and A1C (1.3, 1.2–1.5). In addition, individuals with macroalbuminuria and an estimated GFR <60 ml/min per 1.73 m2 were twice as likely to have died during follow-up as those with preserved kidney function (2.0, 1.1–3.4), independent of other baseline risk factors and the use of preventive therapies (Fig. 3B). The presence of a reduced estimated GFR was equivalent (in terms of mortality risk) to that of preexisting macrovascular disease.
Mortality in individuals with microalbuminuria.
In individuals with microalbuminuria, 5.2% (26 of 504) died during follow-up. This mortality rate was over twice that observed in the general population (adjusted SMR 2.8, 95% CI 2.0–4.2). The major independent predictors of all-cause mortality in microalbuminuria were the duration of diabetes (decades, adjusted HR 2.0, 95% CI 1.3–3.0) and the presence of preexisting macrovascular disease (6.0, 2.4–14.5). All-cause mortality was not significantly associated with treatments, the achieved level of blood pressure, or lipid levels. The estimated GFR was also independently associated with mortality risk (P < 0.01). This relationship was nonlinear and was characterized using a regression spline (Fig. 3C). Individuals with microalbuminuria and estimated GFR <60 ml/min per 1.73 m2 (8%) were more likely to have died during follow-up than those with preserved kidney function (unadjusted mortality 18 vs. 4%, P < 0.01). Equally, individuals with microalbuminuria and an estimated GFR >120 ml/min per 1.73 m2 had increased mortality compared with those with normal renal function (unadjusted mortality 7 vs. 3%, P < 0.01).
Mortality in individuals with normoalbuminuria.
In individuals with normoalbuminuria, only 1.3% (30 of 2,296) died during follow-up. This rate was not significantly different from the general population (adjusted SMR 0.8, 95% CI 0.5–1.1) and was independent of the duration of diabetes. The major independent predictors of mortality in individuals with normoalbuminuria were age (decades, adjusted HR 1.7, 95% CI 1.2–2.4) and the presence of preexisting macrovascular disease (6.7, 2.5–17.6). Again, mortality in these individuals was not associated with lipid levels or blood pressure control. However, the estimated GFR was independently associated with mortality (P < 0.001). The relationship between estimated GFR and the hazard ratio for mortality in individuals with normoalbuminuria again exhibited nonlinearity (Fig. 3D). Although <3% of individuals with normoalbuminuria had an estimated GFR <60 ml/min per 1.73 m2, significantly more of these individuals died during follow-up than those with normal kidney function (unadjusted mortality 5 vs. 1%, P < 0.01). In addition, individuals with an estimated GFR >120 ml/min per 1.73 m2 also displayed increased mortality compared with those with normal renal function (unadjusted mortality 3 vs. 1%, P < 0.01).
Mortality and glycemic control in type 1 diabetes.
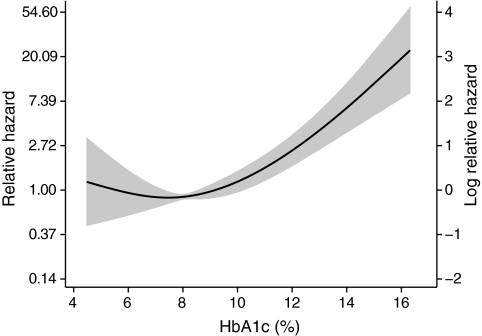
Glycemic control is an important target for the management of individuals with type diabetes. In FinnDiane participants not receiving renal replacement therapy, glycemic control, as estimated by the A1C, was independently and continuously associated with all-cause mortality (adjusted HR 1.3, 95% CI 1.2–1.4). However, the relationship between A1C and the hazard for mortality exhibited some nonlinearity and was modeled as a multivariate cubic spline (Fig. 4). Notably, there was no difference in the relationship between A1C and mortality risk across different subgroups of albuminuria (interaction P > 0.34).
FIG. 4.
Relationship of A1C to the hazard ratio for mortality in individuals with type 1 diabetes without end-stage kidney disease. Dotted line shows point estimate for the cubic regression spline adjusted for other predictive variables. Gray area denotes 95% CI.
Insulin resistance has also been associated with cardiovascular disease in individuals with type 1 diabetes (20), although this relationship is confounded by the strong association between the insulin sensitivity and chronic kidney disease in type 1 diabetes (7). In the FinnDiane cohort, insulin sensitivity, as measured by the estimated glucose disposal rate (20), was inversely associated with all-cause mortality (P < 0.001), independent of age, duration of diabetes, and glycemic control. However, this association was eliminated after adjusting for the presence and severity of chronic kidney disease (P = 0.9).
DISCUSSION
In a large sample of adults with type 1 diabetes from Finland, an independent, graded association was observed between the presence and severity of chronic kidney disease and all-cause mortality. In this population, excess mortality associated with type 1 diabetes was only observed in individuals with chronic kidney disease (Fig. 2), whereas mortality in participants without chronic kidney disease (66% of the total cohort) was identical to the general population. These results from a stable, nationally representative cohort of adults with type 1 diabetes complement and extend evidence from the last 2 decades showing the link between mortality and chronic kidney disease (3–6). These findings highlight the continuing clinical and public health importance of chronic kidney disease and its prevention in the management of type 1 diabetes.
Our findings in individuals with type 1 diabetes are analogous to the relation between chronic kidney disease and mortality previously described in individuals with type 2 diabetes (21) and in nondiabetic individuals (22).The observed association between chronic kidney disease and mortality can be explained by several plausible mechanisms. It has been suggested that kidney damage in individuals with type 1 diabetes reflects more generalized damage to the cardiovascular system leading to cardiovascular disease and subsequently mortality (23). The association of mortality with proliferative retinopathy (Fig. 1C) may be explained in the same way. However, impaired kidney function may also directly contribute to hypertension (24), oxidative stress, insulin resistance (7), inflammation (8), dyslipidemia (25), elevated plasma homocysteine (26), the accumulation of advanced glycation end products (27), anemia (28), left ventricular hypertrophy (29), arterial calcification (30), and endothelial dysfunction (31). Chronic kidney disease is also associated with impaired immune function, which, alongside vascular compromise, probably contributed to the increased frequency of death from infection observed in those with chronic kidney disease.
The observed hazards associated with each stage of kidney disease are similar to those reported in cohort studies >20 years ago (3–6), although the absolute mortality in the FinnDiane cohort is significantly lower. Although it is possible to argue that improvements in diabetic management have not led to changes in the relative risk of mortality in individuals with chronic kidney disease, there is little doubt that the absolute risk reduction has been considerable over this time period, via the prevention of chronic kidney disease, and the management of individuals with proteinuria (32) and/or macrovascular disease, who carry the majority of the risk burden. However, the contemporary hazard associated with chronic kidney disease demonstrated in this study emphasizes that more remains to be done to address this enhanced risk beyond currently available regimens.
This study also demonstrates that estimated GFR is an independent risk factor for mortality in type 1 diabetes. Regardless of the level of albuminuria, those with an estimated GFR <60 ml/min per 1.73 m2 were twice as likely to have died during follow-up. In addition, an elevated estimated GFR was also associated with increased mortality (Fig. 1B), which was identified by nonlinear analysis (Fig. 3). Although it should be noted that the MDRD equation overestimates renal function in this range and that the ability of this formula to discriminate hyperfiltration from normal function is suboptimal (33), particularly as a single measurement, it was nonetheless able to stratify mortality risk. In so far as hyperfiltration precedes progressive kidney disease (34,35), this association is not surprising, and probably reflects early pathological changes both in the kidney and in other parts of the vasculature.
Although hyperglycemia has been implicated in the development of microvascular complications in type 1 diabetes, an association between glycemic control and mortality has only been observed in some (36), but not all studies (20,37). In the FinnDiane cohort, glycemic control was also independently associated with all-cause mortality (Fig. 4). A significant association between A1C and mortality was also present in individuals with a kidney transplant, consistent with previous reports (38). However, when modeled as a linear variable, no clear association was observed between A1C and mortality in individuals with normo- or microalbuminuria (P ≥ 0.38). It is possible that this reflects a type II error due to the lower number of deaths in these subgroups. However, when modeled as a multivariate cubic spline (Fig. 4), there was no interaction between A1C and albuminuria (P > 0.34) in determining mortality, suggesting the impact of poor glycemic control is homogeneous across all levels of albuminuria.
Strengths of our study include its large cohort of individuals with type 1 diabetes, high participation rate, access to subsidized care (75–100% of costs), and contemporary treatment regimens, including a range of insulin regimens, statins, blockers of the RAS, and self-monitoring technologies. Our methods of measuring UAE and serum creatinine were reliable, validated centrally using standardized methodologies. We used validated methods to identify deaths, and all deaths in our cohort were confirmed through death records. Surveillance bias is unlikely, given the uniform vital status follow-up procedures used by our staff masked to participants' chronic kidney disease status levels. In our questionnaire, we had broad data on tobacco or alcohol use, diet, education, socioeconomic status, other possible confounders (e.g., insulin resistance), or the severity of disease (e.g., level of blood pressure). Finally, few changes in diabetes treatment and health care over the short study period will have affected mortality results.
Several study limitations need to be considered. First, our study results may not be generalizable because of selection bias in enrollment and subsequently in ascertainment. Our study was conducted among adults with long-standing diabetes. Consequently, our results may reflect past management practices that are less generalizable to adults with newly diagnosed diabetes. These studies were also limited by the use of broad definitions of diminished kidney function and the inclusion of relatively small numbers of subjects with renal impairment, thus reducing the statistical power to examine different levels of reduced estimated GFR. It is also likely that our clinical history was not sufficiently sensitive to accurately discriminate between the presence or absence of macrovascular disease in this population, given that diabetes is often associated with silent ischemia and that subjects with type 1 diabetes can often have a normal resting electrocardiogram and still have suffered a previous myocardial infarction or have significant coronary lesions (39). Such standardized exercise testing and/or coronary imaging was not feasible in a large study such as FinnDiane. Consequently, the true contribution of underlying macrovascular disease to the observed changes in mortality cannot be ascertained in this study. Nonetheless, a reliance on clinical history is potentially more representative of the true clinical setting. Changes in medications and risk factor control during follow-up cannot be controlled for. Finally, it is possible that our results were biased by residual confounding by factors not measured in the study but potentially related to cardiovascular disease/mortality in type 1 diabetes, including silent cardiovascular disease, lipoprotein subclasses (40), oxidative stress (41), and endothelial (31) and autonomic dysfunction (42).
In conclusion, results from our study clearly demonstrate that chronic kidney disease is the dominant contributor to excess mortality in type 1 diabetes. Consequently, if you have type 1 diabetes, prevention of chronic kidney disease is currently the best way to reduce your risk of a premature death. Modern multifactorial therapeutic approaches, such as those detailed in American Diabetes Association guidelines, are effective in preventing the development of kidney disease. For those with established chronic kidney disease, more intensive multifactorial interventions are also valuable in reducing the progression of chronic kidney disease, and, with it, mortality.
Supplementary Material
Acknowledgments
The FinnDiane study was funded by the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Sigrid Juselius Foundation, European Commission, Medicinska Understödsföreningen Liv och Hälsa, Signe and Ane Gyllenberg Foundation, Waldemar von Frenckell Foundation, EVO governmental grants, and the National Institutes of Health. None of these groups had a role in data collection, analysis, or preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
P.-H.G., J.W., L.M.T., V.-P.M., M.R.-B., M.S., K.H., O.H., and C.F. were involved in data collection. We also acknowledge all the physicians and nurses at each participating center for their invaluable role in patient recruitment and collection of samples and data (previously presented in detail) (8). M.C.T., P.-H.G., C.F., and J.L.M. were involved in data analysis and manuscript preparation. All authors contributed to the final submitted version.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, Matsushima M, Reunanen A, Tuomilehto J, Tajima N: Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care 2003; 26: 2037– 2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, Binder C, Parving HH: Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003; 26: 1258– 1264 [DOI] [PubMed] [Google Scholar]
- 3.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH: Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 1996; 313: 779– 784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messent JW, Elliott TG, Hill RD, Jarrett RJ, Keen H, Viberti GC: Prognostic significance of microalbuminuria in insulin-dependent diabetes mellitus: a twenty-three year follow-up study. Kidney Int 1992; 41: 836– 839 [DOI] [PubMed] [Google Scholar]
- 5.Forsblom CM, Groop PH, Ekstrand A, Groop LC: Predictive value of microalbuminuria in patients with insulin-dependent diabetes of long duration. BMJ 1992; 305: 1051– 1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643– 2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, Waden J, Ronnback M, Rosengard-Barlund M, Bjorkesten CG, Taskinen MR, Groop PH: Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005; 28: 2019– 2024 [DOI] [PubMed] [Google Scholar]
- 8.Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH: Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 2003; 46: 1402– 1407 [DOI] [PubMed] [Google Scholar]
- 9.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1– S266 [PubMed] [Google Scholar]
- 10.Force ICGT: Global Guideline for Type 2 Diabetes Brussels, International Diabetes Federation, 2005 [Google Scholar]
- 11.Kuha J: AIC and BIC: comparisons of assumptions and performance. Sociol Method Res 2005; 33: 188– 229 [Google Scholar]
- 12.Belsley D: Conditioning Diagnostics, Collinearity and Weak Data in Regression New York, Wiley, 1991 [Google Scholar]
- 13.Royston P: A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 2000; 19: 1831– 1847 [DOI] [PubMed] [Google Scholar]
- 14.Royston P, Sauerbrei W: Multivariable modeling with cubic regression splines: a principled approach. Stata J 2008; 7: 45– 70 [Google Scholar]
- 15.Grambsch P, Therneau TM: Proportional hazards tests and diagnostics based upon weighted residuals. Biometrika 1994; 81: 515– 526 [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361– 387 [DOI] [PubMed] [Google Scholar]
- 17.May S, Hosmer DW: Hosmer and Lemeshow type goodness-of-fit statistics for the Cox proportional hazards model. In Advances in Survival Analysis Rao NBCR: Ed. Amsterdam, Elsevier, 2004, p. 383– 394 [Google Scholar]
- 18.Royston P: Explained variation for survival models. Stata J 2006; 6: 83– 96 [Google Scholar]
- 19.Barrett-Connor E, Wingard DL: Sex differential in ischemic heart disease mortality in diabetics: a prospective population-based study. Am J Epidemiol 1983; 118: 489– 496 [DOI] [PubMed] [Google Scholar]
- 20.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ: Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003; 26: 1374– 1379 [DOI] [PubMed] [Google Scholar]
- 21.Bruno G, Merletti F, Bargero G, Novelli G, Melis D, Soddu A, Perotto M, Pagano G, Cavallo-Perin P: Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia 2007; 50: 941– 948 [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Lin J, Solomon CG, Jablonski KA, Rice MM, Steffes M, Domanski M, Hsia J, Gersh BJ, Arnold JM, Rouleau J, Braunwald E, Pfeffer MA: Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation 2007; 116: 2687– 269318025537 [Google Scholar]
- 23.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia 1989; 32: 219– 226 [DOI] [PubMed] [Google Scholar]
- 24.Thomas MC, Atkins RC: Blood pressure lowering for the prevention and treatment of diabetic kidney disease. Drugs 2006; 66: 2213– 2234 [DOI] [PubMed] [Google Scholar]
- 25.Tolonen N, Forsblom C, Thorn L, Waden J, Rosengard-Barlund M, Saraheimo M, Heikkila O, Pettersson-Fernholm K, Taskinen MR, Groop PH: Relationship between lipid profiles and kidney function in patients with type 1 diabetes. Diabetologia 2008; 51: 12– 20 [DOI] [PubMed] [Google Scholar]
- 26.Chico A, Perez A, Cordoba A, Arcelus R, Carreras G, de Leiva A, Gonzalez-Sastre F, Blanco-Vaca F: Plasma homocysteine is related to albumin excretion rate in patients with diabetes mellitus: a new link between diabetic nephropathy and cardiovascular disease? Diabetologia 1998; 41: 684– 693 [DOI] [PubMed] [Google Scholar]
- 27.Thomas MC, Baynes JW, Thorpe SR, Cooper ME: The role of AGEs and AGE inhibitors in diabetic cardiovascular disease. Curr Drug Targets 2005; 6: 453– 474 [DOI] [PubMed] [Google Scholar]
- 28.Thomas MC, MacIsaac RJ, Tsalamandris C, Molyneaux L, Goubina I, Fulcher G, Yue D, Jerums G: Anemia in patients with type 1 diabetes. J Clin Endocrinol Metab 2004; 89: 4359– 4363 [DOI] [PubMed] [Google Scholar]
- 29.Sampson MJ, Chambers J, Sprigings D, Drury PL: Intraventricular septal hypertrophy in type 1 diabetic patients with microalbuminuria or early proteinuria. Diabet Med 1990; 7: 126– 131 [DOI] [PubMed] [Google Scholar]
- 30.Maahs DM, Snell-Bergeon JK, Kinney GL, Wadwa RP, Garg S, Ogden LG, Rewers M: ACE-I/ARB treatment in type 1 diabetes patients with albuminuria is associated with lower odds of progression of coronary artery calcification. J Diabetes Complications 2007; 21: 273– 279 [DOI] [PubMed] [Google Scholar]
- 31.Stehouwer CD, Fischer HR, van Kuijk AW, Polak BC, Donker AJ: Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes 1995; 44: 561– 564 [DOI] [PubMed] [Google Scholar]
- 32.Astrup AS, Tarnow L, Rossing P, Pietraszek L, Riis Hansen P, Parving HH: Improved prognosis in type 1 diabetic patients with nephropathy: a prospective follow-up study. Kidney Int 2005; 68: 1250– 1257 [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim H, Mondress M, Tello A, Fan Y, Koopmeiners J, Thomas W: An alternative formula to the Cockcroft-Gault and the modification of diet in renal diseases formulas in predicting GFR in individuals with type 1 diabetes. J Am Soc Nephrol 2005; 16: 1051– 1060 [DOI] [PubMed] [Google Scholar]
- 34.Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, Dalton RN, Dunger DB: The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: the Oxford Regional Prospective Study. Kidney Int 2005; 68: 1740– 1749 [DOI] [PubMed] [Google Scholar]
- 35.Mogensen CE: Prediction of clinical diabetic nephropathy in IDDM patients: alternatives to microalbuminuria? Diabetes 1990; 39: 761– 767 [DOI] [PubMed] [Google Scholar]
- 36.Shankar A, Klein R, Klein BE, Moss SE: Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol 2007; 166: 393– 402 [DOI] [PubMed] [Google Scholar]
- 37.Weis U, Turner B, Gibney J, Watts GF, Burke V, Shaw KM, Cummings MH: Long-term predictors of coronary artery disease and mortality in type 1 diabetes. QJM 2001; 94: 623– 630 [DOI] [PubMed] [Google Scholar]
- 38.Stadler M, Auinger M, Anderwald C, Kastenbauer T, Kramar R, Feinbock C, Irsigler K, Kronenberg F, Prager R: Long-term mortality and incidence of renal dialysis and transplantation in type 1 diabetes mellitus. J Clin Endocrinol Metab 2006; 91: 3814– 3820 [DOI] [PubMed] [Google Scholar]
- 39.Koistinen MJ: Prevalence of asymptomatic myocardial ischaemia in diabetic subjects. BMJ 1990; 301: 92– 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groop PH, Elliott T, Ekstrand A, Franssila-Kallunki A, Friedman R, Viberti GC, Taskinen MR: Multiple lipoprotein abnormalities in type I diabetic patients with renal disease. Diabetes 1996; 45: 974– 979 [DOI] [PubMed] [Google Scholar]
- 41.Hata I, Kaji M, Hirano S, Shigematsu Y, Tsukahara H, Mayumi M: Urinary oxidative stress markers in young patients with type 1 diabetes. Pediatr Int 2006; 48: 58– 61 [DOI] [PubMed] [Google Scholar]
- 42.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH: Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2006; 29: 334– 339 [DOI] [PubMed] [Google Scholar]
- 43.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ: Can clinical factors estimate insulin resistance in type 1 diabetes? 2000; 49: 626– 632 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.