Abstract
OBJECTIVE
Leptin released from adipocytes plays a key role in the control of food intake, energy balance, and glucose homeostasis. In addition to its central action, leptin directly affects pancreatic β-cells, inhibiting insulin secretion, and, thus, modulating glucose homeostasis. However, despite the importance of glucagon secretion in glucose homeostasis, the role of leptin in α-cell function has not been studied in detail. In the present study, we have investigated this functional interaction.
RESEARCH DESIGN AND METHODS
The presence of leptin receptors (ObR) was demonstrated by RT-PCR analysis, Western blot, and immunocytochemistry. Electrical activity was analyzed by patch-clamp and Ca2+ signals by confocal microscopy. Exocytosis and glucagon secretion were assessed using fluorescence methods and radioimmunoassay, respectively.
RESULTS
The expression of several ObR isoforms (a–e) was detected in glucagon-secreting αTC1-9 cells. ObRb, the main isoform involved in leptin signaling, was identified at the protein level in αTC1-9 cells as well as in mouse and human α-cells. The application of leptin (6.25 nmol/l) hyperpolarized the α-cell membrane potential, suppressing the electrical activity induced by 0.5 mmol/l glucose. Additionally, leptin inhibited Ca2+ signaling in αTC1-9 cells and in mouse and human α-cells within intact islets. A similar result occurred with 0.625 nmol/l leptin. These effects were accompanied by a decrease in glucagon secretion from mouse islets and were counteracted by the phosphatidylinositol 3-kinase inhibitor, wortmannin, suggesting the involvement of this pathway in leptin action.
CONCLUSIONS
These results demonstrate that leptin inhibits α-cell function, and, thus, these cells are involved in the adipoinsular communication.
Among the different hormones released by adipocytes, leptin plays a fundamental role in the control of satiety and body weight by acting on the hypothalamus and, thus, regulating food intake and energy expenditure (1,2). Although several factors modulate the release of leptin from adipocytes, its plasma levels are frequently proportional to body fat mass (1). Its action is mediated by the activation of the leptin receptor (ObR), which is highly expressed in the hypothalamus and cerebellum as well as in other tissues involved in metabolism, such as the endocrine pancreas, the liver, and the adipose tissue (3). The ObR gene produces several splicing variants, yet the long form of the receptor (ObRb) is the main isoform involved in the transduction of intracellular signals (3,4). The activation of ObR induces JAK/STAT (Janus kinase/signal transducer and activator of transcription signaling), which is implicated in transcriptional modulation (4). Additionally, leptin can induce rapid effects on membrane potential and secretion in endocrine cells and neurons by activating the phosphatidylinositol 3-kinase (PI3K) signaling pathway (5–10).
Remarkably, leptin can inhibit insulin expression and secretion in pancreatic β-cells, regulating glucose homeostasis directly through its action on the endocrine pancreas in addition to its central effects (7,11–15). The inhibition of insulin release by leptin is mainly mediated by the hyperpolarization of the membrane potential and a subsequent decrease in Ca2+ signaling in both human and rodent β-cells (8–13). These effects result from ATP-sensitive K+ channel (KATP channel) opening and involve the PI3K pathway (9–11). Since insulin also stimulates leptin release from adipocytes, there is a bidirectional feedback loop between β-cells and the adipose tissue. It has been proposed that the dysregulation of this adipoinsular communication may play a role in the development of diabetes in obese individuals (14,15).
However, despite the importance of glucagon secretion in the regulation of glycemia, the effect of leptin on α-cells has not been studied in detail. The hyperglycemic hormone glucagon increases blood glucose levels essentially by inducing glucose synthesis and mobilization in/from the liver (16,17). Glucagon secretion is the main line of defense against hypoglycemia and, also, counterbalances the effects of insulin on glucose levels (18). In diabetes, glucagon secretion does not respond adequately to glucose changes, which leads to further problems in the control of glucose levels in diabetic patients besides those difficulties derived from β-cell malfunction (17,18). Leptin has been revealed as an important modulator of glucose homeostasis by directly acting on β-cells. Therefore, given that glucagon is an essential player in the islet function and the regulation of glycemia, it is important to determine whether leptin can affect α-cell function. Since defects in the adipoinsular axis may contribute to diabetes associated with obesity (14,15), a better understanding of the communication between the adipose tissue and the two main endocrine cells involved in glucose homeostasis is essential to design potential therapeutic strategies. Several evidences suggest that leptin could have a role in the α-cell. It has been reported that mouse models with defects in leptin signaling can develop hyperglucagonemia (19–21). Additionally, it was recently shown that induction of hyperleptinemia decreases glucagon levels in diabetic mice with hyperglucagonemia (22). However, the direct regulation of glucagon secretion by leptin has not been described so far.
In the present study, we show a direct action of leptin on the α-cell. The presence of ObR was identified in the glucagon-secreting cell line αTC1-9 and in mouse and human α-cells. We found that leptin hyperpolarizes the membrane potential in α-cells, suppressing electrical activity. Additionally, leptin inhibited Ca2+ signaling and glucagon secretion induced by low glucose concentrations. All these findings indicate that the α-cell function can be modulated by the adipose tissue through a leptin signaling pathway.
RESEARCH DESIGN AND METHODS
Islet isolation and cell culture.
All protocols were approved by our animal care committee according to national regulations. Swiss albino OF1 mice (8–10 weeks old) were killed by cervical dislocation, and islets were then isolated by collagenase digestion (23,24). Isolated islets were dispersed into single cells by trypsin enzymatic digestion and then cultured overnight at 37°C in RPMI-1640 (Sigma, Madrid, Spain) supplemented with 10% FCS, 100 IU/ml penicillin, 0.1 mg/ml streptomycin, and 5.6 mmol/l d-glucose (23,24). The glucagon-releasing αTC1-9 cell line was purchased from the American Type Cultures Collection (ATCC CRL-2350; passage 5–15). αTC1-9 cells were grown in Dulbecco's Modified Eagle's medium (Invitrogen) containing (in mmol/l) 4 l-glutamine, 16 glucose, 19 NaHCO3, 10% fetal bovine serum, 15 HEPES, and 0.1 nonessencial amino acids. Human islets from healthy donors were obtained from the human islet isolation facility of the Clinic Hospital of Barcelona after approval of the hospital ethics committee. Islets were cultured in RPMI-1640 at 37°C and 5% CO2. Experiments were performed within 48 h of isolation. Leptin (Calbiochem) was used at 6.25 nmol/l (100 ng/ml) and 0.625 nmol/l (10 ng/ml). Except when indicated, all experiments were carried out at 37°C.
Ca2+ signaling measurements by confocal microscopy.
For Ca2+ experiments, cells were loaded for 1 h at 37°C with the Ca2+ probe Fluo-4 (2 μmol/l) (Invitrogen). Islets were loaded with Fluo-4 (5 μmol/l) for 1 h at room temperature. Islets or cells were placed on a perfusion chamber mounted on the microscope stage (25). Then, they were perfused at a rate of 1.5 ml/min with a modified Ringer solution containing (in mmol/l) 120 NaCl; 5 KCl; 25 NaHCO3; 1.1 MgCl2; and 2.5 CaCl2, pH = 7.4, gassed with 95% O2 and 5% CO2. Ca2+ signals were monitored in individual cells using a Zeiss LSM 510 laser confocal microscope equipped with a ×40 oil immersion objective. The configuration of the system was set to excite the Ca2+ probe at 488 nm and collect the emission with a band-pass filter at 505–530 nm from an optical section of 8 μm. Images were collected at 2-s intervals and treated with a low-pass filter (26). As previously reported, individual cells loaded with Fluo-4 were easily identified at the periphery of the islet where α-cells usually are more abundant (26). Fluorescence records were represented as the percentage of ΔF/F0, where F0 is the fluorescence signal at the beginning of a record and ΔF is F − F0. Background fluorescence was subtracted from F0. The frequency of oscillatory Ca2+ signals was calculated over a 5-min period of the Ca2+ recording, immediately before and 5 min after the application of the stimulus, as previously reported (23). To analyze frequency, a Ca2+ oscillation or spike was defined as a rapid increase in the intracellular Ca2+ concentration higher than twice the SD of the background signal at the intervals between spikes (23). Some data were expressed in percentages with respect to the frequency before the stimulus.
Glucagon secretion.
Batches of 15 islets were preincubated for 60 min at 37°C in 0.5 ml Krebs-Ringer bicarbonate buffer supplemented with 15 mmol/l HEPES, 0.5% bovine serum albumin, and 5.6 mmol/l glucose, pH = 7.4 (23). Then, the islets were incubated at 37°C for 60 min with Krebs-Ringer bicarbonate buffer supplemented with 0.5 mmol/l glucose and additional reagents, as indicated in the results. At the end of the incubation, the medium was aspirated and assayed for glucagon using a commercial radioimmunoassay kit (Linco Research).
Analysis of exocytosis by fluorescence imaging.
The exocytotic response of αTC1-9 cells was monitored at the single-cell level using the styryl dye FM1-43 (Invitrogen), as previously reported (27–29). The cells were incubated for 15 min with FM1-43 (2 μmol/l), allowing its incorporation into the cell membrane (27). The dye was maintained continuously throughout the experiment. This cell-impermeable probe is nonfluorescent in an aqueous solution but emits intense fluorescence after partitioning into the plasma membrane. Consequently, the incorporation of secretory granules into the plasma membrane during secretion increases the cell surface, augmenting FM1-43 fluorescence (27–29). The FM1-43 signal was monitored by exciting the cells at 488 nm and obtaining the emission with a 560-nm long-pass filter. The relative change in fluorescence (ΔF) was represented with respect to time for each analyzed cell. The average rate of fluorescence changes (ΔF/min) was also calculated for each experimental condition. Background signal was subtracted in all cases.
Patch-clamp recordings.
As previously reported, α-cells were identified by their size, membrane capacitance (<4 pF), and their characteristic electrical activity in the absence of glucose (30,31). Membrane potential and whole-cell currents were recorded in the perforated patch whole-cell configuration using an Axopatch 200B amplifier (Axon Instruments). Patch pipettes were pulled from borosilicate capillaries using a flaming/brown micropipette puller P-97 (Sutter Instruments) with a resistance of 3–5 MΩ when filled with the pipette solution (in mmol/l): 76 K2SO4, 10 KCl, 10 NaCl, 1 MgCl2, 5 HEPES (pH = 7.35). The extracellular solution consisted of (in mmol/l) 138 NaCl, 5.6 KCl, 2.6 CaCl2, 1.2MgCl2, and 5 HEPES (pH = 7.4) and supplemented with glucose as indicated (30,31).
Immunocytochemistry.
This protocol was performed as previously reported (23). Briefly, islets or cells were fixed with Bouin's solution for 5 min and then dehydrated for 3 min with 30, 50, and 70% ethanol. Triton X-100 (0.5%) was used for permeabilization. To reduce nonspecific binding, cells were first preincubated with a blocking buffer (5% serum in PBS) for 45 min before applying primary antibodies in a buffer containing 1% serum. Glucagon-containing cells were identified with monoclonal anti-glucagon mouse antibodies (1:200; Sigma, Madrid, Spain). ObR receptors were detected with an anti-ObR goat antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) or an anti-ObRb rabbit antibody (1:500; Alpha Diagnostic, San Antonio, TX). An anti-ObRb rabbit antibody specific for humans was also used (1:100; Millipore). These antibodies were applied overnight at 4°C. After washing, appropriate combinations of secondary antibodies conjugated with Alexa Fluor dyes (1:500; Invitrogen) were applied for 2 h at room temperature. The omission of the first antibody led to the absence of staining.
RT-PCR.
Total RNA was isolated using RNeasy Mini Kit (Quiagen). Extracted RNA was quantified by OD260/280 measurement. Extracted RNA was used to generate cDNA using Expand Reverse Transcriptase (Roche, Mannheim, Germany), and 2 μl of each RT reaction were used as input for the PCR. Primers are detailed in Table 1. PCR amplification was performed using Taq-DNA polymerase (Invitrogen, Barcelona, Spain) under the following conditions: 15 s at 94°C, 30 s at 55°C, and 1 min at 72°C. 18S was amplified as an internal control. PCR products were subjected to agarose-gel electrophoresis.
TABLE 1.
PCR primers for ObR isoforms and 18S
| Name | Sense primer (5′-3′) | Antisense primer (5′-3′) |
|---|---|---|
| ObRa | AGGGCTGTATGTCATTGTACCCAT | AGTTTAGGTTTGTTTCCCTCCATC |
| ObRb | ACAGTTCTGGCTGTCAATTCCC | AGGAGCTGCTAGAAAGACTG |
| ObRc | ACAGTTCTGGCTGTCAATTCCC | GAAAGGATGAACAGGCTTGAGAAC |
| ObRd | AGGGCTGTATGTCATTGTACCCAT | CTTCATGTAAAGATATATCCTTTTCC |
| ObRe | AGGGCTGTATGTCATTGTACCCAT | CCATGAAAAGTACAGTACACATACC |
| 18S | GGGAGGTAGTGACGAAAAATAAC | AATCATGGCCTCAGTTCCGAAA |
Western blot analysis.
Cell pellets were obtained by centrifugation at 1,000g ×10 min and resuspended in 200 μl of cell lysis buffer (Cell Signaling Technology, Danvers, MA). Cell extracts were subjected to SDS-PAGE (12% gels). Prestained SDS-PAGE standards were included for molecular mass estimation. The transfer to polyvinylidene fluoride membranes was performed at 125 mA for 90 min in a buffer with 2.5 mmol/l Tris base, 9 mmol/l glycine, and 20% methanol. After membranes were blocked with 2% nonfat dry milk, they were incubated with the above-mentioned anti-ObR (1:100) or anti-ObRb (1:500) antibodies before being incubated with peroxidase-conjugated donkey anti-goat (Santa Cruz Biotechnology, Santa Cruz, CA) or goat anti-rabbit (GE Healthcare, Barcelona, Spain) antibodies, respectively. Protein bands were revealed by using the ECL Chemiluminiscence Reagents kit (Amersham Biosciences, Barcelona, Spain).
Statistical analysis.
Some data are shown as means ± SE. Student's t test or one-way ANOVA were performed, as appropriate, with a level of significance of P < 0.05.
RESULTS
Identification of leptin receptors in α-cells.
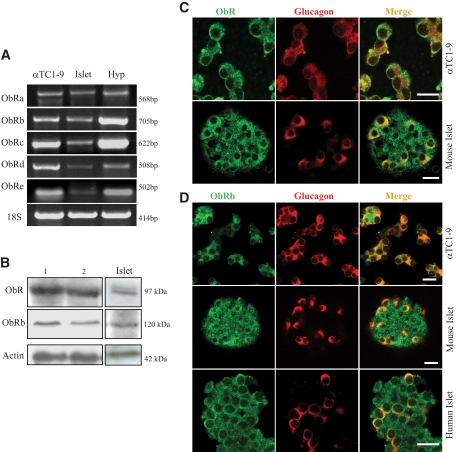
Multiple isoforms have been described for the leptin receptor (ObR) (3,4). Although these variants have a common extracellular domain, the intracellular site varies for each isoform. ObRb is the long full-length isoform, and it is considered the functional receptor in terms of intracellular signaling (4). To investigate the existence of ObR in glucagon-releasing cells, we first performed a PCR analysis in αTC1-9 cells. As shown in Fig. 1A, these cells express mRNA for the majority of ObR isoforms (ObRa–e). The presence of these receptors was also studied at the protein level by Western blot in αTC1-9 cells. We initially explored the existence of ObR using an antibody that recognizes the extracellular domain, which is common in all the ObR isoforms (Fig. 1B). Nonetheless, given the importance of ObRb for leptin signaling, its presence in these cells was also confirmed with a specific antibody against this isoform (Fig. 1B). Finally, the spatial localization of ObR and ObRb was studied using these antibodies and confocal microscopy. As shown in Fig. 1C and D, both αTC1-9 cells and α-cells within intact mouse islets contain these receptors. Remarkaly, ObRb was also identified in human α-cells. These results indicate that, in addition to β-cells, glucagon-containing cells are also equipped with ObR.
FIG. 1.
Expression of leptin receptors in the pancreatic α-cell. A: PCR analysis of ObR transcripts shows that multiple isoforms are expressed in αTC1-9 cells. The expression in the mouse hypothalamus (Hyp) and islets is also illustrated. B: The presence of ObRb was demonstrated in αTC1-9 cells and in mouse islets by Western blot as well. An antibody that recognizes the extracellular domain of all the isoforms was also tested (ObR). Two examples are shown for the αTC1-9 cells. C and D: The spatial localization of ObRb in αTC1-9 cells and in α-cells within mouse and human islets (green) was assayed by immunofluorecence and confocal microscopy. Glucagon staining is shown in red. As in B, we also used an antibody that recognizes all the isoforms (ObR). These results are representative of at least three different experiments for each condition. Scale bar: 20 μm. (A high-quality representation of this figure is available in the online issue.)
Leptin hyperpolarizes α-cell membrane potential and suppresses electrical activity.
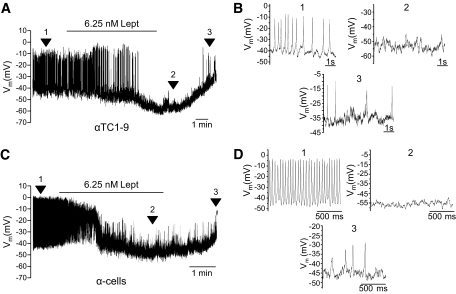
In the absence of glucose or at low concentrations, α-cells produce a characteristic, regenerative electrical activity (30–36) that triggers Ca2+ signals and glucagon secretion. Thus, to study the functional role of leptin in α-cells, we first recorded their electrical activity using the patch-clamp technique in the whole-cell configuration. In 0.5 mmol/l of glucose, all the analyzed αTC1-9 cells displayed a characteristic electrical activity with action potentials that originated from −39.9 ± 0.2 mV (Fig. 2A). The application of leptin (6.25 nmol/l) hyperpolarized the membrane potential by −21.1 ± 1.1 mV and suppressed electrical activity. Although the effect of leptin persisted in some cells, its removal from the bath allowed for the depolarization of the membrane potential and the restoration of electrical activity (Fig. 2A and B). Similar effects were found in mouse α-cells, since leptin hyperpolarized the membrane potential from −37.3 ± 0.6 mV to −60.6 ± 0.4 mV, and decreased electrical activity as well (Fig. 2C and D). Thus, leptin is able to induce short-term effects that modulate electrical responses in α-cells, in agreement with findings in other secretory cells (6,8–11).
FIG. 2.
Leptin induces membrane hyperpolarization and inhibition of electrical activity. A: Recording of membrane potential in whole-cell configuration in αTC1-9 cells. With 0.5 mmol/l glucose, characteristic action potentials originated from a membrane potential of −39.9 ± 0.2 mV (n = 8). The application of leptin (6.25 nmol/l) induced hyperpolarization (21.1 ± 1.1 mV) and suppression of electrical activity. Removal of leptin allowed for a depolarization and recovery of electrical activity. Lept, leptin. B: Expanded records from A of different significant instants (indicated by numbers). C: With 0.5 mmol/l glucose, an intense electrical activity was recorded in mouse α-cells. Leptin (6.25 nmol/l) hyperpolarized these cells from −37.3 ± 0.6 mV to −60.6 ± 0.4 mV (n = 3) and decreased electrical activity. Lept, leptin. D: Expanded records from C of different significant instants (indicated by numbers).
Regulation of Ca2+ signaling by leptin in α-cells.
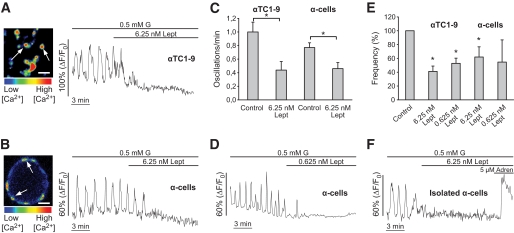
Since glucose-regulated Ca2+ signals in α-cells are mainly sustained by their electrical activity (16,17,36), the effect of leptin on membrane potential should affect Ca2+ oscillations. It has been reported that among the different islet cell types, α-cells isolated in culture or within mouse islets are unique in displaying Ca2+ oscillations at low glucose levels (23,25,37–41). As shown in Fig. 3, α-cells exhibited this characteristic oscillatory Ca2+ pattern with 0.5 mmol/l glucose. The application of leptin (6.25 nmol/l) produced either a complete blockage or a decrease in the frequency of the Ca2+ oscillations in both αTC1-9 cells and α-cells within intact mouse islets (Fig. 3A–C). The average effect of leptin was a decrease of ∼60 and ∼40% in the Ca2+ signaling frequency in αTC1-9 cells and mouse α-cells, respectively (Fig. 3E). A similar response was observed with 3 mmol/l glucose (see supplementary Figure S1 in the online appendix [available at http://dx.doi.org/10.2337/db08-1787]). Removal of leptin from the bath allowed for the restoration of Ca2+ oscillations (supplementary Figure S2). After long periods of stimulation, this reversibility was not complete during the recording time, indicating that the leptin effect may be persistent. The addition of 0.625 nmol/l leptin also produced an inhibitory effect in αTC1-9 cells (∼45% decrease) (Fig. 3E). In contrast, although 0.625 nmol/l leptin blocked Ca2+ signals in ∼57% of α-cells (Fig. 3D), the average effect was not significant because of the heterogeneous response to this concentration (Fig. 3E). To avoid the potential contribution of islet paracrine interactions on the leptin action, some experiments were performed with isolated α-cells (Fig. 3F). In these conditions, leptin produced a similar inhibitory response, proving its direct action on α-cells. The absence of effect on α-cells from db/db mice, which lack functional ObRb, gave further molecular evidence that this receptor is involved in leptin-induced actions (supplementary Figure S3). Finally, the response of α-cells to this hormone was also evaluated in human islets (Fig. 4). Application of leptin at 6.25 and 0.625 nmol/l decreased Ca2+ signals by ∼58 and ∼32%, respectively. This result indicates that ObR is also functional in human α-cells.
FIG. 3.
Leptin inhibits Ca2+ signals induced by low glucose concentrations in αTC1-9 cells and mouse α-cells. Leptin (6.25 nmol/l) blocks or reduces the frequency of Ca2+ signals induced with 0.5 mmol/l glucose in αTC1-9 cells (A) and α-cells (B) (n = 13 and 16, respectively). Images show a culture of αTC1-9 cells (A) and an intact mouse islet (B) loaded with the Ca2+-sensitive probe Fluo-4. Islet images were acquired by confocal microscopy from an optical section close to the equatorial plane. Several individual cells were easily identified at the periphery of the islet (white arrows). C: Average frequency of Ca2+ oscillations with 0.5 mmol/l glucose (control) and in the presence of leptin. D: Effect of 0.625 nmol/l leptin in islet α-cells (n = 20; n = 52 for αTC1-9 cells, not shown). E: Frequency (%) of Ca2+ signals after stimuli compared with control conditions. F: Leptin inhibits Ca2+ signaling in isolated islet α-cells (n = 11). The effect of adrenaline, which is characteristic of this islet cell type (32), is also shown. Data in C and E are shown as means ± SE. *Statistically significant (P < 0.05) compared with control. Adren, adrenaline; G, glucose; Lept, leptin. Scale bar: 20 μm. (A high-quality representation of this figure is available in the online issue.)
FIG. 4.
Leptin decreases Ca2+ signaling in human α-cells. The application of 6.25 nmol/l leptin (A) or 0.625 nmol/l (B and C) blocks or reduces the frequency of Ca2+ signals induced with 0.5 mmol/l glucose in human α-cells. D: Average frequency of Ca2+ oscillations in 0.5 mmol/l glucose (control) and in the presence of leptin at 6.25 and 0.625 nmol/l (n = 12 and 45, respectively). Data are shown as means ± SE. Statistically significant: *P < 0.05; **P < 0.01 vs. controls. G, glucose; Lept, leptin.
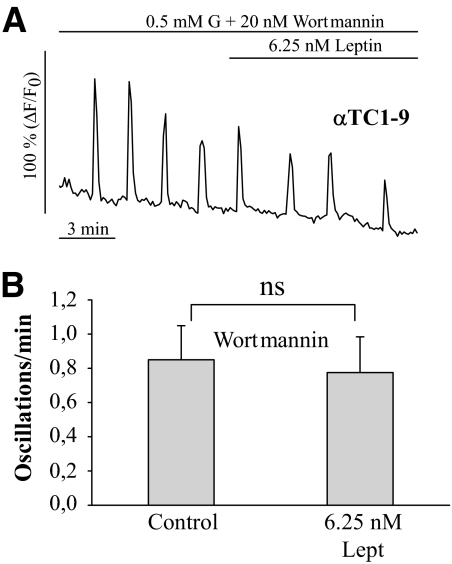
It has been reported that the leptin effect on electrical activity and Ca2+ signals is mediated by the PI3K pathway in pancreatic β-cells and neurons (5,6,8–10). To determine whether this pathway is also involved in the α-cell, some experiments were performed in the presence of the PI3K inhibitor wortmannin (20 nmol/l) (Fig. 5). In these conditions, leptin failed to inhibit Ca2+ signals in αTC1-9 cells (Fig. 5B and C), as shown in other cell types (6,8). Thus, the PI3K pathway may be involved in the leptin effect. The following results further support these observations.
FIG. 5.
Leptin does not affect Ca2+ signals in the presence of wortmannin. A: In the presence of the PI3K inhibitor wortmannin (20 nmol/l), leptin was unable to affect the intracellular Ca2+ oscillations induced with 0.5 mmol/l glucose in αTC1-9 cells (n = 8). B: Average frequency of Ca2+ signals with 0.5 mmol/l glucose (control) and leptin, both in the presence of wortmannin. Data in B and C are shown as means ± SE. G, glucose; Lept, leptin; ns, nonsignificant.
Reduced glucagon secretion in the presence of leptin.
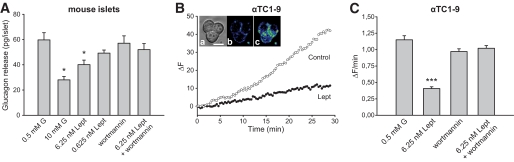
Glucagon secretion depends on Ca2+ signaling (16,17). Thus, all these changes induced by leptin may be associated with alterations in the secretory pattern. To test this possibility, we analyzed glucagon release from isolated mouse islets using radioimmunoassay (Fig. 6A). Glucagon secretion with 0.5 mmol/l glucose is almost maximal in mice (42). Incubation with 10 mmol/l glucose reduced glucagon release by ∼55%, consistent with previous reports (42). In the presence of 6.25 nmol/l leptin, glucagon secretion with 0.5 mmol/l glucose decreased by ∼33%. The inhibitory effect was not statistically significant with 0.625 nmol/l leptin, which probably may result from the heterogeneous response observed in Ca2+ signaling with this concentration (Fig. 3E). In agreement with the previous finding with Ca2+ signals (Fig. 5), wortmannin counteracted the blocking action of 6.25 nmol/l leptin on glucagon secretion (Fig. 6A), further indicating the involvement of the PI3K pathway. Finally, to further prove the influence of leptin on the secretory process, we analyzed exocytosis at the single-cell level by monitoring FM1-43 fluorescence in αTC1-9 cells, as previously reported in β-cells (27–29). FM1-43 emits fluorescence when it partitions into the plasma membrane. Thus, the incorporation of secretory granules into the plasma membrane increases the cell surface and, also, the fluorescence of FM1-43 (27–29). As shown in Fig. 6B and C, the fluorescence augmented with 0.5 mmol/l glucose (control), indicating an active secretory response. This increase was significantly reduced in the presence of leptin (6.25 nmol/l). Again, the application of wortmannin counteracted this effect (Fig. 6C). Thus, all these observations indicate that leptin is working as an inhibitory regulator of α-cell function.
FIG. 6.
Effect of leptin on glucagon secretion. A: Glucagon secretion from mouse islets with 10 mmol/l glucose or with 0.5 mmol/l glucose plus 0.625 nmol/l or 6.25 nmol/l leptin was compared with the control (0.5 mmol/l glucose). The effect of wortmannin (50 nmol/l) on glucagon release with 0.5 mmol/l glucose in the absence and presence of 6.25 nmol/l leptin is also displayed. Data are shown as means ± SE (n = 8–13). B: Changes in FM1-43 fluorescence (ΔF; arbitrary units) versus time in αTC1-9 cells in control conditions (0.5 mmol/l glucose) and in the presence of leptin (6.25 nmol/l). Inset: images illustrating a group of cells in transmitted light (A) and loaded with FM1-43 at the beginning (B) and at the end of the record (C). Scale bar: 10 μm. C: The average rate of fluorescence changes as a function of time (ΔF/min) indicates that leptin reduces the exocytotic response. This effect was counteracted by wortmannin (20 nmol/l). Data are shown as means ± SE (n = 32–45 for each condition). *P < 0.05; ***P < 0.001 vs. control. G, glucose; Lept, leptin. (A high-quality representation of this figure is available in the online issue.)
DISCUSSION
Glucose homeostasis is mainly regulated by the coordinated action of glucagon and insulin secreted from pancreatic α- and β-cells, respectively (17). These two islet populations respond reciprocally to blood glucose changes. Pancreatic α-cells develop spontaneous electrical activity at low glucose concentrations, leading to Ca2+ signals and glucagon secretion, while these processes become inhibited with the elevation of glucose levels (25,26,31,33–35,38). Remarkably, this glucagon secretory response can be impaired in diabetic individuals, aggravating the difficulties in the control of glycemia (17–18). Glucose homeostasis can be further regulated by the adipocyte hormone leptin through its inhibitory action on β-cell secretion (7,11–15). It has been proposed that defects in this communication may be implicated in obesity-induced diabetes (12,14,15). The functional role of leptin in pancreatic α-cells and its involvement in the adipoinsular communication, however, has not been investigated extensively.
In the present study, we have shown that leptin receptors are present in mouse and human glucagon-containing cells. We demonstrate that leptin produces significant changes in the membrane potential, electrical activity, Ca2+ signaling, and glucagon secretion in mouse α-cells as well as glucagon-secreting αTC1-9 cells. This cell line has been previously validated as a good model to study α-cell function (38). Additionally, the blocking action of leptin on Ca2+ signaling was also demonstrated in human α-cells. In a previous report (11), the function of leptin was not studied in α-cells because of the lack of ObR expression in hamster glucagonoma INR1G-9 cells and a weak antibody staining in isolated rat α-cells. Although methodological or interspecies differences may account for this discrepancy, our findings on the presence of ObR in α-cells are further sustained by the demonstration of direct leptin actions at the single-cell level.
It has been reported that leptin induces membrane potential changes that can modulate electrical activity in neurons and in β-cells (5,6,8–11,43). In agreement with these findings, we have observed similar effects in the pancreatic α-cell. In contrast to other islet cell populations, α-cells are electrically active in the absence or at low concentrations of glucose (30,33–35). In these conditions, spontaneous action potentials arise from a membrane potential that varies from −60 to −40 mV, depending on experimental conditions (30,33–35). In αTC1-9 cells and mouse α-cells, we observed this characteristic electrical activity with 0.5 mmol/l glucose, where action potentials arose from a membrane potential of about −40 mV. Consistent with the results obtained in other secretory cell types including β-cells (6,8–11,43), leptin hyperpolarized the membrane potential and decreased electrical activity (Fig. 2), which is voltage-dependent (33,34,40). Additionally, this hormone inhibited intracellular Ca2+ oscillations and glucagon secretion (Figs. 3, 4, and 6). This effect could be anticipated in α-cells, since their electrical activity triggers Ca2+ signals, and exocytosis is Ca2+ dependent (16,17). In the case of the pancreatic β-cell, leptin effects on membrane potential are also reflected in Ca2+ signals and insulin release (11,12). Like leptin, other hormones such as insulin and somatostatin can inhibit glucagon secretion in α-cells by mechanisms that involve membrane hyperpolarization and suppression of electrical activity, affecting Ca2+ signals (38,44,45). It has been proposed that the effects of leptin on β-cell membrane potential are mediated by the opening of KATP channels (11). A similar process may occur in α-cells, since their membrane potential is controlled by the KATP channel and its activation causes the hyperpolarization of these cells (40). In any case, further experiments should be performed since other molecular targets may be involved in leptin actions (3,4).
Leptin can activate multiple signaling cascades (4). Among them, PI3K signaling has an important function in leptin short-term effects on membrane potential, Ca2+ signals, and secretion in pancreatic β-cells and in neurons (5–10). In agreement with these findings, leptin failed to decrease Ca2+ signaling and secretion in α-cells when PI3K was inhibited (Figs. 5 and 6). Therefore, this signaling pathway seems to be involved in the intracellular transduction of leptin short-term effects on the α-cell.
Basal plasma leptin concentrations are in the order of 1–10 ng/ml (∼0.0625–0.625 nmol/l) during fasting state (1–3,46). These values can significantly increase every day by circadian mechanisms (46). These leptin fluctuations are more marked in women, reaching concentrations of ∼15 ng/ml in healthy individuals (47). Factors like feeding behavior, sex, age, and pregnancy or elevation of hormones such as insulin, estrogen, or glucocorticoids can notably increase plasma leptin levels in both rodents and humans (1–3). Leptin concentrations can reach 30–100 ng/ml (∼1.87–6.25 nmol/l) in obesity and are highly elevated in situations such as impaired renal function and inflammatory responses (1–3,46,47). The present study demonstrates a clear inhibitory effect at 6.25 nmol/l leptin. This response was more heterogeneous at 0.625 nmol/l. At this concentration, leptin clearly inhibited Ca2+ signals in αTC1-9 and human α-cells (Fig. 3E and Fig. 4), yet the average effect was not found significant in mouse α-cells (Fig. 3E). Nevertheless, 0.625 nmol/l leptin had a blocking action in several of these cells (∼57%) (Fig. 3D). Thus, these results indicate that α-cells may be less sensitive to leptin in mice than in humans. Additionally, all these observations suggest that leptin actions on α-cells may be more limited at basal plasma leptin levels. However, leptin effects should be more effective in those physiological situations when this hormone increases above basal values and in pathological situations (1–3), as previously mentioned.
The present findings indicate that leptin can directly regulate α-cell function. This suggests that the adipose tissue may modulate glucose homeostasis not only by inhibiting β-cells and insulin release but α-cells and glucagon secretion as well. Thus, the effect of leptin on islet hormonal responses would be similar to that of somatostatin, which limits both insulin and glucagon release (16,17). Since leptin has been detected in mouse α-cells, this hormone may also play a local autoregulatory effect within the islet (48). Interestingly, hyperglucagonemia has been reported in mouse models that have defects in leptin and ObR, which impairs the signaling by this hormone (19–21). Moreover, a similar suppressive action by leptin has been recently observed in vivo in diabetic mice with hyperglucagonemia and hyperglycemia (22). In these rodents, hyperglucagonemia was reduced by adenoviral-induced hyperleptinemia, which normalized glucose levels. Our results indicate that, although several mechanisms could be involved, this effect may be the result of a direct action on α-cells. It has been demonstrated that leptin regulates hepatic glucose fluxes and, remarkably, that this hormone antagonizes the effects of glucagon in the liver, the main target for α-cell function (49,50). Our present observations suggest that leptin could antagonize glucagon action not only in the liver but also directly in the α-cells. In diabetic individuals, an absolute or relative excess of glucagon along with insulin deficiency can generate an excessive hepatic glucose production (17,18). Since this can be a major problem in these patients, several approaches have been developed to limit glucagon secretion and/or action (17,18). In this regard, the direct inhibitory effect of leptin on the α-cell reported in this study may be explored as a therapeutic strategy for the control of glucagon levels, as has been recently suggested for type 1 diabetes (22). However, the specific management of glucagon levels by leptin would be more complex if β-cells are functional, since it could also affect insulin release (10,11). Thus, further research will be necessary in this context to develop approaches to discriminate the secretory responses of α- and β-cells to this hormone. Leptin resistance has been reported in obese individuals, and several mechanisms have been proposed to explain this process at the central level (2–4). Nevertheless, although it has been suggested that leptin resistance may occur directly in the β-cell, there is no information about the molecular determinants involved (12,14,15). It has been proposed that β-cell leptin resistance and dysregulation of the adipoinsular communication may contribute to obesity-induced diabetes (12,14,15). Thus, it would be interesting to analyze whether α-cells may develop leptin resistance in this context and the role that these cells may be playing in this situation.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministerio de Educacion y Ciencia (BFU2007-67607, PCI2005-A7-0131, BFU2008-01492, and SAF2006-07382), the Ministerio de Ciencia e Innovación (ISCIII-Acción Transversal de Terapias Avanzadas), and FAPESP (2008/53811-8). CIBERDEM is an initiative of the Instituto de Salud Carlos III.
No potential conflicts of interest relevant to this article were reported.
The authors thank A.B. Rufete, M.L. Navarro, and M. Julià for their expert technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Margetic S, Gazzola C, Pegg GG, Hill RA: Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 2002; 26: 1407– 1433 [DOI] [PubMed] [Google Scholar]
- 2.Niswender KD, Schwartz MW: Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol 2003; 24: 1– 10 [DOI] [PubMed] [Google Scholar]
- 3.Anubhuti, Arora S: Leptin and its metabolic interactions: an update. Diabetes Obes Metab 2008; 10: 973– 993 [DOI] [PubMed] [Google Scholar]
- 4.Frühbeck G: Intracellular signalling pathways activated by leptin. Biochem J 2006; 393: 7– 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanley LJ, O'Malley D, Irving AJ, Ashford ML, Harvey J: Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol 2002; 545: 933– 944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK: Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 2008; 118: 1796– 1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao AZ, Bornfeldt KE, Beavo JA: Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest 1998; 102: 869– 873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning K, Miller LC, Laidlaw HA, Burgess LA, Perera NM, Downes CP, Leslie NR, Ashford ML: A novel leptin signalling pathway via PTEN inhibition in hypothalamic cell lines and pancreatic beta-cells. EMBO J 2006; 25: 2377– 2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, Ashford ML: Essential role of phosphoinositide 3-kinase in leptin-induced K(ATP) channel activation in the rat CRI-G1 insulinoma cell line. J Biol Chem 2000; 275: 4660– 4669 [DOI] [PubMed] [Google Scholar]
- 10.Harvey J, Hardy SC, Irving AJ, Ashford ML: Leptin activation of ATP-sensitive K+ (KATP) channels in rat CRI-G1 insulinoma cells involves disruption of the actin cytoskeleton. J Physiol 2000; 527: 95– 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF: Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic β-cells. Diabetes 1997; 46: 1087– 1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF: Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab 1999; 84: 670– 676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seufert J, Kieffer TJ, Habener JF: Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci U S A 1999; 96: 674– 679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN: Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest 2007; 117: 2860– 2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ: The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 2006; 4: 291– 302 [DOI] [PubMed] [Google Scholar]
- 16.Gromada J, Franklin I, Wollheim CB: Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007; 28: 84– 116 [DOI] [PubMed] [Google Scholar]
- 17.Quesada I, Tuduri E, Ripoll C, Nadal A: Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 2008; 199: 5– 19 [DOI] [PubMed] [Google Scholar]
- 18.Dunning BE, Gerich JE: The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007; 28: 253– 283 [DOI] [PubMed] [Google Scholar]
- 19.Strowski MZ, Cashen DE, Birzin ET, Yang L, Singh V, Jacks TM, Nowak KW, Rohrer SP, Patchett AA, Smith RG, Schaeffer JM: Antidiabetic activity of a highly potent and selective nonpeptide somatostatin receptor subtype-2 agonist. Endocrinology 2006; 147: 4664– 4673 [DOI] [PubMed] [Google Scholar]
- 20.Stearns SB, Benzo CA: Glucagon and insulin relationships in genetically diabetic (db/db) and in streptozotocin-induced diabetic mice. Horm Metab Res 1978; 10: 20– 23 [DOI] [PubMed] [Google Scholar]
- 21.Dunbar JC, Walsh MF: Glucagon and insulin secretion by islets of lean and obese (ob/ob) mice. Horm Metab Res 1980; 12: 39– 40 [DOI] [PubMed] [Google Scholar]
- 22.Yu X, Park BH, Wang MY, Wang ZV, Unger RH: Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A 2008; 105: 14070– 14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tudurí E, Filiputti E, Carneiro EM, Quesada I: Inhibition of Ca2+ signaling and glucagon secretion in mouse pancreatic alpha-cells by extracellular ATP and purinergic receptors. Am J Physiol Endocrinol Metab 2008; 294: E952– E960 [DOI] [PubMed] [Google Scholar]
- 24.Quesada I, Todorova MG, Soria B: Different metabolic responses in alpha-, beta-, and delta-cells of the islet of Langerhans monitored by redox confocal microscopy. Biophys J 2006; 90: 2641– 2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadal A, Quesada I, Soria B: Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse. J Physiol 1999; 517: 85– 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quesada I, Nadal A, Soria B: Different effects of tolbutamide and diazoxide in α-, β-, and δ-cells within intact islets of Langerhans. Diabetes 1999; 48: 2390– 2397 [DOI] [PubMed] [Google Scholar]
- 27.Smukler SR, Tang L, Wheeler MB, Salapatek AM: Exogenous nitric oxide and endogenous glucose-stimulated β-cell nitric oxide augment insulin release. Diabetes 2002; 51: 3450– 3460 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H: Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science 2002; 297: 1349– 1352 [DOI] [PubMed] [Google Scholar]
- 29.Leung YM, Sheu L, Kwan E, Wang G, Tsushima R, Gaisano H: Visualization of sequential exocytosis in rat pancreatic islet beta cells. Biochem Biophys Res Commun 2002; 292: 980– 986 [DOI] [PubMed] [Google Scholar]
- 30.Barg S, Galvanovskis J, Göpel SO, Rorsman P, Eliasson L: Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells. Diabetes 2000; 49: 1500– 1510 [DOI] [PubMed] [Google Scholar]
- 31.Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P: ATP-sensitive K+ channel–dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes 2004; 53( Suppl. 3): S181– S189 [DOI] [PubMed] [Google Scholar]
- 32.Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renstrom E, Rorsman P: Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol 1997; 110: 217– 228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Göpel SO, Kanno T, Barg S, Rorsman P: Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets. J Physiol 2000; 528: 497– 507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P: Regulation of glucagon release in mouse alpha-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol 2000; 528: 509– 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning Fox JE, Gyulkhandanyan AV, Satin LS, Wheeler MB: Oscillatory membrane potential response to glucose in islet beta-cells: a comparison of islet-cell electrical activity in mouse and rat. Endocrinology 2006; 147: 4655– 4663 [DOI] [PubMed] [Google Scholar]
- 36.Quoix N, Cheng-Xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois MC, Henquin JC, Gilon P: Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse α-cells. Diabetes 2009; 58: 412– 421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quesada I, Todorova MG, Alonso-Magdalena P, Beltra M, Carneiro EM, Martin F, Nadal A, Soria B: Glucose induces opposite intracellular Ca2+ concentration oscillatory patterns in identified α- and β-cells within intact human islets of Langerhans. Diabetes 2006; 55: 2463– 2469 [DOI] [PubMed] [Google Scholar]
- 38.Ravier MA, Rutter GA: Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 2005; 54: 1789– 1797 [DOI] [PubMed] [Google Scholar]
- 39.Berts A, Gylfe E, Hellman B: Ca2+ oscillations in pancreatic islet cells secreting glucagon and somatostatin. Biochem Biophys Res Commun 1995; 208: 644– 649 [DOI] [PubMed] [Google Scholar]
- 40.Macdonald PE, Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P: A KATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 2007; 5: e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiota C, Rocheleau JV, Shiota M, Piston DW, Magnuson MA: Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Am J Physiol Endocrinol Metab 2005; 289: E570– E577 [DOI] [PubMed] [Google Scholar]
- 42.Salehi A, Vieira E, Gylfe E: Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes 2006; 55: 2318– 2323 [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Zubcevic L, Ashcroft FM: Glucose regulates the effects of leptin on hypothalamic POMC neurons. Proc Natl Acad Sci U S A 2008; 105: 9811– 9816 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y: Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 2006; 3: 47– 58 [DOI] [PubMed] [Google Scholar]
- 45.Yoshimoto Y, Fukuyama Y, Horio Y, Inanobe A, Gotoh M, Kurachi Y: Somatostatin induces hyperpolarization in pancreatic islet [alpha] cells by activating a G protein-gated K+ channel. FEBS Lett 1999; 444: 265– 269 [DOI] [PubMed] [Google Scholar]
- 46.Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW: Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med 1997; 3: 575– 579 [DOI] [PubMed] [Google Scholar]
- 47.Licinio J, Negrão AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Negro PP, Mulla A, Veldhuis JD, Cearnal L, Flier JS, Gold PW: Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab 1998; 83: 4140– 4147 [DOI] [PubMed] [Google Scholar]
- 48.Reddy S, Lau EM, Ross JM: Immunohistochemical demonstration of leptin in pancreatic islets of non-obese diabetic and CD-1 mice: co-localization in glucagon cells and its attenuation at the onset of diabetes. J Mol Histol 2004; 35: 511– 519 [DOI] [PubMed] [Google Scholar]
- 49.Aiston S, Agius L: Leptin enhances glycogen storage in hepatocytes by inhibition of phosphorylase and exerts an additive effect with insulin. Diabetes 1999; 48: 15– 20 [DOI] [PubMed] [Google Scholar]
- 50.Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE: Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem 2000; 275: 11348– 11354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.