Abstract
OBJECTIVE
Defining an optimal costimulatory blockade–based immune suppression protocol enabling engraftment and functional development of E42 pig embryonic pancreatic tissue in mice.
RESEARCH DESIGN AND METHODS
Considering that anti-CD40L was found to be thrombotic in humans, we sought to test alternative costimulatory blockade agents already in clinical use, including CTLA4-Ig, anti-LFA1, and anti-CD48. These agents were tested in conjunction with T-cell debulking by anti-CD4 and anti-CD8 antibodies or with conventional immunosuppressive drugs. Engraftment and functional development of E42 pig pancreatic tissue was monitored by immunohistology and by measuring pig insulin blood levels.
RESULTS
Fetal pig pancreatic tissue harvested at E42, or even as early as at E28, was fiercely rejected in C57BL/6 mice and in Lewis rats. A novel immune suppression comprising anti-LFA1, anti-CD48, and FTY720 afforded optimal growth and functional development. Cessation of treatment with anti-LFA1 and anti-CD48 at 3 months posttransplant did not lead to graft rejection, and graft maintenance could be achieved for >8 months with twice-weekly low-dose FTY720 treatment. These grafts exhibited normal morphology and were functional, as revealed by the high pig insulin blood levels in the transplanted mice and by the ability of the recipients to resist alloxan induced diabetes.
CONCLUSIONS
This novel protocol, comprising agents that simulate those approved for clinical use, offer an attractive approach for embryonic xenogeneic transplantation. Further studies in nonhuman primates are warranted.
The potential use of embryonic tissues as a novel source for transplantation, which might be less immunogenic transplantable tissue, has been advocated over the years. During the past decade, several studies (1–5) have suggested that the relative reduced expression of major histocompatibility complex molecules or adhesion molecules, as well as the lack of antigen-presenting cells or endothelial cells in embryonic tissues, are likely associated with reduced immunogenicity. Furthermore, it has been more recently argued that early embryonic porcine precursor tissues could completely evade the immune system upon implantation into recipient rats or nonhuman primates (NHPs) (6–9). We previously attempted to define an optimal window for transplantation of pig embryonic pancreatic tissue, based on the risk of teratoma, growth potential, and immunogenicity (10,11). Assessment of the first two parameters was performed following implantation of embryonic tissues harvested at different gestational time points under the renal capsule of xenogenic NOD-SCID mice that lack a functional adaptive immune system. To evaluate the relative immunogenicity of the different tissues, the recipient mice were infused with human peripheral blood mononuclear cells and homing, and destruction of the embryonic tissues by human T-cells or macrophages was analyzed. Based on this study, pig embryonic pancreatic tissue harvested at embryonic gestational age 42 (E42) was selected as the tissue of choice for transplantation. This choice was based, in particular, on the marked growth potential exhibited by E42 tissue, compared with E28 tissue, while no significant difference in their immunogenicity could be detected. Nevertheless, all early embryonic tissues, including tissue harvested at E28, were fiercely rejected when transplanted into immune competent mice in the complete absence of some form of immune suppression.
While our findings were in sharp contrast to the studies of Hammerman et al. (6,9), who showed engraftment and normalization of glucose levels in rats transplanted with E28–E29 pig pancreas, it is possible that this discrepancy was due to a difference between rats (used in the Hammerman et al. study) and mice (used in our study) in the strength of their rejection response. Therefore, in the present study, we further tested the potential of E28 pig tissue to evade the immune system in rats. As we previously reported in the mouse model, we observed fierce rejection of the implanted tissue, similar to that exhibited upon transplantation of E42 pancreas. These findings suggest that implantation of embryonic pig pancreas from any gestational stage will likely require some form of immune suppression.
Previous transplantation data suggest that costimulatory blockade does not interfere with pancreatic function. Unlike some of the conventional immunosuppressive drugs, such as rapamycin, costimulatory blockade seems preferable for porcine embryonic pancreas transplantation. One approach, recently demonstrated in the NHP model and also in our previous mouse study, has demonstrated the impressive role of a protocol combining CTLA4-Ig and anti-CD40L (11–13). However, the recent observation that anti-CD40L monoclonal antibody treatment is often associated with lethal thrombotic complications (14) suggests that the use of other costimulatory agents already tested in clinical trials may be preferable. In the present study, we indeed demonstrate that combining anti-LFA1 and anti-CD48 could markedly enhance engraftment and development of E42 pancreatic tissue in immune competent mice.
RESEARCH DESIGN AND METHODS
Animals.
Animals were maintained under conditions approved by the institutional animal care and use committee at the Weizmann Institute of Science. NOD-SCID and C57BL mice and “Nude” and Lewis rats aged 8–12 weeks (Weizmann Institute Animal Breeding Center, Rehovot, Israel) were used as hosts for the transplantation studies.
Porcine embryonic pancreatic tissue.
Pig embryos were obtained from the Lahav Institute of Animal Research (Kibbutz Lahav, Israel) as previously described (10,11). Cold ischemia time until transplantation was routinely <2 h. The study protocol was approved by the ethics committees at both the Weizmann Institute of Science and the Lahav Institute of Animal Research.
Implantation.
Implantation under the kidney capsule of mice was performed as previously described (10,11). Implantation intra omentum of rats was performed under general anesthesia (ketamine 60 mg/kg and xylazine 7 mg/kg i.p.). Host omentum was exposed through a midline abdominal incision, and two pancreata were deposited into sutured omental pockets.
Induction of diabetes.
Diabetes was induced by alloxan as previously described (11).
Immune suppression protocols.
The immune-suppressive protocols included T-cell debulking with anti-CD4 and anti-CD8 antibodies (hybridomas GK1.5 and 53.6.72; BioExpress, West Lebanon, NH) administered intraperitoneally on day −3 at a dosage of 300 μg/mouse. The costimulatory blockade agents (200 μg/mouse mouse CTLA4-Ig fusion protein [lot no. 20204; Chimerigen Laboratories], 250 μg/mouse anti-CD48 [hybridoma HM48-1; provided by Dr. Hideo Yagita], and anti-LFA1 [hybridoma FD441.8; Bioexpress] antibodies) were given intraperitoneally on days 0, 2, 4, and 6, and a single injection was repeated biweekly until 3 months posttransplant. FTY720 was administered i.p. daily from day −2 until day +3 and then biweekly at a dosage of 5 mg/kg. Alternatively, 1.5 mg/kg s.c. rapamycin (Sirolimus; Wyeth Europa, Berkshire, U.K.), 1 mg/kg s.c. FK506 (Prograf, Fujisawa, Ireland), or 40 mg/kg p.o. mycophenolate mofetil (MMF) (CellCept; Roche, Mannheim, Germany) was administered daily until the animals were killed. All drugs were administered according to the manufacturer instructions using doses previously shown to be effective in mice (15–17).
Evaluation of transplant growth.
Grafted animals were killed at 4–6 weeks after transplantation. Kidneys bearing the grafts were removed and fixed in 4% paraformaldehyde. Long and short axes of the grafts were measured, and transplant size was calculated by multiplying long and short axes.
Histology and immunohistochemistry.
Tissues were obtained from transplanted animals. Fixation, sectioning, and staining were performed as in previous studies (10,11,18,19). The following first antibodies were applied: guinea pig anti-rabbit insulin (Dako, Giostrup, Denmark), polyclonal rabbit anti-human CD3 (Dako), rat anti-mouse F4/80 antigen (Serotec, Oxford, U.K.), mouse anti-rat ED1 (Serotec), rabbit anti-human glucagon (Dako), mouse anti-human cytokeratin 20 (clone Ks 20.8; Dako), rabbit anti-cytokeratin (wide-spectrum screening; Dako), monoclonal mouse anti-human cytokeratin, clone MNF116 (broad-spectrum cytokeratin; Dako), rat anti-mouse CD31 (BD Pharmingen, Oxford, U.K.), and fluorescein isothiocyanate–conjugated anti-porcine CD31 (Serotec). The following secondary antibodies were used (purchased from Jackson ImmunoResearch Laboratories, West Grove, PA): Texas red anti-mouse, Texas red or Cy3 anti-rat, Texas red or Cy2 anti-rabbit, biotinylated anti–guinea pig following streptavidin aminomethylcoumarin, fluorescein isothiocyanate anti-mouse IgM, and Cy2 anti-mouse IgG.
Enzyme-linked immunosorbent assay measurements of pig insulin.
The porcine/human insulin kit (K6219; Dako), in which the primary pig anti-insulin antibody does not cross-react with mouse insulin, was used to follow pig insulin levels in the serum of transplanted mice according to the manufacturer's instructions.
Statistical analysis.
Differences between groups were evaluated by the Student's t test. Data are expressed as means ± SD and were considered statistically significant if P values were ≤0.05.
RESULTS
Implantation of E28 versus E42 pig pancreatic tissue in immune competent mice and rats in the absence of immune suppression.
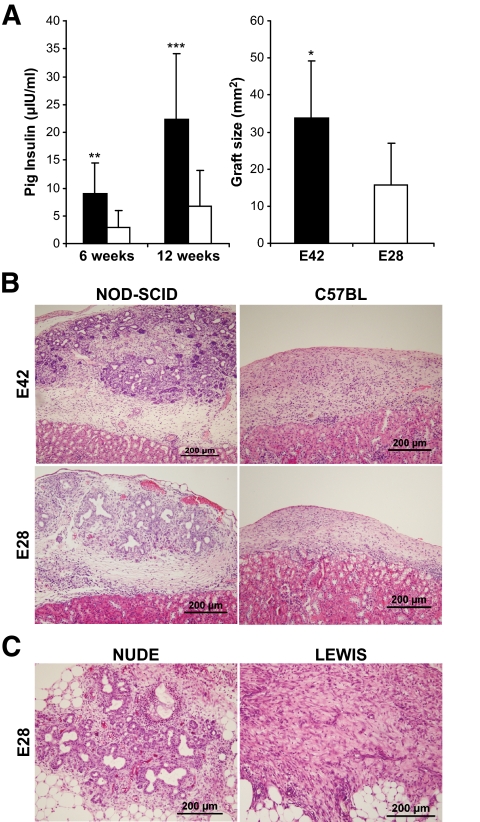
To determine whether early embryonic pig pancreatic tissues can evade the immune system when transplanted into rodents, E28 and E42 pig tissues were implanted under the renal capsule in different strains of mice and rats. The enhanced growth potential of E42 pig pancreatic tissue, compared with that exhibited by E28 tissue, was demonstrated upon implantation in NOD-SCID mice by insulin secretion, by implant size and by histological documentation of ducts and islets (Fig. 1A).
FIG. 1.
Comparison between E42 and E28 pancreas in growth potential, function, and rejection patterns. A: The difference in size and insulin secretion of the E42 and E28 pancreas transplanted under the kidney capsule of NOD-SCID mice (black [E42] and white [E28] bars, respectively). The graft size was determined 6 weeks following transplantation. B: Histological (hematoxylin and eosin) evaluation of E42 and E28 pancreatic grafts 1 month after transplantation under the kidney capsule of NOD-SCID or C57BL mice. C: Histological (hematoxylin and eosin) evaluation of E28 pancreatic grafts 10 days after transplantation in the omentum of Nude or Lewis rats. (A high-quality representation of this figure is available in the online issue.)
However, when transplanted under the kidney capsule of C57BL immune-competent mice without any immune suppression, all early embryonic tissues tested, including tissue harvested at E28 and E42, were rejected. Thus, 4 weeks after transplantation, only fibrosis and massive infiltration in the site of implantation in the C57BL mice could be detected (six of six recipients in each group) (Fig. 1B).
Considering that our findings in the mouse model were in sharp contrast to the results of Rogers and colleagues (6,9), who suggested that E28 pancreas can evade the immune response in rats, we attempted to repeat these transplantation experiments in rats, using the same strain of recipients, namely immune-competent Lewis rats, and the same site of implantation, namely the omentum. As a control for the growth capacity of the implanted tissue, we used immune-deficient Nude rats.
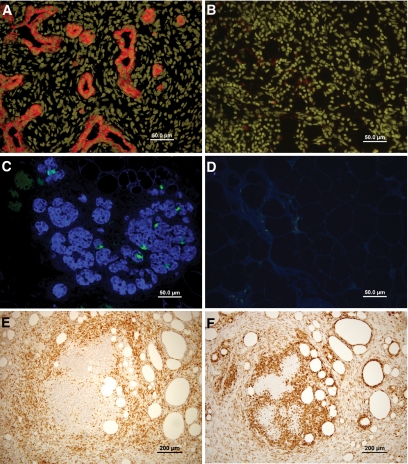
As can be seen in Fig. 1C, following transplantation of two E28 pancreata into the omentum of Nude rats, normal growth and development was attained with grafts containing pancreatic elements without leukocyte infiltration in the pancreas parenchyma. Thus, at all time points tested, pig pancreatic epithelial cells, stained specifically by anti-pig cytokeratin 20, were present in the growing grafts (Fig. 2A). As previously demonstrated in the mouse model, within 5–15 days posttransplant, most of the pancreatic tissue included pancreatic ducts. Within 2–5 months after transplantation, the grafts were composed mostly of endocrine pancreatic elements, and in seven of eight Nude rats, insulin and glucagon-producing cells arranged in islet structures or scattered as single cells in adipose tissue were clearly visible (Fig. 2C).
FIG. 2.
Immunostaining of E28 pig pancreatic tissue implanted in the omentum of Nude and Lewis rats. Pig pancreatic epithelial cells, 15 days after transplantation in nude (A) or Lewis (B) rats, visualized using cytokeratin 20 (reddish color)-specific staining. Nuclei are stained with yellow Hoechst. Pig endocrine cells, 4 months after transplantation in Nude (C) or Lewis (D) rats, visualized using insulin (blue) and glucagon (green)-specific staining. Infiltration of the omentum in transplanted Lewis rats 15 days after transplantation is visualized using CD3 for detection T-cells (E) and F4/80 for detection of macrophages (F). (A high-quality representation of this figure is available in the online issue.)
In sharp contrast, rapid rejection of the E28 pancreas was documented following implantation into immune-competent Lewis rats. Histological examination of the grafts at all time points, between 5 days and 5 months posttransplant, revealed no pancreatic elements at the site of implantation, similar to our observations in the mouse model (Fig. 1). Starting 5 days after transplantation, the grafts were heavily infiltrated, and long-term follow-up revealed large areas of fibrosis and necrosis. Specific staining for CD3+ lymphocytes and macrophages, 2 weeks after transplantation, revealed massive infiltration of both cell types in the omentum of the transplanted Lewis rats (Fig. 2E and F). Moreover, there were no cells stained for cytokeratin 20, nor could any insulin- or glucagon-producing cells be detected in any of the rats transplanted (0 of 10) (Fig. 2B and D).
Accordingly, during the entire follow-up period low, but significant, blood levels of pig insulin were detected by specific enzyme-linked immunosorbent assays in the Nude recipients but not in the immune-competent recipients. Thus, 3 months after transplantation, the blood level of pig insulin in the Nude rats was 1.048 ± 0.887 μIU/ml (n = 6), whereas in all six Lewis rats tested, it was below the detection threshold (<0.5 μIU/ml).
Although substantial pig insulin blood levels were recorded in the Nude rat recipients, they were significantly lower compared with those exhibited in SCID mice (Fig. 1A). This could be caused, in part, by the different implantation site used for transplantation, namely omentum versus renal capsule, respectively.
Engraftment and functional growth in immune-competent mice following implantation of E42 pig pancreatic tissue in conjunction with immune suppression.
Considering that E42 embryonic pig tissue exhibits a more robust growth potential, and that E28 tissue, similarly to E42 tissue, is vigorously rejected in immune-competent mice or rats, further studies defining optimal minimally toxic immune suppression protocols were warranted. Very recently, we demonstrated the curative potential of the E42 tissue in the NHP model, demonstrating its ability to induce complete insulin independence in streptozotocin-treated animals (20). To demonstrate this proof of concept, we used a rather intensive immune-suppression protocol based on conditioning with a short treatment with anti-thymocyte globulin (ATG) and rituxan, followed by maintenance with RAD (a derivative of rapamycin), CTLA4-Ig, and FTY720. Clearly, while this protocol demonstrates the proof of concept for the curative potential of E42 pig pancreatic tissue, further reduction of its toxicity, if possible, would bring us closer to considering such transplants in human diabetic patients. One potential approach to reduce the toxicity of the protocol could entail replacing RAD and FTY720 with costimulatory blockade agents.
Indeed, the effectiveness of anti-CD40L has been recently demonstrated in the context of pig islet transplantation in the NHP model (12,13). However, in initial clinical attempts in renal transplantation, this agent was found to be associated with lethal thrombotic complications (14). Thus, we searched for alternative agents that have been given to patients without such toxicities including anti-LFA1 and anti-CD48, which are already used safely in patients with psoriasis (21,22). These antibodies were tested in combination with CTLA4-Ig, in the presence or absence of T-cell debulking monoclonal antibodies (anti-CD4 and anti-CD8) or FTY720.
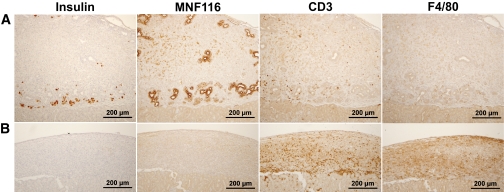
As can be seen in Table 1, a protocol combining all these agents led to 100% (eight of eight) engraftment of E42 pancreas in C57BL mice. Histological examination of the grafts in the first days posttransplantation revealed significant differences between untreated and treated mice (Fig. 3). Thus, in the absence of any immune suppression, CD3+ lymphocytes heavily infiltrated the grafts 3 days after transplantation, leading to complete rejection by 2 weeks posttransplantation, at which time only massive CD3+ lymphocyte and macrophage infiltrates were evident and no insulin or cytokeratin-expressing cells could be detected.
TABLE 1.
Outcomes of E42 pig pancreatic tissue implantation in C57BL/6 mice under different immune suppression protocols
| Engraftment* | Porcine insulin† | |
|---|---|---|
| Tests of immune suppressive combinations to identify maximum effective protocol | ||
| Debulking, FTY720, CTLA4-Ig, anti-LFA1, and anti-CD48‡ | 8/8 | 5.3 ± 4.3 |
| Debulking, CTLA4-Ig, anti-LFA1, and anti-CD48 | 1/7 | 0.28 ± 0.48 |
| Debulking, FTY720, anti-LFA1, and anti-CD48 | 7/7 | 16.9 ± 10.4 |
| FTY720, CTLA4-Ig, anti-LFA1, and anti-CD48 | 5/5 | 27.9 ± 29 |
| FTY720, anti-LFA1, and anti-CD48 | 4/4 | 8.5 ± 7.7 |
| CTLA4-Ig, anti-LFA1, and anti-CD48 | 0/6 | 0.1 ± 0.2 |
| The role of FTY720 with individual costimulatory blockade agents | ||
| FTY720 and CTLA4-Ig | 0/4 | 0 |
| FTY720 and anti-CD48 | 0/4 | 0 |
| FTY720 and anti-LFA1 | 2/4 | 0.8 ± 0.9 |
| FTY720, CTLA4-Ig, and anti-CD48 | 3/4 | 3.3 ± 5.1 |
| FTY720, CTLA4-Ig, and anti-LFA1 | 3/4 | 5.7 ± 8.2 |
| FTY720, anti-LFA1, and anti-CD48 | 11/11 | 13.8 ± 10 |
| FTY720, CTLA4-Ig, anti-LFA1, and anti-CD48 | 13/13 | 15.6 ± 22 |
| The effect of various conventional immunosuppressive drugs with anti-LFA1 and anti-CD48 | ||
| FK506, CTLA4-Ig, anti-LFA1, and anti-CD48 | 0/3 | 2.2 ± 3.5 |
| Rapamycin, CTLA4-Ig, anti-LFA1, and anti-CD48 | 0/7 | 0.6 ± 0.8 |
| MMF, CTLA4-Ig, anti-LFA1, and anti-CD48 | 0/4 | 0.1 ± 0.1 |
Data are means ± SD.
*Mice were defined engrafted according to histology and/or porcine insulin in the serum. Mice were defined positive for porcine insulin if porcine insulin level in the serum of the mice 8 weeks posttransplantation was ≥1uIU/ml.
†Porcine insulin in the serum of transplanted mice 8 weeks posttransplantation.
‡Anti-CD4 and anti-CD8 monoclonal antibodies on day −3.
FIG. 3.
Histological examination of E42 pig pancreatic tissue 2 weeks after transplantation under the renal capsule of C57BL with (A) and without (B) immune-suppression with costimulatory blockade (anti-LFA1, anti-CD48, and CTLA4-Ig), FTY720, and T-cell debulking. Slides were stained for pig insulin, cytokeratin (MNF116), CD3+ lymphocytes, and macrophages (F4/80), as indicated. (A high-quality representation of this figure is available in the online issue.)
In contrast, mice that were treated with the maximum immune suppression protocol of T-cell debulking, FTY720, CTLA4-Ig, anti-LFA1, and anti-CD48, exhibited at 2 weeks posttransplantation, large pancreatic grafts with insulin- and cytokeratin-expressing cells, and with minimal infiltration of CD3+ lymphocytes or macrophages. Moreover, 2 months after E42 pancreas transplantation in mice treated with the maximal protocol, the grafts contained all pancreatic elements and were functional, as indicated by posttransplant blood levels of pig insulin (15.3 ± 14.2 μIU/ml) similar to the level exhibited in NOD-SCID control mice (9.7 ± 11 μIU/ml) (n = 16).
Interestingly, CTLA4-Ig or T-cell debulking could be removed from the maximal immune suppression protocol without loss of engraftment or graft functionality (five of five and seven of seven engrafted, respectively), while FTY720 was found to be essential to the engraftment-sustaining effect of CTLA4-Ig, anti-LFA1, and anti-CD48 (one of seven engrafted). Based on these studies, a novel reduced intensity protocol associated with 100% graft acceptance and with functional grafts has been defined. This protocol comprises anti-CD48, anti-LFA1, and FTY720 (four of four engrafted). Replacement of FTY720 with CTLA4-Ig in this protocol led to marked rejection (zero of six engrafted).
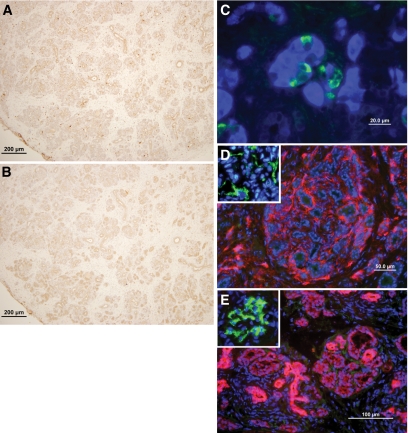
Mice implanted under immune suppression with anti-CD48, anti-LFA1, and FTY720 exhibited robust implants at 2 months posttransplant. Thus, as can be seen in Fig. 4, large islets with insulin- and glucagon-secreting cells were clearly detected by immunohistological staining (Fig. 4C). All the blood vessels in the pig pancreatic implant were of mouse origin, without evidence of pig endothelial cells (Fig. 4D). The grafts exhibited minimal CD3+ lymphocytes and macrophage infiltration in the periphery (Fig. 4A and B); however, the stained cells did not penetrate the pancreatic parenchyma. Moreover, we could not detect significant levels of IgG and IgM antibody deposits inside the pancreatic epithelium, despite minimal presence of bound immunoglobulin in the surrounding tissue, which was lower compared with that exhibited in the host kidney glomeruli that served as a positive control (Fig. 4E).
FIG. 4.
Histological examination of E42 pig pancreatic tissue 2–10 months after transplantation under the renal capsule of C57BL mice treated with the costimulatory blockade protocol (anti-LFA1, anti-CD48, and FTY720). Slides were stained for CD3+ lymphocytes (A), macrophages (F4/80) (B), insulin (blue) and glucagon (green) (C), mouse blood vessels (CD31, red), and pig blood vessels (CD31, green) (D), and cytokeratin (broad spectrum, red) and IgM and IgG deposits (green) (E). The inset in D demonstrates positive staining for pig endothelial cells (green) in E42 pancreas. The inset in E demonstrates positive normal staining for IgM and IgG deposits in the glomeruli of the kidney. The data are representative of the experiment shown in Table 1 (n = 11). (A high-quality representation of this figure is available in the online issue.)
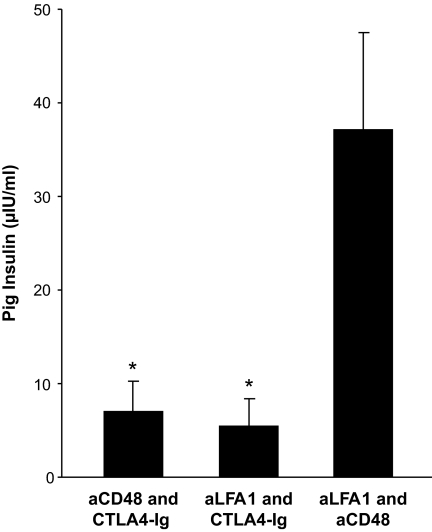
Further analysis of the relative role of anti-LFA1 and anti-CD48 showed that both are critical for achieving 100% success rate. As can be seen in Table 1, addition of anti-CD48 alone to FTY720, similarly to CTLA4-Ig along with FTY720, could not prevent rejection, and 8–10 weeks after transplantation all the grafts were completely rejected. Interestingly, the use of FTY720 with anti-LFA1 alone led to 50% engraftment. Thus, it seems that anti-LFA1 is more effective than anti-CD48 or CTLA4-Ig when added to FTY720. Furthermore, in each of the FTY720-based protocols, addition of CTLA4-Ig to either anti-LFA1 (three of four engrafted) or anti-CD48 (three of four engrafted) appeared to be less effective compared with the combined use of anti-LFA1 and anti-CD48 (eleven or eleven engrafted). The advantage of the anti-LFA1 and anti-CD48 combination is statistically significant as reflected by the pig insulin blood levels in the serum of transplanted mice 10 weeks after transplantation (Fig. 5) (37.1 ± 34.8 μIU/ml, compared with 5.5 ± 5.7 μIU/ml [P < 0.05] and 6.9 ± 6.8 μIU/ml [P < 0.05]) found upon addition of CTLA4-Ig to anti-LFA1 or to anti-CD48.
FIG. 5.
Porcine insulin blood levels 10 weeks after transplantation of E42 pancreas into immunosuppressed C57BL/6 mice. Recipients were treated with different combinations of costimulatory blockade agents (anti-LFA1, anti-CD48, and CTLA4-Ig) and FTY720, with or without T-cell debulking. Data are presented as means ± SE. *P ≤ 0.05.
The strikingly high levels of porcine insulin attained in the serum of implanted mice can likely be attributed to the molecular difference between pig and mouse insulin, associated with a reduced affinity of pig insulin to mouse insulin receptor. Thus, as we previously demonstrated when defining optimal gestation time window for transplantation (11), the relatively high pig insulin blood levels in transplanted mice is not associated with hypoglycemia or with an abnormal weight increase. Accordingly, correction of hyperglycemia in two different models of diabetes, induced by alloxan or by streptozotocin, requires higher pig insulin levels than those effective in humans (11). Interestingly, in line with the closer homology of pig and primate insulin (only one amino acid difference), ongoing experiments in the nonhuman primate model show that correction of hyperglycemia is attained by pig insulin blood levels more similar to those found in normal humans (∼10μIU/ml).
Since FTY720 is not approved for use in clinical transplantation, it was of interest to test whether it can be replaced with other conventional immunosuppresive drugs. As can be seen in Table 1, FK506 (1 mg/kg), rapamycin (1.5 mg/kg), or MMF (40 mg/kg), administered on a daily basis (using doses shown by other studies to be effective in mice [15–17]) could substitute FTY720 and prevent rejection in conjunction with costimulatory blockade.
Cessation of immune suppression.
The immune-suppressive protocol of choice comprising anti-LFA1, anti-CD48, and FTY720 was further tested for graft acceptance and insulin levels during a follow-up period of >6 months, in the presence or absence of T-cell debulking, prior to transplantation.
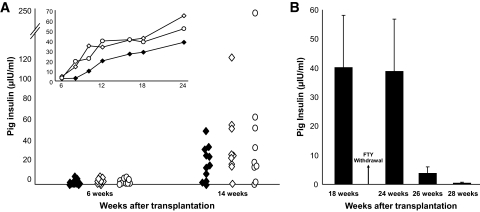
As can be seen in Fig. 6A, similar and significant blood levels of pig insulin were attained during the entire follow-up period, in the presence or absence of induction with T-cell debulking prior to transplantation. Thus, induction with anti-CD4 and anti-CD8 antibodies is not required. Furthermore, in these experiments, anti-LFA1 and anti-CD48 treatment was stopped 3 months after transplantation, and mice were maintained on a twice-weekly dose of FTY720 alone. These mice remained positive for pig insulin for >8 months posttransplantation.
FIG. 6.
A: Porcine insulin levels in the serum of C57BL mice transplanted with E42 pancreas and treated with costimulatory blockade agents (anti-LFA1, anti-CD48, and ±CTLA4-Ig), FTY720, with (◇) or without (○) debulking, at different time points after transplantation. Treatment with costimulatory antibodies was stopped at 3 months posttransplant, and graft maintenance was continued twice weekly only with FTY720. Insulin levels in the serum of NOD-SCID mice transplanted with E42 pancreas served as a positive control (♦). The inset demonstrates average pig insulin levels in transplanted mice over a course of 6 months. No statistical difference could be found between the tested groups. B: Porcine insulin levels in the serum of C57BL mice transplanted with E42 pancreas and treated with costimulatory blockade agents with or without debulking at different time points after FTY720 withdrawal. Data are presented as means ± SE.
In some mice (n = 9), FTY720 treatment was also stopped at 5 months posttransplant (Fig. 6B). Four weeks after FTY720 cessation, pig insulin levels remained stable (40.1 ± 54 μIU/ml before withdrawal of FTY720 vs. 38.9 ± 53.8 μIU/ml 4 weeks after withdrawal), although CD4+ and CD8+ cell levels in the peripheral blood were elevated from undetectable levels to 9.7 ± 6% and 10.6 ± 5.7% of CD4+ and CD8+ cells, respectively. However, after 2 additional weeks (i.e., 6 weeks after FTY720 discontinuation), pig insulin blood levels were drastically reduced (3.7 ± 6.8μIU/ml) and reached undetectable levels in seven of nine mice tested.
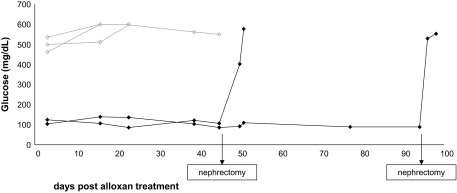
A detailed analysis of the implant functionality in response to glucose challenge and its ability to maintain normoglycemia in different diabetes models has been described recently (11). In the present study, the immune suppression of choice was selected based largely on histological evaluation and pig insulin blood levels, which we found previously to be correlated with graft rejection or acceptance. However, the curative capacity of the pig embryonic implants, maintained long term under just a biweekly treatment with FTY720, was also verified in the two remaining mice by exposure to alloxan treatment at 6 months posttransplant (alloxan selectively destroys rodent, but not human or porcine, β-cells [(23)]). Moreover, the role of the implant in maintaining normoglycemia following alloxan treatment is further proven by removal of the implant, which abruptly leads to marked elevation of glucose levels.
As can be seen in Fig. 7, the mice indeed maintained normal glucose levels, in sharp contrast to control mice that did not receive a transplant. Removal of the implant by nephrectomy of the kidney harboring the pig implant was associated with loss of normoglycemia, confirming that maintenance of glucose levels following alloxan treatment was mediated by the implanted pig embryonic pancreatic tissue.
FIG. 7.
Long-term engrafted (♦) as opposed to nonengrafted (◇) mice resist the alloxan challenge. At the indicated time points, the implanted mice were subjected to nephrectomy of the kidney harboring the implant, leading to prompt induction of hyperglycemia.
DISCUSSION
In the rat transplantation model, our data extends our previous observation in the mouse model that embryonic pig pancreatic tissue harvested at different gestational time points, including E28 and E42, are fiercely rejected upon transplantation into immune-competent recipients. Considering that E42 tissue exhibits more robust growth potential, the new data presented here further support the suggestion that E42 pancreatic pig tissue is best suited for transplantation in diabetic recipients, at least in this rodent xenotransplantation model. Our results are in contrast to the previous suggestion of Rogers and colleagues (6,9) that E28 pancreatic tissue can evade the immune system in Lewis rats. Our failure to find in these rats any detectable trace of pig pancreatic tissue cannot be due to growth deficiencies, as the same E28 tissue exhibited growth and development in immune deficient Nude rats.
This discrepancy between our results and those of Rogers and colleagues (6,9) are intriguing. However, it should be noted that in the latter study pig insulin blood levels were not monitored to establish the role of the pig implant in attaining normoglycemia, and, therefore, it is conceivable that autologous recovery of the rat islets might have occurred as suggested in other recent studies (24,25). While unable to evade the immune system in our models, early embryonic pig or human tissues were shown to be less immunogenic compared with their adult counterparts (1,2,10,11,18,19,26). Thus, it was important to define a minimally toxic immunosuppressive protocol for transplantation of embryonic pig tissues.
In our initial attempt to demonstrate the proof of concept in the NHP model, we chose a conventional protocol comprising induction with ATG and rituxan, followed by maintenance with CTLA4-Ig, RAD, and FTY720. This relatively intensive immune suppression enabled us to demonstrate that, indeed, implanted E42 pig pancreatic tissue can grow, differentiate, and induce complete insulin independence in diabetic NHP (20). However, further reduction of this immune suppression protocol is desirable before applying this approach for studies in diabetic primates or humans.
Our previous experience in mice, similar to results in NHP in the context of islet transplantation (11–13), demonstrated that costimulatory blockade using CTLA4-Ig and anti-CD40L can prevent E42 pancreatic graft rejection. However, anti-CD40L was shown to be associated with thrombotic complications in patients (14). To eliminate this agent in the present study, we combined elements from the primate protocol, such as FTY720 and rapamycin, with alternative costimulatory blockade agents such as anti-LFA1 and anti-CD48 (the murine homologue of human CD58 (LFA3) (27,28). Previous studies (29–32) demonstrated that each agent could be used in strategies aimed at controlling graft-versus-host disease or graft rejection following allogeneic bone marrow transplant or solid organ transplantation in rodents.
Efalizumab (humanized anti LFA1) and alefacept (human LFA3 fusion protein) are new immune-suppressive agents approved by the Food and Drug Administration in 2003 for the treatment of psoriasis (Food and Drug Administration application no. BLA 125075 and BL 125036). Both are currently in phase II/III clinical trials in the transplantation setting (33,34).
Our results, comparing different combinations of anti-LFA1 and anti-CD48, suggest that optimal engraftment and development of E42 pig pancreatic tissue could be attained upon immune suppression with a protocol comprising anti-LFA1 and anti-CD48, in combination with low doses of FTY720. Substitution of FTY720 by other conventional immune-suppressive drugs, including rapamycin, MMF, and FK506, failed to overcome rejection in our model. This special attribute of FTY720 might be associated with its unique mechanism of action, affecting trafficking of lymphocytes. Thus, while all other agents do not have additive activity to that of costimulatory blockade agents (i.e., suppressing proliferation or activation of T cells), FTY720 might add an additional function that might be critical for survival of embryonic grafts. However, further studies with these agents using different dosing are still required to establish whether this finding reflects a true mechanistic difference.
Collectively, immune suppression with anti-LFA1 and anti-CD48, in conjunction with low-dose FTY720, might offer unique advantages in NHP and in humans, being free of the thrombotic side effects of anti-CD40L, the toxicity of ATG and rituxan, and the proven deleterious effects of rapamycin on pancreatic graft function and insulin secretion (35,36). Our ability to prevent rejection of E42 pig pancreas in the mouse model for >6 months in C57BL/6 recipients and to attain secreted pig insulin blood levels, similar to the levels secreted from grafts that were transplanted in SCID mice, is indeed very encouraging. Furthermore, the treatment with anti-LFA1 and anti-CD48 monoclonal antibodies could be stopped at 11 weeks after transplantation, and the grafts will remain functional upon minimal maintenance with FTY720, administered twice weekly. However, cessation of this low-dose FTY720 maintenance led to complete rejection within 1 month, suggesting that immune tolerance had not been attained.
In renal transplantation FTY720 was tested together with a reduced dose of cyclosporine A (CSA) and was found to be associated with macular edema cases and lower creatinine clearance. Thus, when compared with a protocol composed of MMF, in conjunction with CSA, the latter was found to be safer, and FTY720 was not licensed for use in this setting (37). However, in the present study we found that the use of FTY720 as a single agent could be very effective if administered in conjunction with a transient treatment with costimulatory blockade agents. Thus, our results suggest a new application as a single agent and not together with a nephrotoxic agent such as CSA. Indeed, the use of FTY720 as a single agent was found to be free of renal toxicity in a recent phase 2b randomized trial in patients with multiple sclerosis (38), and a further phase 3 trial is currently underway. Considering that several conventional immunosuppressive drugs including FK506 and MMF could not replace FTY720 in our model, the latter represents an important potential single agent modality for the implantation of E42 pig pancreatic tissue in humans, and, therefore, further studies in the NHP model are warranted.
The ability of costimulatory blockade protocols to induce durable immune tolerance in allogeneic and xenogeneic solid-organ transplantation is highly controversial. A few studies have claimed permanent acceptance and donor specific tolerance after short treatment with costimulatory blockade agents (29,31,39,40), while more commonly, this protocol was shown to induce prolonged engraftment without establishment of tolerance (41–50). This discrepancy could be attributed, in part, to strain or species differences investigated in the different studies reported, as well as to the different assays used to define rejection (32,51).
The novel combination of anti-LFA1, anti CD48, and FTY720, introduced here, while short of inducing durable acceptance of the grafts, could likely serve as a reasonable starting point for new tolerance-induction strategies, without relying on anti-CD40L. This platform is especially attractive, considering that clinical-grade agents for neutralizing LFA1 and CD48 in humans are available. Further studies using this protocol in the NHP model for E42 pancreatic tissue transplantation is expected to lead to the development of a safer transplantation protocol in patients with type 1 diabetes.
Acknowledgments
This work was supported in part by Tissera (to Y.R.) and by National Institutes of Health R01 HL56067, R01 34495, R01 HL63452, and P01 AI056299 (to B.R.B.)
Y.R. is a scientific consultant and holds equity with Tissera, which supported this work, and holds the Henry H. Drake Professional Chair in Immunology. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y: Human and porcine early kidney precursors as a new source for transplantation. Nat Med 2003; 9: 53– 60 [DOI] [PubMed] [Google Scholar]
- 2.Dekel B, Burakova T, Marcus H, Shezen E, Polack S, Canaan A, Passwell J, Reisner Y: Engraftment of human kidney tissue in rat radiation chimera: I. A new model of human kidney allograft rejection. Transplantation 1997; 64: 1541– 1550 [DOI] [PubMed] [Google Scholar]
- 3.Foglia RP, DiPreta J, Statter MB, Donahoe PK: Fetal allograft survival in immunocompetent recipients is age dependent and organ specific. Ann Surg 1986; 204: 402– 410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Statter MB, Foglia RP, Parks DE, Donahoe PK: Fetal and postnatal testis shows immunoprivilege as donor tissue. J Urol 1988; 139: 204– 210 [DOI] [PubMed] [Google Scholar]
- 5.Metzger R, Parasta A, Joppich I, Till H: Organ-specific maturation of the major histocompatibility antigens in rats. Pediatr Surg Int 2002; 18: 640– 647 [DOI] [PubMed] [Google Scholar]
- 6.Rogers SA, Chen F, Talcott M, Hammerman MR: Islet cell engraftment and control of diabetes in rats after transplantation of pig pancreatic anlagen. Am J Physiol Endocrinol Metab 2004; 286: E502– E509 [DOI] [PubMed] [Google Scholar]
- 7.Rogers SA, Chen F, Talcott M, Liapis H, Hammerman MR: Glucose tolerance normalization following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic ZDF rats. Transpl Immunol 2006; 16: 176– 184 [DOI] [PubMed] [Google Scholar]
- 8.Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M, Hammerman MR: Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation 2007; 14: 591– 602 [DOI] [PubMed] [Google Scholar]
- 9.Rogers SA, Liapis H, Hammerman MR: Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transpl Immunol 2005; 14: 67– 75 [DOI] [PubMed] [Google Scholar]
- 10.Eventov-Friedman S, Katchman H, Shezen E, Aronovich A, Tchorsh D, Dekel B, Freud E, Reisner Y: Embryonic pig liver, pancreas, and lung as a source for transplantation: optimal organogenesis without teratoma depends on distinct time windows. Proc Natl Acad Sci U S A 2005; 102: 2928– 2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eventov-Friedman S, Tchorsh D, Katchman H, Shezen E, Aronovich A, Hecht G, Dekel B, Rechavi G, Blazar BR, Feine I, Tal O, Freud E, Reisner Y: Embryonic pig pancreatic tissue transplantation for the treatment of diabetes. PLoS Med 2006; 3: e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP: Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 2006; 12: 304– 306 [DOI] [PubMed] [Google Scholar]
- 13.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ: Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006; 12: 301– 303 [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB: Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000; 6: 114. [DOI] [PubMed] [Google Scholar]
- 15.Molano RD, Pileggi A, Berney T, Poggioli R, Zahr E, Oliver R, Malek TR, Ricordi C, Inverardi L: Long-term islet allograft survival in nonobese diabetic mice treated with tacrolimus, rapamycin, and anti-interleukin-2 antibody. Transplantation 2003; 75: 1812– 1819 [DOI] [PubMed] [Google Scholar]
- 16.Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Hara M, Sasayama S: FTY720, a new immunosuppressant, promotes long-term graft survival and inhibits the progression of graft coronary artery disease in a murine model of cardiac transplantation. Circulation 1999; 100: 1322– 1329 [DOI] [PubMed] [Google Scholar]
- 17.Blaha P, Bigenzahn S, Koporc Z, Schmid M, Langer F, Selzer E, Bergmeister H, Wrba F, Kurtz J, Kiss C, Roth E, Muehlbacher F, Sykes M, Wekerle T: The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood 2003; 101: 2886– 2893 [DOI] [PubMed] [Google Scholar]
- 18.Aronovich A, Tchorsh D, Katchman H, Eventov-Friedman S, Shezen E, Martinowitz U, Blazar BR, Cohen S, Tal O, Reisner Y: Correction of hemophilia as a proof of concept for treatment of monogenic diseases by fetal spleen transplantation. Proc Natl Acad Sci U S A 2006; 103: 19075– 19080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katchman H, Tal O, Eventov-Friedman S, Shezen E, Aronovich A, Tchorsh D, Cohen S, Shtabsky A, Hecht G, Dekel B, Freud E, Reisner Y: Embryonic porcine liver as a source for transplantation: advantage of intact liver implants over isolated hepatoblasts in overcoming homeostatic inhibition by the quiescent host liver. Stem Cells 2008; 26: 1347– 1355 [DOI] [PubMed] [Google Scholar]
- 20.Hecht G, Eventov-Friedman S, Rosen C, Shezen E, Tchorsh D, Aronovich A, Freud E, Golan H, El-Hasid R, Katchman H, Hering BJ, Zung A, Kra-Oz Z, Shakel-Mishan P, Yusim A, Shtabsky A, Idelevich D, Tober A, Harmelin A, Bachar-Lustig E, Reisner Y: Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci U S A 2009; 106: 8659– 8664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schon MP: Efalizumab in the treatment of psoriasis: mode of action, clinical indications, efficacy, and safety. Clin Dermatol 2008; 26: 509– 514 [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama H, McCormick TS, Cooper KD, Korman NJ: Alefacept in the treatment of psoriasis. Clin Dermatol 2008; 26: 503– 508 [DOI] [PubMed] [Google Scholar]
- 23.Tyrberg B, Andersson A, Borg LA: Species differences in susceptibility of transplanted and cultured pancreatic islets to the beta-cell toxin alloxan. Gen Comp Endocrinol 2001; 122: 238– 251 [DOI] [PubMed] [Google Scholar]
- 24.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007; 117: 2553– 2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamamoto Y, Tsuura Y, Fujimoto S, Nagata M, Takeda T, Mukai E, Fujita J, Yamada Y, Seino Y: Recovery of function and mass of endogenous beta-cells in streptozotocin-induced diabetic rats treated with islet transplantation. Biochem Biophys Res Commun 2001; 287: 104– 109 [DOI] [PubMed] [Google Scholar]
- 26.Brands K, Colvin E, Williams LJ, Wang R, Lock RB, Tuch BE: Reduced immunogenicity of first-trimester human fetal pancreas. Diabetes 2008; 57: 627– 634 [DOI] [PubMed] [Google Scholar]
- 27.Davis SJ, van der Merwe PA: The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today 1996; 17: 177– 187 [DOI] [PubMed] [Google Scholar]
- 28.Kato K, Koyanagi M, Okada H, Takanashi T, Wong YW, Williams AF, Okumura K, Yagita H: CD48 is a counter-receptor for mouse CD2 and is involved in T cell activation. J Exp Med 1992; 176: 1241– 1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isobe M, Yagita H, Okumura K, Ihara A: Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science 1992; 255: 1125– 1127 [DOI] [PubMed] [Google Scholar]
- 30.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Vallera DA: Coblockade of the LFA1:ICAM and CD28/CTLA4:B7 pathways is a highly effective means of preventing acute lethal graft-versus-host disease induced by fully major histocompatibility complex-disparate donor grafts. Blood 1995; 85: 2607– 2618 [PubMed] [Google Scholar]
- 31.Qin L, Chavin KD, Lin J, Yagita H, Bromberg JS: Anti-CD2 receptor and anti-CD2 ligand (CD48) antibodies synergize to prolong allograft survival. J Exp Med 1994; 179: 341– 346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolls MR, Coulombe M, Yang H, Bolwerk A, Gill RG: Anti-LFA-1 therapy induces long-term islet allograft acceptance in the absence of IFN-gamma or IL-4. J Immunol 2000; 164: 3627– 3634 [DOI] [PubMed] [Google Scholar]
- 33.Vincenti F, Mendez R, Pescovitz M, Rajagopalan PR, Wilkinson AH, Butt K, Laskow D, Slakey DP, Lorber MI, Garg JP, Garovoy M: A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant 2007; 7: 1770– 1777 [DOI] [PubMed] [Google Scholar]
- 34.Vincenti F, Kirk AD: What's next in the pipeline. Am J Transplant 2008; 8: 1972– 1981 [DOI] [PubMed] [Google Scholar]
- 35.Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH: Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes 2006; 55: 2429– 2436 [DOI] [PubMed] [Google Scholar]
- 36.Bussiere CT, Lakey JR, Shapiro AM, Korbutt GS: The impact of the mTOR inhibitor sirolimus on the proliferation and function of pancreatic islets and ductal cells. Diabetologia 2006; 49: 2341– 2349 [DOI] [PubMed] [Google Scholar]
- 37.Salvadori M, Budde K, Charpentier B, Klempnauer J, Nashan B, Pallardo LM, Eris J, Schena FP, Eisenberger U, Rostaing L, Hmissi A, Aradhye S: FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant 2006; 6: 2912– 2921 [DOI] [PubMed] [Google Scholar]
- 38.Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW: Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355: 1124– 1140 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB: Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med 1999; 5: 1298– 1302 [DOI] [PubMed] [Google Scholar]
- 40.Tran HM, Nickerson PW, Restifo AC, Ivis-Woodward MA, Patel A, Allen RD, Strom TB, O'Connell PJ: Distinct mechanisms for the induction and maintenance of allograft tolerance with CTLA4-Fc treatment. J Immunol 1997; 159: 2232– 2239 [PubMed] [Google Scholar]
- 41.Dai Z, Konieczny BT, Baddoura FK, Lakkis FG: Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J Immunol 1998; 161: 1659– 1663 [PubMed] [Google Scholar]
- 42.Ferrari-Lacraz S, Zheng XX, Kim YS, Li Y, Maslinski W, Li XC, Strom TB: An antagonist IL-15/Fc protein prevents costimulation blockade-resistant rejection. J Immunol 2001; 167: 3478– 3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishimoto K, Dong VM, Issazadeh S, Fedoseyeva EV, Waaga AM, Yamada A, Sho M, Benichou G, Auchincloss H, Jr, Grusby MJ, Khoury SJ, Sayegh MH: The role of CD154-CD40 versus CD28–B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest 2000; 106: 63– 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, Kenyon NS, Ricordi C, Burkly LC, Inverardi L: Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes 2001; 50: 270– 276 [DOI] [PubMed] [Google Scholar]
- 45.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW: Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol 2001; 2: 591– 596 [DOI] [PubMed] [Google Scholar]
- 46.Shirasugi N, Adams AB, Durham MM, Lukacher AE, Xu H, Rees P, Cowan SR, Williams MA, Pearson TC, Larsen CP: Prevention of chronic rejection in murine cardiac allografts: a comparison of chimerism- and nonchimerism-inducing costimulation blockade-based tolerance induction regimens. J Immunol 2002; 169: 2677– 2684 [DOI] [PubMed] [Google Scholar]
- 47.Kumagai-Braesch M, Ekberg H, Wang F, Osterholm C, Ehrnfelt C, Sharma A, Lindeborg E, Holgersson J, Corbascio M: Anti-LFA-1 improves pig islet xenograft function in diabetic mice when long-term acceptance is induced by CTLA4Ig/anti-CD40L. Transplantation 2007; 83: 1259– 1267 [DOI] [PubMed] [Google Scholar]
- 48.Arefanian H, Tredget EB, Rajotte RV, Korbutt GS, Gill RG, Rayat GR: Combination of anti-CD4 with anti-LFA-1 and anti-CD154 monoclonal antibodies promotes long-term survival and function of neonatal porcine islet xenografts in spontaneously diabetic NOD mice. Cell Transplant 2007; 16: 787– 798 [DOI] [PubMed] [Google Scholar]
- 49.Lehnert AM, Mottram PL, Han W, Walters SN, Patel AT, Hawthorne WJ, Cowan PJ, d'Apice AJ, O'Connell PJ: Blockade of the CD28 and CD40 pathways result in the acceptance of pig and rat islet xenografts but not rat cardiac grafts in mice. Transpl Immunol 2001; 9: 51– 56 [DOI] [PubMed] [Google Scholar]
- 50.Elwood ET, Larsen CP, Cho HR, Corbascio M, Ritchie SC, Alexander DZ, Tucker-Burden C, Linsley PS, Aruffo A, Hollenbaugh D, Winn KJ, Pearson TC: Prolonged acceptance of concordant and discordant xenografts with combined CD40 and CD28 pathway blockade. Transplantation 1998; 65: 1422– 1428 [DOI] [PubMed] [Google Scholar]
- 51.Schaub M, Stadlbauer TH, Chandraker A, Vella JP, Turka LA, Sayegh MH: Comparative strategies to induce long-term graft acceptance in fully allogeneic renal versus cardiac allograft models by CD28–B7 T cell costimulatory blockade: role of thymus and spleen. J Am Soc Nephrol 1998; 9: 891– 898 [DOI] [PubMed] [Google Scholar]