Abstract
OBJECTIVE
To evaluate mechanisms underlying diabetic neuropathy progression using indexes of sural nerve morphometry obtained from two identical randomized, placebo-controlled clinical trials.
RESEARCH DESIGN AND METHODS
Sural nerve myelinated fiber density (MFD), nerve conduction velocities (NCVs), vibration perception thresholds, clinical symptom scores, and a visual analog scale for pain were analyzed in participants with diabetic neuropathy. A loss of ≥500 fibers/mm2 in sural nerve MFD over 52 weeks was defined as progressing diabetic neuropathy, and a MFD loss of ≤100 fibers/mm2 during the same time interval as nonprogressing diabetic neuropathy. The progressing and nonprogressing cohorts were matched for baseline characteristics using an O'Brien rank-sum and baseline MFD.
RESULTS
At 52 weeks, the progressing cohort demonstrated a 25% decrease (P < 0.0001) from baseline in MFD, while the nonprogressing cohort remained unchanged. MFD was not affected by active drug treatment (P = 0.87), diabetes duration (P = 0.48), age (P = 0.11), or BMI (P = 0.30). Among all variables tested, elevated triglycerides and decreased peroneal motor NCV at baseline significantly correlated with loss of MFD at 52 weeks (P = 0.04).
CONCLUSIONS
In this cohort of participants with mild to moderate diabetic neuropathy, elevated triglycerides correlated with MFD loss independent of disease duration, age, diabetes control, or other variables. These data support the evolving concept that hyperlipidemia is instrumental in the progression of diabetic neuropathy.
Twenty-three million Americans have diabetes, and the incidence is increasing by 5% per year. The most common complication of diabetes is peripheral neuropathy, occurring in ∼60% of all diabetic patients (1–3). In the U.S., diabetic neuropathy is the leading cause of diabetes-related hospital admissions and nontraumatic amputations (1–3). Current methods used to confirm diabetic neuropathy and measure its progression include presence of symptoms, clinical signs, deficits in nerve conduction studies (NCVs), and quantitative sensory measures (1–3). Changes in these parameters correlate with anatomical evidence of decreased large and small myelinated fiber densities (MFDs) in the sural nerve (4,5) and the epidermis (intraepidermal nerve fiber density) (6). Although several risk factors for diabetic neuropathy are identified in prior randomized or observational clinical trials (7,8), a comprehensive understanding of their relationship and relevance for risk assessment is still lacking.
Diabetic neuropathy is positively correlated with the most common marker of hyperglycemia, A1C (9). Recent clinical evidence suggests that dyslipidemia is also associated with diabetic neuropathy. The EURODIAB study established a significant association between cholesterol and fasting triglycerides and the development of diabetic neuropathy (10) and cardiac autonomic neuropathy (11). A review by Steinmetz (12) summarizes data from the U.K. Prospective Diabetes Study Group and the Fenofibrate Intervention and Event Lowering in Diabetes Study indicate that lipid-lowering therapy reduces the incidence of macrovascular complications and microvascular complications including retinopathy, nephropathy, and autonomic neuropathy.
We are in possession of a unique repository of samples and data, including human sural nerve biopsies with matched blood chemistries, electrophysiology, and nerve function tests, from participants in a large, randomized placebo-controlled clinical diabetic neuropathy trial testing acetyl-l-carnitine (ALC). Based on this material, we assessed predictors of diabetic neuropathy by correlating the change in sural nerve MFD, assessed at study onset and again at study completion 1 year later, with baseline participant characteristics. Our results indicate that those participants with progressing diabetic neuropathy exhibited significantly elevated triglyceride levels and deficits in peroneal motor NCV at baseline. These results indicate a role for dyslipidemia in the progression of diabetic neuropathy and demonstrate that changes in motor NCV may precede sensory nerve fiber loss.
RESEARCH DESIGN AND METHODS
We analyzed deidentified data obtained from two identical double-blind, placebo controlled, multicenter, 52-week clinical diabetic neuropathy trials of two ALC doses (1.5 and 3.0 g/day), conducted and supported by Sigma-Tau Research (13). Both placebo and drug-treated participants were considered in the analyses. Design of these trials is described elsewhere (13). Eligibility criteria included A1C >5.9%, age between 18 and 70 years, diabetes duration of >1 year, and diabetic neuropathy as defined by the San Antonio Conference (13,14). Because the data analyzed in this report were deidentified, the University of Michigan Institutional Review Board concluded that no human subjects were involved in this project.
Blood chemistry, clinical symptoms, and electrophysiology.
Blood samples were collected at baseline, and A1C, triglycerides, cholesterol, albumin, and hematocrit were recorded. Clinical symptoms, including pain, numbness, paresthesia, muscle weakness, postural dizziness, problems with sweating, gastrointestinal problems, and sexual dysfunction were recorded at baseline and at 52 weeks and scored on a scale of 0 (no symptoms) to 3 (incapacitating symptoms). In addition, the participants' own assessment of their most troublesome symptom at baseline was recorded.
Vibration perception thresholds of the index finger and great toe were assessed bilaterally in triplicate (15) at baseline and at 52 weeks using a Vibratron (Physitemp Instruments, Clifton, NJ) (16). These measures were completed during a 4-week run-in period prior to randomization (13). Electrophysiological measurements included bilateral sural nerve amplitude and conduction velocity, peroneal amplitude and NCV on the dominant side, and median motor and sensory amplitudes and NCVs on the nondominant side. Sural nerve amplitude ≥1 μV was required for inclusion.
Sural nerve biopsies.
Sural nerve biopsies were collected from the majority of the study participants. A biopsy was taken from one ankle at the beginning of the study, and a second biopsy was taken from the opposite ankle after 52 weeks. Morphometric parameters measured included total myelinated fiber number, fascicular area, mean fiber size, MFD, fiber occupancy, and axon-to-myelin ratio (17). MFD (fibers/mm2) in the largest fascicle was determined in semi-thin, paraphenylene diamine–stained sections. Details of counting methods have been previously described (4,5).
Primary outcome measure.
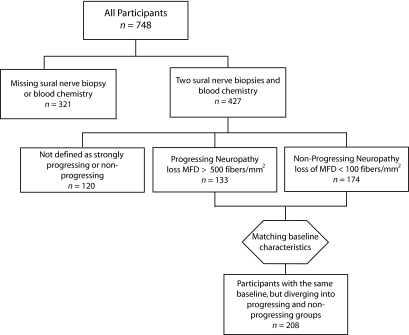
The primary outcome measure of the present study was the difference between the initial and 52-week sural nerve MFD. Participants without both a primary and secondary sural nerve biopsy or blood chemistry data were excluded from our current data analyses. Of 748 participants in the study, 321 were excluded due to missing data. A total of 427 participants were included in the primary data analysis of the present study (Fig. 1).
FIG. 1.
Schematic outline of participant selection. This flow diagram represents the decision process for including or excluding participants at each stage of the analysis.
Data analysis.
The analysis of these data were divided into two stages. In the primary data analysis, variables were tested for a simple correlation with the rate of MFD loss. However, a simple correlation assumes a consistent, linear progression of diabetic neuropathy, which may not be the case. In the secondary analysis, we balanced groups based on initial diabetic neuropathy status and tested the significant variables correlated with divergent outcomes.
Primary data analysis.
In the cohort of 427 participants, 99.5% of clinical symptoms, vibration perception, and electrophysiological measures were available. The small number of missing values were imputed by the k-nearest-neighbor technique (18). The O'Brien rank-sum (19) of each patient was calculated at baseline using the values for NCV, amplitude, vibration perception, and the clinical symptom score. Continuous variables (e.g., A1C) from the initial time point were correlated with change in sural nerve MFD using the Spearman nonparametric method, and a significance value of the correlation was calculated. Categorical variables (treatment, sex, diabetes type, most bothersome symptom at baseline, and insulin treatment) were tested for significant differences in sural nerve MFD by a Mann-Whitney test (20) (two categories) or Kruskal-Wallis (21) (more than two categories).
Participant selection for the secondary data analysis.
To identify factors driving diabetic neuropathy progression, two groups of participants with a similar sural nerve MFD and diabetic neuropathy at baseline, but differing degrees of sural nerve MFD at 52 weeks, were defined. A Perl program evaluated the change in sural nerve MFD and identified participants with an absolute loss of 500 fibers/mm2 over 52 weeks as having rapidly progressing diabetic neuropathy. Participants with a loss of 100 fibers/mm2 or less over 52 weeks were identified as having nonprogressing diabetic neuropathy. Participants with a 52-week sural nerve MFD >1,000/mm2 greater than baseline were excluded. Diabetic neuropathy was also estimated in the participants using the O'Brien rank-sum score, a nonparametric combination of neuropathy measures. The O'Brien rank-sum is composed of a linear combination of the variables listed in Table 1, excluding the “most bothersome symptom at baseline,” demographic data and drug treatment information. These 16 variables describe the NCV in three nerves, along with the corresponding nerve amplitudes, vibration perception thresholds in the fingers and toes, and the total clinical symptom score. The program matched each participant with rapidly progressing diabetic neuropathy with a nonprogressing diabetic neuropathy participant with similar sural nerve MFD and O'Brien score at baseline. The maximum difference in MFD between matching participants was set as 1,000 fibers/mm2, and the maximum difference in the O'Brien was set as 1,000. This O'Brien threshold required that at least 3 of 16 measures of neuropathy differ by a large degree between the participants at baseline. When multiple participants were under the similarity thresholds defined, the samples with the most similar initial fiber densities were matched.
TABLE 1.
Significance of correlation or association between possible risk factors and MFD loss
| Nominal P value | Correlation | |
|---|---|---|
| Most bothersome symptom | 0.58 | NA |
| Total clinical symptom score | 0.44 | −0.038 |
| Toe vibration perception | 0.40 | −0.041 |
| Left-finger vibration perception | 0.43 | −0.038 |
| Right-finger vibration perception | 0.96 | −0.003 |
| Dominant peroneal motor NCV | 0.005 | 0.136 |
| Dominant peroneal motor amplitude (knee) | 0.71 | 0.018 |
| Dominant peroneal motor amplitude (ankle) | 0.77 | 0.014 |
| Sural sensory NCV | 0.05 | 0.095 |
| Sural sensory nerve amplitude | 0.42 | 0.039 |
| Nondominant median motor NCV | 0.02 | 0.110 |
| Nondominant median motor amplitude (elbow) | 0.90 | 0.006 |
| Nondominant median motor amplitude (wrist) | 0.83 | −0.011 |
| Nondominant median sensory NCV (elbow) | 0.08 | 0.086 |
| Nondominant median sensory amplitude (elbow) | 0.05 | 0.094 |
| Nondominant median sensory NCV (wrist) | 0.36 | 0.045 |
| Nondominant median sensory amplitude (wrist) | 0.27 | 0.053 |
| A1C | 0.012 | −0.115 |
| Triglycerides | 0.02 | −0.110 |
| BMI | 0.54 | −0.029 |
| Cholesterol | 0.27 | −0.053 |
| Hematocrit | 0.84 | 0.010 |
| Serum albumin | 0.46 | −0.036 |
| Diabetes duration | 0.07 | 0.086 |
| Age | 0.13 | −0.074 |
| Insulin | 0.46 | NA |
| Sex | 0.10 | NA |
| Drug treatment | 0.87 | NA |
NA, not applicable.
Based on these unbiased criteria, type 1 diabetic and insulin-treated participants were overrepresented in the nonprogressing group. This enrichment was not statistically significant (diabetes type P = 0.19, insulin treatment P = 0.11). However, because insulin is known to be neuroprotective (22,23), the two groups were then explicitly balanced for diabetes type and insulin treatment to prevent a potential confounding effect. After balancing, 104 rapidly progressing and 104 nonprogressing participants were identified for further analysis, for a total n = 208 (Fig. 1).
Secondary data analysis.
In the secondary data analysis, rapidly progressing participants were compared with nonprogressing participants. Variables that were significantly correlated or associated with decreased sural nerve MFD in the primary analysis were advanced to the secondary analysis. The variables were tested for significant differences between the rapidly progressing and nonprogressing groups using the Mann-Whitney nonparametric test (20).
Machine-learning analysis.
Machine-learning analysis was performed according to the American Diabetes Association Consensus Statement on Computer Modeling of Diabetes (24). The rapidly progressing and nonprogressing groups (Fig. 1) were used as a “training” set for seven machine-learning techniques (Naïve Bayes, k-nearest-neighbor, support vector machine, linear regression, random Forest, classification tree and CN2 rule based) (25). The accuracy, sensitivity, and specificity of each model was estimated using leave-one-out cross validation (26). To ensure that overfitting did not take place, the highest-performing and most sensitive models were then tested on an independent dataset from the same population. The dataset was taken from 56 participants who fit the criteria of rapidly progressing (28 participants) or nonprogressing (28 participants) but who were not included in the secondary analysis cohort. A classification confidence threshold was chosen using this independent set, creating a third category of “unclassified” participants that the model lacked confidence to classify. Classification accuracy was reported based on this independent set. All analyses were performed by one investigator (T.D.W.) using GraphPad Prism 5.01 for Windows (San Diego, CA) and Orange (Ljubljana, Slovenia) (25).
RESULTS
The dataset included 748 participants in the ALC clinical trials, but blood chemistries, initial sural nerve MFD, and 52-week sural nerve MFD were only available from 427 participants (Fig. 1). There were no significant demographic, treatment, or metabolic differences between the excluded cohort (321 participants) and those with the necessary data for the primary analysis (427 participants). ALC treatment did not affect sural nerve MFD loss (P = 0.87); therefore, data from ALC-treated participants were pooled with placebo-treated participants in tests related to outcome. The participants included in the primary analysis were primarily male (67%), the majority had type 2 diabetes (78%), and the majority (59%) were treated with insulin (Table 2).
TABLE 2.
Patient characteristics at baseline
| All participants | Participants with complete data | Matched rapidly progressing and nonprogressing participants | |
|---|---|---|---|
| n | 748 | 427 | 208 |
| Treatment | |||
| 1,000 mg ALC | 252 | 150 | 69 |
| 500 mg ALC | 252 | 143 | 71 |
| Placebo | 244 | 134 | 68 |
| Sex | |||
| Male | 475 | 284 | 150 |
| Female | 273 | 143 | 58 |
| Diabetes type | |||
| Type 1 | 168 | 95 | 40 |
| Type 2 | 580 | 332 | 168 |
| Most bothersome symptom at baseline | |||
| Burning | 65 | 39 | 21 |
| Numbness | 213 | 114 | 55 |
| Pain | 213 | 126 | 58 |
| Paresthesia | 32 | 12 | 5 |
| Tingling | 88 | 59 | 29 |
| Weakness | 9 | 7 | 5 |
| Other | 128 | 126 | 36 |
| Insulin treatment | |||
| Yes | 447 | 251 | 118 |
| No | 301 | 176 | 90 |
| Age (years) | 53.0 ± 11 | 53.1 ± 10 | 53.4 ± 11 |
| Diabetes duration (years) | 12.3 ± 8 | 12.3 ± 8 | 11.6 ± 8 |
| BMI (kg/cm2) | 29.7 ± 6 | 29.7 ± 6 | 29.7 ± 6 |
| A1C (%) | 8.8 ± 1.8 | 8.8 ± 1.7 | 8.8 ± 1.7 |
Data are means ± SD for continuous variables, unless otherwise indicated.
In the primary analysis, the baseline values of the patient symptoms, functional neurological exams, blood chemistries, and demographics were tested for association with change of sural nerve MFD between the initial and 52-week sural nerve biopsies (Table 1). Five baseline variables were significantly correlated with a loss of sural nerve MFD over the 52 weeks of the study. They were dominant peroneal motor NCV (R = 0.13, P = 0.005), nondominant median motor NCV (R = 0.11, P = 0.02), sural sensory NCV (R = 0.10, P = 0.05), A1C (R = −0.12, P = 0.02), and triglyceride level (R = −0.11, P = 0.02). The primary analysis was potentially confounded by the effect of initial sural nerve MFD on MFD change. There was a positive correlation between initial sural nerve MFD and the size of the decrease of sural nerve MFD over 52 weeks (R = 0.14). To account for this confounding factor, the variables with a nominal P value <0.05 were tested in a subset of this dataset controlled for initial MFD.
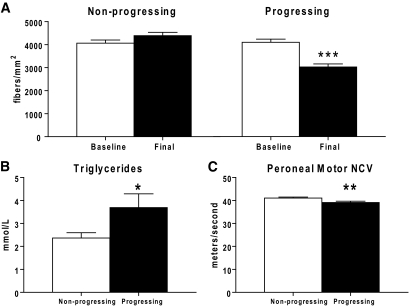
Using the methodology in “Participant selection for the secondary data analysis” in research design and methods, two groups of participants were selected (n = 208). The groups did not differ significantly in mean initial sural nerve MFD (P = 0.87), O'Brien rank-sum for neuropathy (P = 0.09), duration of diabetes (P = 0.48), age (P = 0.11), or BMI (P = 0.30). At 52 weeks, the rapidly progressing group had significantly decreased mean sural nerve MFD (i.e., sural nerve MFD had decreased by >25% over the course of the study) (P < 0.0001) (Fig. 2A). In contrast, the nonprogressing participants had no significant change in sural nerve MFD over the study period. The divergence of diabetic neuropathy progression between these two groups over the 52-week study period served as the basis for the secondary data analysis.
FIG. 2.
MFD of the rapidly progressing and nonprogressing participants and significant changes between the groups. A: The nonprogressing dataset shows a no change in MFD (fibers/mm2) over 52 weeks, while the progressing dataset shows a highly significant decrease in MFD. Baseline measurements of triglyceride levels (B) and peroneal motor NCV (C) are significantly different between the progressing and nonprogressing participants. *P < 0.05; ** P < 0.01; *** P < 0.0001.
When comparing the rapidly progressing and nonprogressing participants, baseline triglycerides were significantly higher in the progressing group (P = 0.04) (Fig. 2B). Baseline peroneal motor NCV was significantly lower in the rapidly progressing participants (P = 0.008) (Fig. 2C), despite the similar sural nerve MFD of the groups at baseline. There was no significant effect of median motor NCV, sural sensory NCV, or A1C in the secondary analysis.
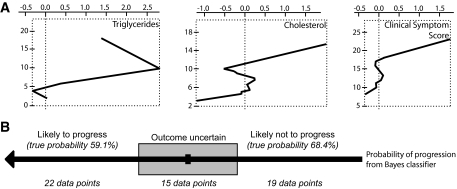
A machine-learning paradigm was used to test the hypothesis that multiple factors may combine to predict the outcome of participants with diabetic neuropathy. Using the same diverging groups of participants defined for the secondary analysis, blood chemistry, demographic data, and clinical symptom score were assigned significance values with regard to decreasing or stable sural nerve MFD. This process was repeated seven times using different machine-learning techniques (see research design and methods). The Naïve Bayes classifier achieved the highest sensitivity in detecting rapidly progressing participants (57%) based on their baseline characteristics (Fig. 3A). The three most influential measures in this model for predicting patient outcome were triglycerides, cholesterol, and the clinical symptom score. A refinement of this classifier creates a third category of unclassified, where the model lacks confidence to classify the participant as more likely progressing or nonprogressing. If only those participants with a high classification confidence (>56%) were assigned a prediction, overall accuracy increased to 63% (Fig. 3B), which was greater than any other model tested.
FIG. 3.
Naïve Bayes classifier performance in an independent group of participants. A: Important variables in the model are triglycerides, cholesterol, and clinical symptom score. B: The model assigns a probability of progressing to each participant. When the probability is >56% or <44%, a specific outcome is predicted.
DISCUSSION
Of 748 initially recruited participants in this randomized placebo-controlled ALC trial, we were able to analyze data from 427 participants using the change in sural nerve MFD over the 1-year study period as the primary outcome measure. The primary analysis showed a correlation between the outcome measure and baseline motor and sensory NCVs, A1C, and triglycerides. To identify factors specific to the rate of diabetic neuropathy progression as measured by MFD, participants with the same degree of diabetic neuropathy at baseline were divided into two groups with either no disease progression or rapid progression over the 1-year study. Triglyceride levels and peroneal motor NCV were the only factors that significantly differed between the nonprogressing and rapidly progressing participants. A model for predicting the progression of diabetic neuropathy based on these data performed with 63% overall classification accuracy.
Our primary outcome measure, MFD, is a quantitative, highly reproducible measure of nerve health (13,27–29). MFD correlates strongly with both motor and sensory NCV in diabetic neuropathy and other neuropathies (13,27–29), but unlike motor NCV, MFD does not vary with acute metabolic disturbances (30–32).
Our primary data analysis on the 427 participants revealed correlations between decreased sural nerve MFD over the one year study period and peroneal motor, median motor and sural sensory NCV, A1C and levels of triglycerides. While statistically significant, these correlations were not robust. This may be partially due to the fact that these data were confounded by the effect of initial sural nerve MFD. Variation in initial sural nerve MFD may be due to type and duration of diabetes, age and sex, the range of A1C values at baseline, and other metabolic variables. Similar variability in baseline diabetic neuropathy has been reported in other clinical, observational, and treatment trials (4,5). Our goal was to examine parameters associated specifically with diabetic neuropathy progression; therefore, the study cohort was separated into two distinct populations with similar baseline diabetic neuropathy and the presence or absence of a significant loss of sural nerve MFD over the 52 weeks of the study. By defining our groups as having a similar degree of diabetic neuropathy at baseline as defined by the O'Brien score, we may underestimate the true statistical significance of NCV differences at baseline. However, this underestimation is necessary to ensure that we have truly diverging groups. Comparisons of these two groups indicate that participants with rapidly progressing diabetic neuropathy (MFD loss >500/mm2) exhibited elevated triglycerides and greater deficits in peroneal NCV at baseline than the nonprogressing participants.
The Diabetes Control and Complications Trial and its continuation, the Epidemiology of Diabetes and its Complications (7,8), established hyperglycemia as the primary cause of diabetes complications. Consistent with these studies, we initially found that elevated A1C correlated with loss of sural nerve MFD. However, when directly comparing participants with a similar degree of baseline diabetic neuropathy (i.e., similar sural nerve MFD), A1C did not differ between rapidly progressing and nonprogressing participants; it was not a specific marker for diabetic neuropathy progression in this study. This suggests that other factors may underlie variation in the progression of diabetic neuropathy. In the last decade, abnormalities in insulin signaling, caused by insulin deficiency, as in type 1 diabetes, or insulin resistance, as in type 2 diabetes, have been invoked as additional pathogenetic components in diabetic neuropathy. This is underscored by the data from the longitudinal Rochester Study, in which type 1 diabetes was found to be a major risk factor for severity of diabetic neuropathy (33). Experimental studies also suggest that insulin deficiency is a major contributor to diabetic neuropathy, because of the prominent neurotrophic effects of insulin (22,23). For this reason, the number of participants with type 1 diabetes and those treated with insulin was balanced when defining the progressing and nonprogressing groups.
In contrast to A1C, baseline serum triglycerides were significantly elevated in the rapidly progressing compared with the nonprogressing groups. Triglycerides are components of HDL, LDL, and VLDL lipid transporters. When measured in serum, free triglycerides are a surrogate marker of endogenous lipid transport pathway activity. Free triglycerides are released from VLDL, leading to their conversion to LDL (34).
Our findings support the emerging idea that dyslipidemia contributes to the development of diabetic neuropathy. This hypothesis may explain the earlier incidence of diabetic neuropathy in individuals with type 2 diabetes compared with type 1 diabetes. Dyslipidemia develops later in the course of type 1 diabetes, and the delayed development of an abnormal lipid profile coincides with the delayed onset and progression of diabetic neuropathy (35,36). In this study, triglycerides were significantly elevated in those participants exhibiting diabetic neuropathy progression independent of diabetes type or insulin treatment. These data confirm reports from several large-scale trials of participants with type 2 diabetes that also point to early dyslipidemia as a major independent risk factor for the progression of diabetic neuropathy (37,38). Correction of dyslipidemia with statins has an ameliorative affect on the development and progression of diabetic neuropathy (39,40).
Peroneal motor NCV also differed between the progressing and nonprogressing groups at baseline. This finding is more indicative of concordant damage to peroneal and sural nerve function than of a specific mechanism for that damage. Multiple studies (13,27–29) agree with our findings and report a correlation between MFD and NCV. However, in the current study, decreased peroneal motor NCV was detectable prior to the loss of a significant amount of sural nerve sensory fibers, as assessed by sural nerve MFD. This most likely reflects metabolic nerve dysfunction in the peroneal nerve rather than earlier nerve fiber loss and is consistent with experimental models of diabetic neuropathy (31). While NCV and fiber density are closely related, factors other than fiber density, such as acute metabolic disruption (41,42), affect NCV without resulting in nerve fiber loss.
Modeling done on this dataset was motivated by the desire to identify noninvasive predictors of the loss of MFD. The American Diabetes Association has issued guidelines (24) for the use of modeling and machine learning that specify that validation of a model should be done in three ways: the model should first be validated on the initial dataset, then the data should be validated on an independent set from the same experiment, and finally an independent set from a different experiment from which the same parameters were collected. In this study, only the first two parts of the recommended validation could be completed due to the lack of additional published datasets with serial sural nerve biopsies. We found that a model for predicting the progression of diabetic neuropathy using the American Diabetes Association guidelines for modeling and machine learning performed with 63% overall classification accuracy. The three most influential measures in this model for predicting patient outcome were triglycerides, cholesterol, and the clinical symptom score. Interestingly, despite being significantly different between progressing and nonprogressing patients, NCV was not a major contributor to this predictive model. This may be because the difference between the two groups, while significant, was ∼5%. The Baysean model used may not be sensitive enough to include this subtle change. A specialized learning algorithm or a measure with greater dynamic range may allow us to include this important predictor in future modeling. Future informatics studies on diabetic neuropathy hold promise and are being proposed on the Diabetes Control and Complications Trial/Epidemiology of Diabetes and its Complications cohort.
In summary, both elevated triglycerides and reduced peroneal motor NCV are predictive of a dramatic decrease in sural nerve MFD over a 1-year period. The correlation between triglycerides and diabetic neuropathy progression suggests that hyperglycemia and aberrant glucose metabolism are not the only factors contributing to nerve damage. The exact mechanism underlying triglyceride mediated injury has yet to be elucidated but may dysregulated lipid metabolism within motor and/or sensory neurons. These same factors, along with acute metabolic flux, may explain the correlation between reduced peroneal motor NCV and rapidly progressing diabetic neuropathy. We have also demonstrated that given an adequate dataset, predictive models of diabetic neuropathy progression may be trained using standard machine learning techniques.
Acknowledgments
This work was supported by the National Institutes of Health (U54-DA021519 [to E.L.F. and T.D.W.], NS04765 [to E.L.F. and R.P.B.], and DK43884 [to A.A.F.S.]) and the Juvenile Diabetes Research Foundation (JDRF) Center for the Study of Complications in Diabetes (to E.L.F., K.A.S., and R.P.B.), the JDRF (to A.A.F.S.), the American Diabetes Association (to E.L.F., K.A.S., and R.P.B.), the Thomas Foundation (to A.A.F.S.), Sigma Tau (to A.A.F.S.), and the A. Alfred Taubman Medical Research Institute and the Program for Neurology Research and Discovery (to E.L.F., K.A.S., and T.D.W.).
No potential conflicts of interest relevant to this article were reported.
The authors acknowledge Judith Boldt for expert administrative assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Edwards JL, Vincent AM, Cheng HT, Feldman EL: Diabetic neuropathy: Mechanisms to management. Pharmacol Ther 2008; 120: 1– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman EL, Stevens MJ, Russell JW, Peltier A, Inzucchi S, Porte JD, Sherwin RS, Baron A: Somatosensory neuropathy. In The Diabetes Mellitus Manual New York: McGraw-Hill, 2005, p. 366– 384 [Google Scholar]
- 3.Feldman EL: Diabetic neuropathy. Curr Drug Targets 2008; 9: 1– 2 [DOI] [PubMed] [Google Scholar]
- 4.Sima A, Cherian V, Albers JW, Greene DA: the Tolrestat Study Group Nerve fiber loss in diabetic neuropathy correlates with impaired evoked potential amplitudes and nerve conduction velocity. Diabetologia 1992; 35: 606 [Google Scholar]
- 5.Greene DA, Arezzo JC, Brown MB: Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Zenarestat Study Group. Neurology 1999; 53: 580– 591 [DOI] [PubMed] [Google Scholar]
- 6.Lauria G, Lombardi R: Skin biopsy: a new tool for diagnosing peripheral neuropathy. BMJ 2007; 334: 1159– 1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The DCCT Research Group Factors in development of diabetic neuropathy: baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). Diabetes 1988; 37: 476– 481 [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial Cohort Epidemiology of Diabetes Interventions and Complications (EDIC): design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial Cohort. Diabetes Care 1999; 22: 99– 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisholm DJ: The Diabetes Control and Complications Trial (DCCT): a milestone in diabetes management. Med J Aust 1993; 159: 721– 723 [PubMed] [Google Scholar]
- 10.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH: Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341– 350 [DOI] [PubMed] [Google Scholar]
- 11.Kempler P, Tesfaye S, Chaturvedi N, Stevens LK, Webb DJ, Eaton S, Kerenyi Z, Tamas G, Ward JD, Fuller JH: Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med 2002; 19: 900– 909 [DOI] [PubMed] [Google Scholar]
- 12.Steinmetz A: Lipid-lowering therapy in patients with type 2 diabetes: the case for early intervention. Diabetes Metab Res Rev 2008; 24: 286– 293 [DOI] [PubMed] [Google Scholar]
- 13.Sima AA, Calvani M, Mehra M, Amato A: Acetyl-l-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28: 89– 94 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Clinical practice recommendations 1995. Diabetes Care 1995; 18( Suppl. 1): 1– 96 [PubMed] [Google Scholar]
- 15.Sima AA, Laudadio C: Design of controlled clinical trials for diabetic polyneuropathy. Semin Neurol 1996; 16: 187– 191 [DOI] [PubMed] [Google Scholar]
- 16.Arezzo JC: New developments in the diagnosis of diabetic neuropathy. Am J Med 1999; 107: 9S– 16S [DOI] [PubMed] [Google Scholar]
- 17.Sima AAF, Blaivas M: Peripheral neuropathies. In Neuropathology: The Diagnostic Approach Garcia J, McKeevar P, Sima AAF: Eds. Philadelphia, Mosby, 1997, p. 758– 809 [Google Scholar]
- 18.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB: Missing value estimation methods for DNA microarrays. Bioinformatics 2001; 17: 520– 525 [DOI] [PubMed] [Google Scholar]
- 19.Huang P, Woolson RF, O'Brien PC: A rank-based sample size method for multiple outcomes in clinical trials. Stat Med 2008; 27: 3084– 3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann HB, Whitney DR: On a test of whether one of two random variables is sochastically larger than the other. Ann Math Stat 1947; 18: 50– 60 [Google Scholar]
- 21.Gaddis GM, Gaddis ML: Introduction to biostatistics: part 5, statistical inference techniques for hypothesis testing with nonparametric data. Ann Emerg Med 1990; 19: 1054– 1059 [DOI] [PubMed] [Google Scholar]
- 22.Toth C, Brussee V, Zochodne DW: Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia 2006; 49: 1081– 1088 [DOI] [PubMed] [Google Scholar]
- 23.Toth C, Brussee V, Martinez JA, McDonald D, Cunningham FA, Zochodne DW: Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience 2006; 139: 429– 449 [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care 2004; 27: 2262– 2265 [DOI] [PubMed] [Google Scholar]
- 25.Demsar J, Zupan B, Leban G: Orange: From Experimental Machine Learning to Interactive Data Mining. Faculty of Computer and Information Science, University of Ljubljana; 2004 [Google Scholar]
- 26.Kearns M, Ron D: Algorithmic stability and sanity-check bounds for leave-one-out cross-validation. Neural Comput 1999; 11: 1427– 1453 [DOI] [PubMed] [Google Scholar]
- 27.Veves A, Malik RA, Lye RH, Masson EA, Sharma AK, Schady W, Boulton AJ: The relationship between sural nerve morphometric findings and measures of peripheral nerve function in mild diabetic neuropathy. Diabet Med 1991; 8: 917– 921 [DOI] [PubMed] [Google Scholar]
- 28.Russell J, Karnes J, Dyck P: Sural nerve myelinated fiber density differences associated with meaningful changes in clinical and electrophysiological measurements. J Neurol Sci 1996; 135: 114– 117 [DOI] [PubMed] [Google Scholar]
- 29.Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ: Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol (Berl) 2001; 101: 367– 374 [DOI] [PubMed] [Google Scholar]
- 30.Saini AK, Arun KH, Kaul CL, Sharma SS: Acute hyperglycemia attenuates nerve conduction velocity and nerve blood flow in male Sprague-Dawley rats: reversal by adenosine. Pharmacol Res 2004; 50: 593– 599 [DOI] [PubMed] [Google Scholar]
- 31.Sima AA, Kamiya H: Diabetic neuropathy differs in type 1 and type 2 diabetes. Ann N Y Acad Sci 2006; 1084: 235– 249 [DOI] [PubMed] [Google Scholar]
- 32.Ward JD, Barnes CG, Fisher DJ, Jessop JD, Baker RW: Improvement in nerve conduction following treatment in newly diagnosed diabetics. Lancet 1971; 1: 428– 430 [DOI] [PubMed] [Google Scholar]
- 33.Dyck PJ, Davies JL, Litchy WJ, O'Brien PC: Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester diabetic neuropathy study cohort. Neurology 1997; 49: 229– 239 [DOI] [PubMed] [Google Scholar]
- 34.Walker KH, Hall DW, Hurst WJ: Clinical Methods: The History, Physical and Laboratory Examinations Boston: Butterworths, 1990 [PubMed] [Google Scholar]
- 35.Tomlin AM, Dovey SM, Tilyard MW: Risk factors for hospitalization due to diabetes complications. Diabetes Res Clin Pract 2008; 80: 244– 252 [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ, Fuller JH, Songini M, Kohner EM: Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 2001; 24: 284– 289 [DOI] [PubMed] [Google Scholar]
- 37.Pennathur S, Heinecke JW: Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 2007; 7: 257– 264 [DOI] [PubMed] [Google Scholar]
- 38.Tsuzura S, Ikeda Y, Suehiro T, Ota K, Osaki F, Arii K, Kumon Y, Hashimoto K: Correlation of plasma oxidized low-density lipoprotein levels to vascular complications and human serum paraoxonase in patients with type 2 diabetes. Metabolism 2004; 53: 297– 302 [DOI] [PubMed] [Google Scholar]
- 39.Fried LF, Forrest KY, Ellis D, Chang Y, Silvers N, Orchard TJ: Lipid modulation in insulin-dependent diabetes mellitus: effect on microvascular outcomes. J Diabetes Complications 2001; 15: 113– 119 [DOI] [PubMed] [Google Scholar]
- 40.Davis TM, Yeap BB, Davis WA, Bruce DG: Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2008; 51: 562– 566 [DOI] [PubMed] [Google Scholar]
- 41.Vinik AI, Bril V, Litchy WJ, Price KL, Bastyr EJ, 3rd: Sural sensory action potential identifies diabetic peripheral neuropathy responders to therapy. Muscle Nerve 2005; 32: 619– 625 [DOI] [PubMed] [Google Scholar]
- 42.Grewal J, Bril V, Lewis G, Perkins BA: Objective evidence for the reversibility of nerve injury in diabetic neuropathic cachexia. Diabetes Care 2006; 29: 473– 474 [DOI] [PubMed] [Google Scholar]