Abstract
OBJECTIVE
The significant roles of brown adipose tissue (BAT) in the regulation of energy expenditure and adiposity are established in small rodents but have been controversial in humans. The objective is to examine the prevalence of metabolically active BAT in healthy adult humans and to clarify the effects of cold exposure and adiposity.
RESEARCH DESIGN AND METHODS
In vivo 2-[18F]fluoro-2-deoxyglucose (FDG) uptake into adipose tissue was measured in 56 healthy volunteers (31 male and 25 female subjects) aged 23–65 years by positron emission tomography (PET) combined with X-ray computed tomography (CT).
RESULTS
When exposed to cold (19°C) for 2 h, 17 of 32 younger subjects (aged 23–35 years) and 2 of 24 elderly subjects (aged 38–65 years) showed a substantial FDG uptake into adipose tissue of the supraclavicular and paraspinal regions, whereas they showed no detectable uptake when kept warm (27°C). Histological examinations confirmed the presence of brown adipocytes in these regions. The cold-activated FDG uptake was increased in winter compared with summer (P < 0.001) and was inversely related to BMI (P < 0.001) and total (P < 0.01) and visceral (P < 0.001) fat areas estimated from CT image at the umbilical level.
CONCLUSIONS
Our findings, being against the conventional view, indicate the high incidence of metabolically active BAT in adult humans and suggest a role in the control of body temperature and adiposity.
Mammals have two types of adipose tissue, white (WAT) and brown (BAT) adipose tissues. These two tissues have quite opposite roles in whole-body energy metabolism; that is, WAT is for energy storage and BAT is for cold- and diet-induced thermogenesis, which significantly contributes to the control of body temperature and energy expenditure (1). BAT thermogenesis is principally dependent on the β-adrenergically mediated activation of lipolysis and subsequent degradation of fatty acids via uncoupling protein 1 (UCP1), which uncouples mitochondrial oxidative phosphorylation to dissipate the electrochemical gradient as heat instead of ATP synthesis. Thus, the β-adrenoceptor–UCP1 system has been expected as an intriguing target for the control of whole-body energy balance, adiposity, and obesity (2–5).
Almost all views about BAT thermogenesis have come from studies using small rodents such as the mouse, rat, and hamster. In humans, significant amounts of BAT are present in newborns and may contribute to body temperature regulation during the neonatal period, probably in the same way as in small rodents. However, BAT seems to disappear rapidly during postnatal periods and in adults is rather difficult to identify by conventional anatomical examinations. Thus, it has been a general contention that BAT is absent or of minute amounts and plays negligible, if any, roles in adult humans (6–8).
The existence of metabolically active BAT in adult humans has been suggested by the current clinical studies using fluoro-deoxyglucose (FDG)–positron emission tomography (PET), one of the powerful diagnostic tools for malignant tumors; that is, PET sometimes detects symmetrical FDG uptake in the shoulder and thoracic spine regions, where no tumor is present. By simultaneous examinations with PET and X-ray computed tomography (CT), the site of FDG uptake was identified as adipose tissue (9). Such FDG uptake is increased at lower environmental temperatures (10–12) and reduced by pretreatment with β-adrenergic blockers (13,14). These findings collectively suggest that the FDG uptake in adipose tissue at the specific regions reflects the metabolic activity of BAT (15).
However, almost all human studies thus far reported seemed for more accurate diagnosis of cancer and not for the detection and evaluation of BAT itself. In this study, we performed FDG-PET/CT examinations in adult healthy volunteers under a warm and cold condition to clarify the effects of cold exposure, age, season, and other body parameters including body fat. Our data indicate an unexpected high incidence of cold-activated BAT in adult healthy humans and suggest a role in the regulation of metabolic thermogenesis and body fat content.
RESEARCH DESIGN AND METHODS
Subjects recruited for this study were 56 healthy volunteers (31 male and 25 female subjects) aged 23–65 years (Table 1). All subjects were carefully instructed about the study and gave their informed consent to participate. After a standardized health examination, they underwent FDG-PET/CT and other examinations once or twice from August of 2006 to the following March. The protocol was approved by the institutional review boards of Tenshi College.
TABLE 1.
Subject profiles
| Male subjects | Female subjects | |
|---|---|---|
| n | 31 | 25 |
| Age (years) | 35.8 ± 9.0 (23–65) | 38.8 ± 8.8 (25–65) |
| Height (cm) | 170.7 ± 5.2 (154–181) | 158.0 ± 4.4 (148–166) |
| Body weight (kg) | 68.8 ± 7.6 (53.2–91.0) | 52.5 ± 5.4 (40.6–63.4) |
| BMI (kg/m2) | 23.8 ± 2.6 (17.7–31.0) | 21.1 ± 2.3 (16.2–26.9) |
| Body fat (kg) | 16.4 ± 4.8 (5.7–36.8) | 14.9 ± 4.4 (7.4–26.2) |
| Blood parameters | ||
| Glucose (mg/dl) | 88.7 ± 5.6 (71–102) | 83.4 ± 5.1 (71–96) |
| A1C (%) | 4.8 ± 0.2 (4.5–5.5) | 4.8 ± 0.2 (4.0–5.1) |
| Total cholesterol (mg/dl) | 195 ± 21 (115–251) | 203 ± 22 (159–272) |
| HDL cholesterol (ng/dl) | 61.6 ± 11.8 (35–90) | 73.0 ± 12.0 (51–98) |
| Triglycerides (mg/dl) | 113 ± 57 (19–395) | 67 ± 25 (29–183) |
| Insulin (ng/ml) | 4.5 ± 2.5 (1.1–10.4) | 3.5 ± 1.3 (1.3–5.3) |
| Leptin (ng/ml) | 5.0 ± 4.1 (0.3–26.1) | 11.1 ± 7.1 (1.4–28.6) |
| Adiponectin (μg/ml) | 10.3 ± 3.2 (3.9–18.0) | 15.1 ± 4.6 (3.3–24.7) |
| T3 (pg/ml) | 2.0 ± 0.6 (1.0–3.7) | 1.9 ± 0.8 (0.5–4.4) |
Data are means ± SD, with minimum and maximum values in parentheses.
FDG-PET/CT.
After fasting for 6–12 h, the subjects were kept in an air-conditioned room at 19°C with light clothing (usually a T-shirt with underwear) and put their legs on an ice block intermittently (usually for 4 min every 5 min). After 1 h under this cold condition, they were given an intravenous injection of 18F-fluoro-2-deoxyglucose (FDG) (259 MBq) and kept under the same cold condition. In some cases, the subjects were kept at 26–28°C with standard clothing and without leg icing (warm condition). One hour after the 18F-FDG injection, whole-body PET/CT scans were performed on a PET/CT system (Aquiduo; Toshiba Medical Systems, Otawara, Tochigi, Japan) in a room at 24°C. With the CT parameters of 120 kv and real-exposure control, unenhanced low-dose spiral axial 2-mm collimated images were obtained. This was used for PET attenuation correction as well as anatomic localization. Subsequently, full-ring PET was performed in six incremental table positions, each ∼15 cm in thickness. The total time of these scans was ∼30 min.
PET and CT images were coregistered and analyzed by a VOX-BASE workstation (J-MAC System, Sapporo, Japan). Two experienced blinded observers assessed the FDG uptake, particularly in both sides of the neck and paravertebral regions, by visually judging the radioactivity greater than background. In parallel, the FDG uptake in the neck region was quantified and expressed as relative to that in the whole brain.
Anthropometric and body fat measurement and blood analysis.
BMI was calculated as body weight in kilograms divided by the square of height in meters (kg/m2), and percent of body fat was estimated by the multifrequency bioelectric impedance method (InBody 320 Boy Composition Analyzer; Biospace, Seoul, Korea). The abdominal and subcutaneous fat areas at the level of L4–L5 were estimated from the CT images. Serum levels of leptin and adiponectin were measured using respective enzyme-linked immunosorbent assay (ELISA) kits (Human leptin ELISA kit, B-Bridge, Mountain View, CA; Human adiponectin ELISA kit, Otsuka Pharmaceutical, Tokyo, Japan). Other blood parameters were analyzed by a company of laboratory testing services (SRL, Tokyo, Japan).
Histological examinations.
An autopsy tissue specimen was obtained from fat depots of the supraclavicular region of a 21-year-old man and stained with hematoxylin-eosin or an anti-serum against rat UCP1 (16).
Data analysis.
Data are expressed as means ± SD and analyzed by paired or Student's t test. χ2 test was used for the data of the prevalence of BAT. SPSS softwear package (version 10.0; Chicago, IL) was used for correlation analysis between BAT and adiposity-related parameters.
RESULTS
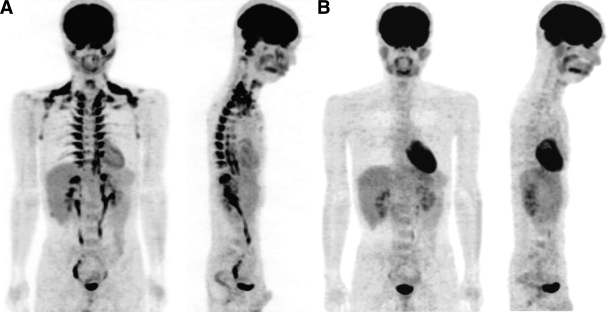
Since one of the typical methods to activate BAT in rodents is cold exposure, we first examined the acute effects of cold exposure on FDG uptake. For this, healthy subjects were overnight fasted and kept in a room at 28°C (warm condition) or at 19°C with light clothes and intermittent application of an ice-cooled footrest (cold condition). Two hours later, they underwent FDG-PET/CT examination. Under the warm condition, a clear FDG uptake was detected in the brain, heart, and the oropharengeal region, while no FDG signal in adipose tissue (Fig. 1B). When the same subjects were kept under the 2-h cold condition, a clear and intense FDG uptake was found in adipose tissue at the supraclavicular and paraspinal regions (Fig. 1A).
FIG. 1.
Whole-body FDG-PET images under cold or warm condition. A: A 25-year-old male subject fasted for 12 h and was kept in an air-conditioned room at 19°C with light clothing and put his legs on an ice block intermittently (for ∼4 min at every 5 min). After 1 h under this cold condition, he was given an intravenous injection of 18F-FDG and kept under the same cold condition. One hour after the 18F-FDG injection, whole-body PET/CT scans were performed in a room at 24°C. B: Two weeks after the first examination in the cold condition (A), the same subject underwent FDG-PET/CT examination as previously, but he was kept at 27°C with standard clothing and without leg icing (warm condition) for 2 h before the examination.
During a 7-month period from August of 2006 to the following March, we performed a total of 71 FDG-PET/CT examinations under the 2-h cold condition for 56 healthy volunteers aged 23–65 years. As summarized in Table 2, cold-activated FDG uptake in the supraclavicular region was detected in 18 of 55 subjects (33%) in winter from January to March. Although the incidence of such FDG uptake seemed not to show apparent sex difference, it changed with age (P < 0.01 by χ2 test), being 52% (16 of 31) and 8% (2 of 24) in younger (aged 23–35 years) and elderly (aged 38–65 years) subjects, respectively. Table 2 confirms again the stimulatory effect of acute cold exposure on FDG uptake as typically shown in Fig. 1; that is, eight subjects who showed clear FDG uptake under the cold condition were tested again under the warm condition at 28°C in 2 weeks, but none of them showed detectable FDG uptake in the adipose tissue, including supraclavicular and paraspinal regions, regardless of the test seasons.
TABLE 2.
Prevalence of cold-activated BAT in adult humans
| Summer | Autumn | Winter | ||
|---|---|---|---|---|
| Outdoor temperature (°C) | 21.3 (19.6–28.3) | 11.7 (7.6–16.1) | −1.6 | (−5.2 to 1.8) |
| n | 8 | 8 | 31 | 24 |
| Age (years) | 23–31 | 23–32 | 23–35 | 38–65 |
| Prevalence | ||||
| Total | 2/8 (0/1) | 3/8 (0/3) | 16/31 (0/4) | 2/24 |
| Male subjects | 1/5 | 2/6 (0/2) | 8/19 (0/3) | 2/12 |
| Female subjects | 1/3 (0/1) | 1/2 (0/1) | 8/12 (0/1) | 0/12 |
Data are mean outdoor temperature (range) or n apparent FDG uptake into BAT (adipose tissue in the supraclavicular region)/total n. During a 7-month period from August of 2006 to the following March, a total of 71 FDG-PET/CT examinations were conducted under the 2-h cold condition for 56 healthy volunteer subjects as in Fig. 1A. Some (the number in parentheses) underwent the examination again under the warm condition as in Fig. 1B 2 weeks after that under the cold condition. Summer: 29 August to 28 September 2006. Autumn: 5 October to 19 October 2006. Winter: 29 January to 6 March 2007.
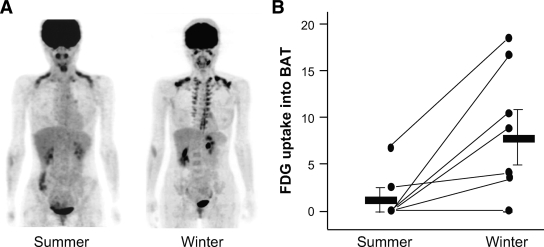
Table 2 also suggests some seasonal variations of the total prevalence, being low in summer from August to September. This was clearly demonstrated from the results of some individual subjects; that is, in summer, the FDG uptake was detected in two of eight subjects. When the same subjects were examined again in winter, it was found in six subjects, four of which showed no FDG uptake in summer and the other two showed lower uptake in summer (Fig. 2B). Moreover, FDG uptake was sometimes detected both at the supraclavicular and paraspinal regions in winter but only in the supraclalvicular region in summer (Fig. 2A). Thus, the incidence and intensity of cold-activated FDG uptake showed seasonal variations, being higher in winter.
FIG. 2.
FDG uptake into BAT in summer and winter. A: A 30-year-old female subject underwent FDG-PET/CT examination under the cold condition as in Fig. 1 on 29 August 2006 (summer) and again on 22 February 2007 (winter). B: Eight subjects (five male and three female subjects) underwent FDG-PET/CT examination under the cold condition in summer (29 August to 28 September 2006) and again in winter (29 January to 6 March 2007). FDG uptake into the supraclavicular region was densitometrically quantified, normalized, and expressed as relative to that in the brain. Thick bars are means ± SD.
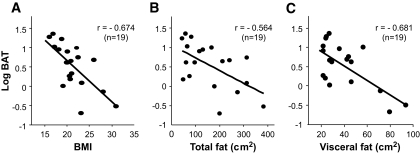
In small rodents, BAT thermogenesis is recognized as a significant component of whole-body energy expenditure and thereby contributes to the regulation of body fat content (1,2). To examine whether this is also the case in humans, we first analyzed the relationship between the cold-activated FDG uptake into BAT and adiposity in a total of 19 subjects bearing detectable BAT. As shown in Fig. 3, FDG uptake into the supraclavicular adipose tissue showed significant inverse relations to BMI (r = −0.674, P < 0.001), total fat (r = −0.564, P < 0.01), and visceral fat (r = −0.681, P < 0.001). Weaker but significant inverse relationships were also found to subcutaneous fat (r = −0.492, P < 0.05) and plasma insulin level (r = −0.473, P < 0.05) but not to plasma levels of leptin, adiponectin, and T3 (data not shown).
FIG. 3.
Cold-activated BAT and adiposity. Cold-activated FDG uptake into the supraclavicular region of 19 subjects was quantified as in Fig. 2 and plotted against BMI and total and visceral fat areas estimated from CT images at the umbilical (L4 and L5) level.
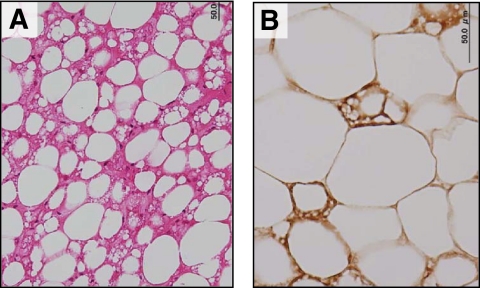
We also compared the similar parameters in subjects bearing detectable and undetectable amounts of cold-activated BAT (Table 3). As expected from the results in Table 2, the mean age was significantly lower in the group bearing detectable BAT than that without BAT. Body weight, BMI, and visceral and subcutaneous fat areas estimated from CT tended to be lower in the group bearing detectable BAT, but the difference was not statistically significant. Since these parameters of adiposity are much influenced by aging, being higher in elderly subjects, next, we compared the two groups of 32 younger subjects (aged 23–35 years) (Table 2). The mean age was comparable in the two groups (aged 27.3 vs. 29.1 years in male subjects, aged 30.3 vs. 29.5 years in female subjects); the effects of aging may be canceled. There was no difference in height, body weight, and blood parameters examined between the two groups in both male and female subjects. The group bearing detectable BAT tended to show decreased BMI, body fat content, and visceral fat area compared with that without BAT, but again the difference was not statistically significant (data not shown). In a separate study, we examined histologically an autopsy sample of fat depots obtained from the supraclavicular region and found numerous multilocular adipocytes expressing UCP1 protein (Fig. 4).
TABLE 3.
Comparison between subjects bearing detectable (+) and undetectable (−) amounts of BAT
| BAT | Male subjects |
Female subjects |
||
|---|---|---|---|---|
| − | + | − | + | |
| n | 21 | 10 | 16 | 9 |
| Age (years) | 38.4 ± 10.0 | 31.3 ± 6.7* | 43.6 ± 8.6 | 30.3 ± 1.7* |
| Height (cm) | 170 ± 6 | 170 ± 4 | 158 ± 5 | 159 ± 3 |
| Body weight (kg) | 71.3 ± 6.8 | 65.3 ± 6.6 | 54.5 ± 4.6 | 49.0 ± 5.7 |
| BMI (kg/m2) | 24.4 ± 2.3 | 22.7 ± 2.8 | 22.0 ± 2.2 | 19.4 ± 2.1 |
| Body fat (%) | 24.5 ± 5.5 | 20.6 ± 5.0 | 29.4 ± 5.1 | 24.5 ± 5.4 |
| Body fat (kg) | 17.7 ± 4.8 | 13.8 ± 4.6 | 16.2 ± 3.6 | 12.5 ± 4.0 |
| Fat area (cm2) | ||||
| Total | 236 ± 82 | 152 ± 87 | 222 ± 85 | 159 ± 76 |
| Visceral | 71 ± 27 | 47 ± 19 | 51 ± 24 | 30 ± 7 |
| Subcutaneous | 165 ± 64 | 104 ± 68 | 171 ± 67 | 129 ± 71 |
Data are means ± SD.
*P < 0.05 vs. BAT (−) by t test.
FIG. 4.
Histological identification of UCP1-positive brown adipocytes in fat depots obtained from the supraclavicular region. Tissue sections were stained with hematoxylin and eosin (A) or anti-serum against rat UCP1 (B). (A high-quality representation of this figure is available in the online issue.)
DISCUSSION
In this study, we performed FDG-PET/CT examination in adult healthy volunteers, particularly focusing on the effects of cold exposure and adiposity-related parameters on FDG uptake into adipose tissue in some specific regions, where UCP1-positive brown adipocytes were detected. The major findings were 1) FDG uptake in adipose tissue in the supraclavicular and paraspinal regions was negligible in warm conditions but markedly increased after a 2-h cold exposure, 2) cold-activated FDG uptake was increased in winter compared with summer, 3) it was detected in about half of younger subjects but in much fewer elderly subjects, and 4) it decreased with increasing adiposity assessed by BMI and body fat content.
It has been reported in rodents that cold exposure markedly increased 2-deoxy-d-glucose (2-DG) uptake into BAT but only slightly into WAT (17–20). The stimulatory effects of cold exposure were mimicked by electrical stimulation of sympathetic nerves into BAT and β-adrenergic agonist administration but were abolished by surgical severing of the sympathetic nerves or β-adrenergic blockade.
We (21) demonstrated that the β-adrenergically stimulated 2-DG uptake into BAT is totally dependent on the activation of UCP1 and is thereby a metabolic marker of BAT. Our present findings of increased FDG uptake into adipose tissues of the specific regions after 2-h cold exposure are quite similar to those in rodents and thus support the idea that it reflects the activation of BAT and that adult humans have, more or less, metabolically active BAT. These seem consistent with previous case reports about patients with malignant lymphoma and some other types of tumors, where the incidence of FDG uptake in the supraclavicular region is higher when the examination was performed at lower room temperatures (10–12).
In our study, cold-activated FDG uptake was markedly increased when examined in winter compared with summer. Cohade et al. (10) reported that the incidence of FDG uptake into the supraclavicular region showed seasonal variations, being higher from January to March. It is known in mice and rats that chronic cold exposure (cold acclimation) results in hyperplasia of BAT and increased 2-DG uptake (1,18–20). Moreover, cold acclimation has been shown in rodents to induce an apparent transdifferentiation of WAT to BAT (22–24). Considering that the average outdoor temperature in our study was −1.6°C in winter and 21.3°C in summer, it is quite likely that the increased FDG uptake in winter is attributable to hyperplasia of BAT and/or transdifferentiation of WAT to BAT. Collectively, it can be concluded that the cold-activated FDG uptake in the supraclavicular and paraspinal regions is an index of the amount and activity of BAT.
It is to be noted that about half of younger (aged 23–35 years) subjects have metabolically active BAT, being quite against the widely accepted view that the amount and activity of BAT are negligible in adult humans. Although the prevalence of BAT was much decreased in elderly subjects (aged 38–65 years), the prevalence in our study is much higher than those previously reported in clinical studies (10), where FDG uptake in the shoulder region is detected in some patients (<10%) at relatively low room temperatures (∼22°C) but not found at higher temperatures (>25°C). The reason for the high prevalence in our study must be that our subjects were kept at a lower temperature (19°C) and stimulated by intermittent ice cooling of legs. In other words, our condition of cold exposure may be more appropriate to detect BAT. More interesting in our results is the relationship between BAT and adiposity of the subjects; that is, cold-activated FDG uptake into BAT showed significant inverse relations to BMI, body fat, and visceral fat. Weaker but significant inverse relations were also found to subcutaneous fat and plasma insulin level.
This seems quite compatible with the observation in rodents that the amount and activity of BAT is decreased in obesity. There is ample evidence that BAT thermogenesis is a significant component of whole-body energy expenditure and thereby contributes to the regulation of energy balance and body fat content (1). Our present results thus imply a significant role of BAT in the control of adiposity via the regulation of energy expenditure in humans in the same way as in rodents. Being consistent with this idea, the subjects bearing detectable BAT tended to be low in BMI and body fat content compared with those without BAT, although the difference did not reach statistically significant levels.
Another point to be noted is that the incidence of cold-activated BAT decreased in elderly subjects. In a total of 24 elderly subjects, BAT was detected in only 2 male subjects (Table 2). It is interesting that these 2 male subjects are rather lean, with BMI 22.2 and 20.6 kg/m2, respectively, compared with the other 10 male subjects bearing no BAT with BMI of 24.4 kg/m2. Although these results are preliminary and are yet to be confirmed in larger numbers of subjects, they may imply that the disappearance of BAT accelerates the development of obesity with aging. This idea seems consistent with our previous observation that UCP1 deficiency in mice increases susceptibility to diet-induced obesity with age (25).
The present results thus collectively suggest that cold-activated BAT participates, more or less, in the control of energy expenditure and adiposity in humans, as it does in small rodents. This may require reevaluation of the physiological relevance of BAT in humans. For example, it is not rare that experimentally and/or clinically estimated basal metabolic rate, as well as postprandial thermogenesis (diet-induced thermogenesis), varies considerably among individuals even after correcting possible contributing factors such as sex, age, and body dimensions. It may be possible that such variations are due to the individual differences in BAT activity. Moreover, our results may highlight again a pharmacological approach to obesity treatment, which has repeatedly been confirmed in experimental animals (3,4,26,27) but not yet approved in humans; that is, agonists specific to the β3-adrenergic receptor effectively reduce adiposity in small rodents and dogs but to a much lesser extent in humans. Based on our results, it may be likely that β3-adrenergic agonists are effective only for individuals who keep active BAT, as in wild-type but not UCP1-deficient mice (4). Our present findings suggest revisiting such intriguing subjects.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15081201). A part of this study was reported in 15th European Congress on Obesity, 22–25 April 2007, Budapest, Hungary, with an abstract that appeared in the International Journal of Obesity 31:S32, 2007.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1482.
REFERENCES
- 1.Cannon B, Nedergaard J: Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277– 359 [DOI] [PubMed] [Google Scholar]
- 2.Lowell BB, Spiegelman BM: Towards a molecular understanding of adaptive thermogenesis. Nature 2000; 404: 652– 660 [DOI] [PubMed] [Google Scholar]
- 3.Lowel BB, Backman ES: β-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem 2003; 278: 29385– 29388 [DOI] [PubMed] [Google Scholar]
- 4.Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, Saito M: Indispensable role of mitochondrial UCP1 for antiobesity effect of β3-adrenergic stimulation. Am J Physiol Endocrinol Metab 2006; 290: E1014– E1021 [DOI] [PubMed] [Google Scholar]
- 5.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J: UCP1 ablation induces obesity and abolishes diet-induced thermogenesis inmice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009; 9: 203– 209 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Barroso MDM, Ricquier D, Cassard-Doulcier AM: The human uncoupling protein-1 gene (UCP): present status and perspectives in obesity research. Obesity Rev 2000; 1: 61– 72 [DOI] [PubMed] [Google Scholar]
- 7.Himms-Hagen J: Does brown adipose tissue (BAT) have a role in the physiology or treatment of human obesity? Rev Endocr Metab Dsiord 2001; 2: 395– 401 [DOI] [PubMed] [Google Scholar]
- 8.Cinti S: The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis 2006; 16: 569– 574 [DOI] [PubMed] [Google Scholar]
- 9.Cohade C, Osman M, Pannu HK, Wahl RL: Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 2003; 44: 170– 176 [PubMed] [Google Scholar]
- 10.Cohade C, Mourtzikos KA, Wahl RL: “USA-Fat:” prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 2003; 44: 1267– 1270 [PubMed] [Google Scholar]
- 11.Christensen CR, Clark PB, Morton KA: Reversal of hypermetabolic brown adipose tissue in F-18 FDG PET imaging. Clin Nucl Med 2006; 31: 193– 196 [DOI] [PubMed] [Google Scholar]
- 12.Garcia CA, Nostrand DV, Atkins F, Acio E, Butler C, Isposito G, Kulkarni K, Majd M: Reduction of brown fat 2-deoxy-2[F-18]fluoro-d-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol 2006; 8: 24– 29 [DOI] [PubMed] [Google Scholar]
- 13.Parysow O, Mollerach AM, Jager V, Racioppi S, Roman JS, Gerbaudo VH: Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 2007; 32: 351– 357 [DOI] [PubMed] [Google Scholar]
- 14.Soderlund V, Larsson SA, Jacobsson H: Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 2007; 34: 1018– 1022 [DOI] [PubMed] [Google Scholar]
- 15.Nedergaard J, Bengtsson T, Cannon B: Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444– E452 [DOI] [PubMed] [Google Scholar]
- 16.Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, Kawada T, Saito M: Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic b3-adrenergic agonist. J Clin Invest 1996; 97: 2898– 2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrete A, Bukowiecke LJ: Stimulation of glucose transport by insulin and norepinephrine in isolated rat borwn adipocytes: Am J Physiol 1989; 257: C714– C721 [DOI] [PubMed] [Google Scholar]
- 18.Vallerand AL, Pesusse F, Bukowiecki LJ: Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol 1990; 259: R1043– R1049 [DOI] [PubMed] [Google Scholar]
- 19.Shimizu Y, Nikami H, Saito M: Sympathetic activation of glucose utilization in brown adipose tissue in rats. J Biochem 1991; 110: 688– 692 [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y, Nikami H, Tsukazaki K, Machado UF, Yano H, Seino Y, Saito M: Increased expression of glucose transporter GLUT-4 in brown adipose tissue of fasted rats after cold exposure. Am J Physiol Endocrinol Metab 1993; 264: E890– E895 [DOI] [PubMed] [Google Scholar]
- 21.Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M: Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 2005; 54: 1385– 1391 [DOI] [PubMed] [Google Scholar]
- 22.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L: Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103: 931– 942 [DOI] [PubMed] [Google Scholar]
- 23.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP: Emergence of brown adipocytes in white fat in mice is under genetic control: effects on body weight and adiposity. J Clin Invest 1998; 102: 412– 420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cinti S: The adipose organ. Prostaglandins Leukot Essent Fatty Acids 2005; 73: 9– 15 [DOI] [PubMed] [Google Scholar]
- 25.Kontani Y, Wang K, Kimura K, Inokuma K, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H: UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 2005; 4: 147– 155 [DOI] [PubMed] [Google Scholar]
- 26.Arch JRS, Wilson S: Prospects for β3-adrenoceptor agonists in the treatment of obesity and diabetes. Int J Obes 1996; 20: 191– 199 [PubMed] [Google Scholar]
- 27.Omachi A, Matsushita Y, Kimura K, Saito M: Role of uncoupling protein 1 in the anti-obesity effect of β3-adrenergic agonist in the dog. Res Vet Sci 2008; 85: 214– 219 [DOI] [PubMed] [Google Scholar]