Abstract
OBJECTIVE
Anti-tissue transglutaminase (TG2) antibodies are the serological marker of celiac disease. Given the close association between celiac disease and type 1 diabetes, we investigated the production and deposition of anti-TG2 antibodies in the jejunal mucosa of type 1 diabetic children.
RESEARCH DESIGN AND METHODS
Intestinal biopsies were performed in 33 type 1 diabetic patients with a normal mucosal architecture: 14 had high levels (potential celiac disease patients) and 19 had normal levels of serum anti-TG2 antibodies. All biopsy specimens were investigated for intestinal deposits of IgA anti-TG2 antibodies by double immunofluorescence. In addition, an antibody analysis using the phage display technique was performed on the intestinal biopsy specimens from seven type 1 diabetic patients, of whom four had elevated and three had normal levels of serum anti-TG2 antibodies.
RESULTS
Immunofluorescence studies showed that 11 of 14 type 1 diabetic children with elevated levels and 11 of 19 with normal serum levels of anti-TG2 antibodies presented with mucosal deposits of such autoantibodies. The phage display analysis technique confirmed the intestinal production of the anti-TG2 antibodies; however, whereas the serum-positive type 1 diabetic patients showed a preferential use of the VH5 antibody gene family, in the serum-negative patients the anti-TG2 antibodies belonged to the VH1 and VH3 families, with a preferential use of the latter.
CONCLUSIONS
Our findings demonstrate that there is intestinal production and deposition of anti-TG2 antibodies in the jejunal mucosa of the majority of type 1 diabetic patients. However, only those with elevated serum levels of anti-TG2 antibodies showed the VH usage that is typical of the anti-TG2 antibodies that are produced in patients with celiac disease.
Insulin-dependent diabetes (type 1 diabetes) is characterized by an autoimmune destruction of the pancreatic islet β-cells that results in a loss of insulin secretion. T-cells that are reactive against specific β-cell antigens infiltrate the endocrine pancreas and destroy the β-cells (1). Both genetic susceptibility and environmental factors contribute to the pathogenesis of type 1 diabetes.
Mounting evidence suggests that the gut immune system is involved in the development of autoimmune diabetes. An inflammatory state has been demonstrated to be present in the structurally normal intestine of patients with type 1 diabetes (2,3), and the abnormal intestinal permeability that has been found in these patients could represent a contributing factor (4). Higher intestinal levels of proinflammatory cytokines, such as interleukin-1α and also interleukin-4, have been reported (3). Recently, we used immunohistochemistry to demonstrate signs of activated cell-mediated mucosal immunity in the lamina propria of the small intestine of type 1 diabetic patients (5); furthermore, the epithelial compartment shows signs of increased infiltration by CD3+ and γδ+ cells (5).
Type 1 diabetes has been found to be associated with other autoimmune diseases, including celiac disease (6–8). Celiac disease is an immune-mediated disease that is triggered by the ingestion of gliadin and other toxic prolamines. It is characterized by a dysregulated immune response at the gut level (9) that results in enteropathy. Several autoantibodies, of which anti-tissue transglutaminase (TG2) autoantibodies are the most frequently observed, are present in the serum of patients with untreated celiac disease. Several studies that have used phage display libraries suggest that these autoantibodies are primarily produced in the small bowel mucosa and that there is a preferential use of heavy-chain variable regions belonging to the VH5 gene family in patients with celiac disease (10). At the mucosal level, anti-TG2 antibodies are found to be deposited on extracellular TG2 (11).
It is possible that type 1 diabetes and celiac disease are more than simply associated; gluten may also have a causative role in type 1 diabetes. This hypothesis has been suggested by the observation of an altered intestinal immune response to gluten in type 1 diabetes. In type 1 diabetic patients, we reported that there is local mucosal recruitment of lymphocytes after rectal instillation of gliadin (12); we also observed an enhanced immune response to gliadin after in vitro gluten challenge in biopsy specimens from type 1 diabetic patients negative for serum anti-human TG2 antibodies (5). These subjects with signs of a deranged immune response to gliadin may be considered potential celiac disease patients (13); in fact, some of the type 1 diabetic patients who are negative for celiac disease–associated autoantibodies may later become seropositive and may eventually develop frank enteropathy (14).
It has recently been shown that specific celiac disease autoantibodies against TG2 are deposited in the normal jejunal mucosa before they can be detected in the circulation and that their deposition precedes the gluten-induced jejunal lesion (15). This finding raises the possibility that the anti-TG2 antibodies might be located only at the small mucosal level in some type 1 diabetic patients.
In this study, we investigated the production and deposition of anti-TG2 autoantibodies in the small intestinal mucosa of type 1 diabetic children, irrespective of the presence of this autoantibody in their serum, with the aim of elucidating both the full spectrum of intestinal immunological derangement in type 1 diabetes and the possible relation with dietary gluten.
RESEARCH DESIGN AND METHODS
We studied 33 patients with type 1 diabetes who were consuming a gluten-containing diet at the time of biopsy (median age 11 years, range 3–22 years). All diabetic patients presented with normal jejunal architecture (stage T0/T1 according to the Marsh classification modified by Oberhuber et al. [(16)]) and were divided into two groups. Group A consisted of 14 patients with raised serum levels of anti-TG2 antibodies (TG2+), and group B consisted of 19 patients with normal serum levels of specific celiac disease autoantibodies (TG2−).
Twelve patients with untreated celiac disease, who had the diagnosis of celiac disease on the basis of biopsy findings, high serum levels of anti-TG2 antibodies, and a positive response to a gluten-free diet, were included. Twenty-eight subjects without celiac disease (final diagnoses: iron deficiency anemia, failure to thrive, gastroesophageal reflux, and recurrent abdominal pain) were enrolled as the control group. Finally, 18 patients with inflammatory bowel diseases (IBDs) and 9 with food allergies were enrolled to form an additional control group that represented subjects with other conditions characterized by gut inflammation.
The patients or the parents, where appropriate, gave their consent for the biopsy sampling for the study. The University Ethics Committee approved the use of the biopsy specimens in the study.
HLA typing.
DNA extracted from blood samples was used to genotype for HLA-II DQ2 and DQ8 haplotypes in the type 1 diabetic patients. In control subjects, DNA was extracted from the biopsy specimens using PrepMan Ultra Sample Preparation Reagent (Applied Biosystems). The Eu-DQ kit (Eurospital) was used for typing.
Jejunal biopsy and immunohistochemical analysis.
Jejunal biopsy specimens were obtained with a gastroscope from all of the patients. One fragment was treated for histological analysis as described previously (17); a second fragment was immediately embedded in an optimal cutting temperature compound (BioOptica) and used in the detection of mucosal anti-TG2 antibody deposits. From seven of the diabetic patients, we obtained a third fragment used for total RNA purification. Immunohistochemistry, staining, and morphometric analyses were performed as described previously (17).
Serum IgA anti-TG2 antibodies.
Serum levels of IgA anti-TG2 antibodies were determined by an enzyme-linked immunosorbent assay (ELISA) using a kit based on recombinant human TG2 (Eu-tTg IgA kit; Eurospital) as described previously (17). Values were considered positive if they were ≥7 arbitrary units/ml.
Intestinal anti-TG2 antibody IgA deposits by double immunofluorescence and confocal analysis.
All the of patients were investigated for mucosal deposition of anti-TG2 antibody IgA. The technique described by Korponay-Szabo et al. (11) was implemented with minor modifications. Acetone-fixed, 5-μm frozen sections from each patient were examined by double immunofluorescence. After a 15-min preincubation with normal rabbit serum (1:100; Dako), the sections were covered with a monoclonal mouse antibody against guinea pig TG2 (CUB 7402, 1:200; NeoMarkers) for 1 h at room temperature in a humidified chamber. The sections were washed with PBS and then incubated with a mixture of a fluorescein isothiocyanate–labeled rabbit antibody against human IgA (1:100; Dako) to detect (in green) IgA and an R-phycoerythrin–labeled rabbit anti-mouse antibody (1:40; Dako) to detect (in red) TG2 for 30 min in the dark. Finally, the sections were washed in PBS and mounted with glycerol/PBS (1:10). The preparations were analyzed with an Axioscope2 (Zeiss) microscope linked to an analysis image system (Siemens). The colocalization of IgA mucosal deposits and TG2 resulted in a yellow image at the fluorescence microscope. The colocalization was confirmed by confocal microscopy (LSM510; Zeiss).
Phage display antibody libraries.
Jejunal specimens from seven of the type 1 diabetic patients (three with normal levels of serum anti-TG2 autoantibodies) were used for the purification of tissue total RNA using TRIzol (Gibco Life Technologies). cDNA was synthesized using random hexamers and SuperScript III reverse transcriptase (Gibco Life Technologies). Two types of intestinal B-lymphocyte phage display antibody libraries were constructed: an IgA-VH5 gene family library and an IgA-whole gene family library. Ig VH5 regions were amplified using a specific V-region primer designed against the first 36 nucleotide bases of the VH5 family (18). Conversely, in the IgA whole gene family library, VH and VL regions were amplified using a 3′- and 5′-primer set as described previously (19). In the first library, the PCR fragments were gel purified and digested with XhoI and NheI (New England Biolabs) for cloning into pDAN5 that had been modified with a VL region. In the latter library, the PCR fragments were assembled in a single-chain fragment variable (scFv) and digested with BsshII and NheI before cloning into the same vector. The phage propagation was carried out in the strain Escherichia coli DH5αF′. Rescue of phagemid particles was carried out as described previously (20). Panning was performed by adding phages diluted in 2% nonfat milk/PBS to immunotubes (Nunc) coated with purified recombinant human TG2 and α-gliadin (10 μg/ml); the next steps were performed as reported previously (18). After two rounds of panning, up to 93 individual clones were screened for reactivity to the antigens. Phages from individual colonies were grown in 96-well plates (20). ELISAs were performed in microtiter plates coated with antigens at 10 μg/ml (18). Purified α-gliadin was prepared as described previously (21). Recombinant human TG2 was obtained by amplifying as described previously (22). The V genes from the different anti-TG2 scFv clones were sequenced (BigDye Terminator v3.1 Cycle Sequencing kit; Applied Biosystems), and the VH gene families used were assessed by screening against the V BASE (http://vbase.mrc-cpe.cam.ac.uk) database (23). Immunofluorescence analysis with the different gene family anti-TG2 scFvs was performed on histological sections of monkey esophagus (MeDiCa, Encinitas, CA).
Statistical analysis.
A χ2 test was used to compare percentage values. P < 0.05 was considered significant.
RESULTS
Immunohistochemical analysis of type 1 diabetic jejunal biopsy specimens.
All of the type 1 diabetic patients were observed to be HLA-DQ2 and/or -DQ8 positive. Signs of activated cell-mediated mucosal immunity were present in approximately one-third of the type 1 diabetic subjects. In group A (TG2+), 4 of 14 patients (29%) showed an increased density of CD25+ mononuclear cells (>4 CD25+cells/mm2) in the lamina propria. Crypt epithelium HLA-DR and intercellular adhesion molecule (ICAM)-1 expressions were enhanced in 10 of 14 (71%) and 4 of 14 (29%) patients, respectively. In the epithelial compartment, 6 of 14 patients (43%) presented with a density of CD3+ intraepithelial lymphocytes (IELs) that was higher than the cutoff (>34 cells/mm epithelium), and 9 of 14 patients (64%) presented with a density of γδ+ IELs that was higher than the cutoff (>3.4 cells/mm epithelium). In addition, 5 of 14 patients (36%) presented with three or more of these above-mentioned altered values.
In group B (TG2−), 7 of 19 patients (37%) showed an increased density of CD25+ mononuclear cells and 11 of 19 (58%) and 6 of 19 (31%) presented with increased HLA-DR and ICAM-1 expression, respectively. With respect to the epithelial compartment, 4 of 19 (21%) and 5 of 19 patients (26%) presented with an increased density of CD3+ and γδ+ IELs, respectively (Table 1). Again, 5 of 19 patients (26%) presented with three or more of the above-mentioned altered values. Only the percentage of subjects with an increased number of γδ+ IELs was significantly higher in group A than in group B (P < 0.05).
TABLE 1.
Immunohistochemical findings in the small intestine of type 1 diabetic patients enrolled in this study
| Group A | Group B | P | |
|---|---|---|---|
| CD3+ >34/mm | 6/14 (43) | 4/19 (21) | NS |
| γδ+ >3.4/mm | 9/14 (64) | 5/19 (26) | <0.05 |
| CD25+ >4/mm2 | 4/14 (29) | 7/19 (37) | NS |
| HLA-DR (++/+++) | 10/14 (71) | 11/19 (58) | NS |
| ICAM-1 (++/+++) | 4/14 (29) | 6/19 (31) | NS |
Group A consisted of type 1 diabetic patients with elevated serum levels of anti-TG2 antibodies (TG2+). Group B consisted of type 1 diabetic patients with normal serum levels of anti-TG2 antibodies (TG2−). CD3+ and γδ+ IELs are expressed per millimeter of epithelium; CD25+ cells are expressed per square millimeter of lamina propria. The expression of crypt epithelial HLA-DR and the expression of lamina propria ICAM-1 were evaluated in terms of their staining intensity and graded on an arbitrary scale of staining level, where − = no staining, + = weak staining, ++ = strong staining, and +++ = very strong staining. The data were analyzed by a χ2 test. P < 0.05 was considered significant.
Immunofluorescence studies.
Jejunal mucosa from most of the control subjects without celiac disease showed IgA antibodies, only inside the plasma cells and the epithelial cells, which were labeled in green (Fig. 1A), and only 4 of 28 subjects had patchy IgA mucosal deposits. With regard to HLA status, 9 of the 28 (32%) control subjects were HLA-DQ2 and/or -DQ8 positive, but only one of the four individuals who were positive for mucosal deposits was HLA-DQ2 positive. In the group of patients with other inflammatory conditions of the gut, only three of the subjects with IBD (16.6%) showed patchy deposits.
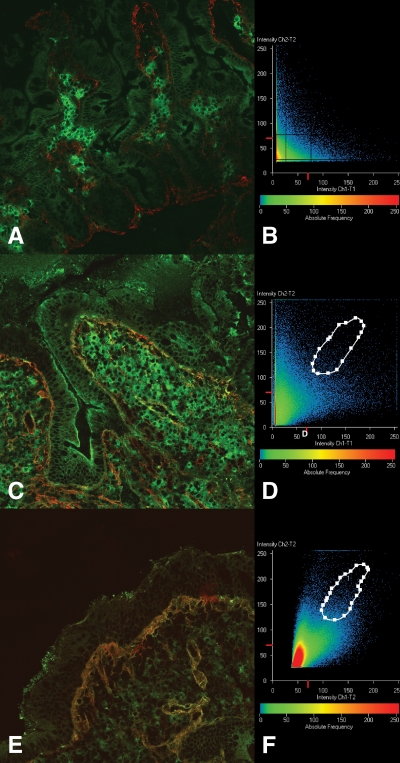
FIG. 1.
A and B: Jejunal section from a subject without celiac disease. It is a negative sample. In A, IgA deposits (in green) are detected only inside plasma cells, whereas tissue transglutaminase (in red) is evident around the crypts and in the subepithelial area. B: Confocal analysis with a scatter plot of the image. In this plot, no area of colocalization is evident around the diagonal of the Cartesian graphic. C and D: Jejunal section from a type 1 diabetic patient with a high serum level of anti-TG2 autoantibodies. In C, IgA deposits (in yellow-orange) are present in a patchy distribution in the subepithelial area and around mucosal vessels. These IgA/anti-TG2 antibody colocalization areas have been analyzed by confocal microscopy. The related scatter plot is shown in D, and the image of this area of colocalization is represented by orange dots. E and F: Jejunal section from a patient with untreated celiac disease with positive serum anti-TG2 antibodies and EMAs, in which thick IgA anti-TG2 antibody deposits are evident just under the superficial epithelium and around the vessels (E). This subepithelial area has been studied by confocal microscopy, and the related scatter plot (F) shows an extensive area of IgA deposits/TG2 colocalization. (A high-quality digital representation of this figure is available in the online issue.)
All 12 patients with untreated celiac disease showed evident IgA deposits below the villous and crypt basement membranes and around the mucosal vessels, corresponding to the intestinal localization of TG2 (in red), apart from the IgA inside the plasma cells. IgA-specific anti-TG2 antibody deposits appeared in yellow-orange because of colocalization with TG2 (Fig. 1E).
Not unexpectedly, in group A (TG2+), 11 of the patients (78%) were observed to have IgA anti-TG2 antibody mucosal deposits that had a patchy distribution. Surprisingly, we found that a high percentage of patients in group B (TG2−) exhibited an IgA deposit–positive pattern; in fact, 11 of the 19 (58%) group B subjects showed IgA anti-TG2 antibody mucosal deposits (Fig. 1C). Four of these 11 patients (36%) presented with a density of γδ+ IELs that was higher than the cutoff.
Most of the specimens with a patchy IgA deposit pattern, all biopsy specimens from patients with untreated celiac disease, and several normal biopsy specimens were analyzed by confocal microscopy to further assess the colocalization of IgA deposits with TG2 (Fig. 1,B, D, and F). In all of the specimens, confocal analysis confirmed the observations described previously. All data are summarized in Table 2.
TABLE 2.
Prevalence of IgA anti-TG2 mucosal deposits in the study population
| Patients | Presence of anti-TG2 mucosal deposits | % Positivity |
|---|---|---|
| Type 1 diabetic patients | ||
| TG2+ | 11/14 | 78 |
| TG− | 11/19 | 58 |
| Patients with untreated celiac disease | 12/12 | 100 |
| Control subjects | 4/28 | 14 |
| Patients with IBD | 3/18 | 16.6 |
| Patients with food allergies | 0/9 | 0 |
TG2+ type 1 diabetic patients had increased serum levels of anti-TG2 antibodies; TG2− type 1 diabetic patients had normal serum levels of anti-TG2 antibodies.
Phage display antibody libraries.
To further confirm the presence of anti-TG2 antibodies in the IgA deposits that colocalized with TG2 in the jejunal specimens of type 1 diabetic patients, we performed an analysis based on antibody phage display. In a previous article, we demonstrated the prevalent use of the VH5 segment to make antibodies against TG2 by the intestinal lymphocytes of patients with celiac disease (10). Also, the reactivity to TG2 was demonstrated to be restricted to the VH chain alone because shuffling of the VL chain with unrelated genes did not affect the antibody reactivity. This feature was shared by the antibodies to TG2 from all of the patients with celiac disease examined (18). For this reason, we are inclined to think that the antibodies to TG2 belonging to the VH5 family are a distinctive marker of celiac disease. Thus, in the present study, a first analysis was performed on intestinal biopsy cDNAs by cloning the VH5 family alone.
Seven IgA-VH5 gene family phage display libraries from type 1 diabetic patients (three with normal levels of anti-TG2 serum autoantibodies and four with elevated levels, here reported as TG2− and TG2+ type 1 diabetic patients, respectively) were constructed using a phagemid vector with resident VL genes compatible with TG2 recognition (18). In all cases, the library diversity resulted in at least 106 clones expressing human antibody fragments. After two rounds of selection at a high level of stringency, 45 individual clones were analyzed for their reactivity to recombinant human TG2 by a phage ELISA. In the libraries obtained from the intestinal biopsy lymphocytes of TG2+ patients, the number of positive clones ranged from 28 to 84% (Table 3). Conversely, using the three libraries constructed from the lymphocytes of TG2− patients, the number of clones recognizing TG2 was rather low, comprising between 0 and 6% of the tested clones. Because the same antibody might be represented many times within the selected clones, the diversity of the positive clones was assessed by fingerprinting the PCR-amplified VH5 region. The diversity was confirmed for the large majority of the clones, attesting to the polyclonal IgA response to TG2.
TABLE 3.
VH5 gene family–restricted IgA phage display libraries
| VH5 gene family IgA phage display libraries |
||||||||
|---|---|---|---|---|---|---|---|---|
| Type 1 diabetic TG2+ |
Type 1 diabetic TG2− |
|||||||
| Library code | N1 | N2 | N3 | N4 | N5 | N6 | N7 | |
| Library size | 3.1 × 106 | 3.2 × 106 | 1.6 × 106 | 4.1 × 106 | 6.3 × 106 | 4.6 × 106 | 2.6 × 106 | |
| Anti-TG2 antibodies | 84 | 28 | 73 | 42 | 2 | 0 | 6 | |
Data are the percentage of positive clones. VH5 gene family–restricted IgA phage display libraries from type 1 diabetes patient lymphocytes were selected for human TG2. The library size represents the number of bacterial clones expressing an antibody fragment. After two rounds of panning, 45 clones for each library were individually tested by ELISA for TG2. Only serologically TG2+ type 1 diabetic patients have a significant number of recombinant α-human TG2 antibodies using the VH5 gene family.
Considering the high percentage of TG2− patients who were observed to have intestinal deposits of anti-TG2 antibodies, we decided to determine whether an anti-TG2 antibody response could be ascribed to non-VH5 antibodies, which were not present in the first version of the antibody libraries. The whole antibody repertoire of the seven type 1 diabetic patients and three control subjects without celiac disease was cloned, assembling all of the VH and VL gene family sequences. The results of the library selections for TG2, reported in Table 4, show a significant number of clones that are positive for recombinant human TG2 isolated from the four libraries of type 1 diabetic patients with anti-TG2 serum antibodies (values ranging from 46 to 88%). Unexpectedly, a comparable result was obtained with the three libraries from the type 1 diabetic patients who did not have peripheral antibodies to TG2, with a number of bacterial clones ranging from 71 to 86%. The antibody diversity, measured by fingerprinting, was high in all of the clusters, attesting to the humoral response to TG2 involving different antibodies. Very few positive clones were found in the control subjects without celiac disease, with 0, 10, and 3 anti-TG2 antibody-positive clones of 90 tested.
TABLE 4.
Whole VH gene family IgA phage display libraries
| Whole VH gene family IgA phage display libraries |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetic TG2+ |
Type 1 diabetic TG2− |
Control subjects without celiac disease |
||||||||
| Library code | N1 | N2 | N3 | N4 | N5 | N6 | N7 | H1 | H2 | H3 |
| Library size | 8.6 × 107 | 8.8 × 108 | 3.8 × 108 | 7.5 × 107 | 6.9 × 107 | 1.8 × 107 | 2.8 × 107 | 5.7 × 107 | 5.7 × 107 | 6 × 107 |
| Anti-TG2 antibodies | 86 | 88 | 73 | 46 | 71 | 68 | 86 | 10 | 3 | 0 |
| Anti–α-gliadin antibodies | 80 | 97 | 77 | 53 | 57 | 93 | 82 | 3 | 11 | 0 |
Data are the percentage of positive clones. IgA phage display libraries from the lymphocytes isolated from type 1 diabetes patients and control subjects without celiac disease were constructed using all of the VH gene families. The libraries were selected for human TG2 and α-gliadin. After two rounds of selection, 45 clones for each type 1 diabetes library and 90 clones for each control library were individually tested by ELISA for the corresponding antigen. All of the libraries from type 1 diabetic patients who were either serologically positive or negative for TG2 show a high percentage of positive clones.
Furthermore, the whole IgA antibody libraries were selected on native gliadin. Both TG2+ and TG2− type 1 diabetic patients showed an elevated number of anti-gliadin antibodies, with a positivity ranging from 53 to 97% of the tested clones. In addition, the antibody diversity was high in all cases. All data are summarized in Table 4.
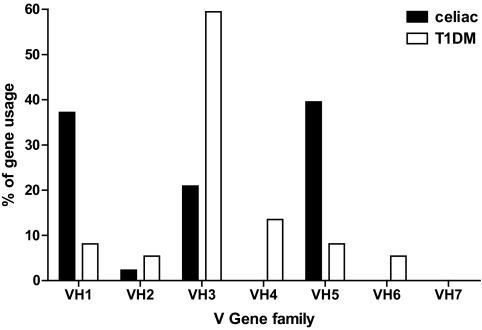
Finally, assignment to the seven VH gene family members of the positive antibodies to TG2 that were selected from the whole libraries was determined by DNA sequencing. As reported in Fig. 2, >50% of the phage antibodies from the type 1 diabetic patients belonged to the VH3 family, with minor usage of the other gene families. For comparison, the antibodies to TG2 selected from the phage display libraries from the intestinal B lymphocyte of three patients with celiac disease that were constructed previously were sequenced for gene family assignment. As reported in Fig. 2, the results showed a more distributed usage of the VH gene families with a stronger trend for VH1 and VH5.
FIG. 2.
Percentage of VH gene family usage in the recombinant anti-TG2 antibodies selected from seven intestinal B lymphocytes of type 1 diabetic patients (T1DM) and three intestinal B lymphocytes of patients with celiac disease.
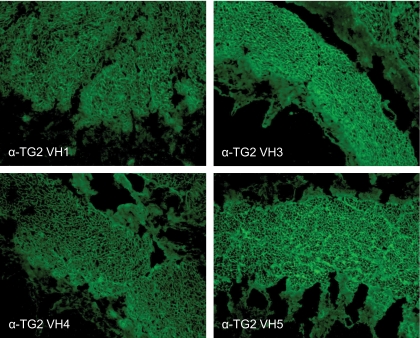
To further confirm the specificity of the anti-TG2 antibodies from the intestinal biopsy specimens of the type 1 diabetic patients, four soluble antibody fragments belonging to VH3, VH1, and VH4 families were assayed for anti-endomysium structure reactivity in monkey esophagus specimens. As shown in Fig. 3, the VH5 antibody (bottom right) showed a pattern that is typical of anti-endomysium antibodies (EMAs), and of all the other antibodies, attesting to their specificity to TG2.
FIG. 3.
Monkey esophagus sections stained with anti-TG2 scFv containing a VH1, VH3, and VH4 gene segment. An anti-TG2 VH5 scFv is shown as a control. All of the scFvs recognize native TG2–anti-endomysium structures in the oesophagus muscularis mucosa according to the reticular motif, which is similar to the results observed for serum anti-endomysium antibodies. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
In this study, given the reported derangement of the intestinal immunity in type 1 diabetic patients (2,3,5) and the association of type 1 diabetes and celiac disease (6–8), we investigated the production and presence of anti-TG2 antibodies, which are a hallmark of celiac disease, in the intestine of type 1 diabetic patients (24). Only patients with normal jejunal architecture were included in this study, which excluded patients with villous atrophy and a diagnosis of overt celiac disease. The results obtained show the presence of anti-TG2 antibodies in the jejunal mucosa of most patients with type 1 diabetes. However, a more precise analysis of the features of these antibodies, particularly the use of the VH genes, has shown that only in type 1 diabetic patients with serum titers of anti-TG2 antibodies that are higher than the cutoff do the anti-TG2 antibodies have the same features that they have in patients with celiac disease.
The immunofluorescence technique we used to detect intestinal deposits of anti-TG2 antibodies (11) is based on the colocalization of antibodies to IgA and to TG2; in this study, we validated this immunofluorescence technique with the use of confocal microscopy to confirm the colocalization. We have previously shown the presence of these deposits (25) in patients with villous atrophy as well as in subjects who had normal mucosa but other signs suggestive of potential celiac disease, such as the presence of EMAs in the serum (17) or an increased density of IELs expressing the γδ receptor (17). In the latter patients, these deposits have been shown by others to predict the future evolution to frank disease (15). In the potential celiac disease patients, we observed that these deposits were often observed in a patchy distribution and were less thick (25). The patchy pattern has also been observed in the patients with type 1 diabetes in this study. In fact, in the type 1 diabetic patients with normal mucosa and a high serum titer of anti-TG2 antibodies, these titers were still lower than those in subjects with villous atrophy; these lower titers and probably the lower antibody affinity might explain the patchy distribution and the thinner line of intestinal deposits.
The presence of these antibodies at the intestinal level strongly suggests that they are locally produced. As a matter of fact, the intestinal production of these antibodies has been demonstrated in patients with celiac disease by means of organ culture (26) and by the detection and measurement of these antibodies in intestinal juices (27) and feces (26). The results obtained with the phage display antibody libraries definitely support the above data on the presence of deposits and local production of anti-TG2 antibodies in the intestine of type 1 diabetic patients. The first attempt, in which we aimed to construct and select antibody libraries that used only the VH5 gene family, yielded many clones isolated from type 1 diabetic patients with a detectable serum titer of antibodies to TG2, whereas very few VH5 antibodies were selected from type 1 diabetic patients without elevated serum antibodies to TG2. This result is not in contrast with our previous studies on celiac disease anti-TG2 antibody profiling, in which we found that all the patients shared an atypical VH5 gene family usage in making antibodies to TG2 (10). However, in these patients, other antibodies belonging to the VH1 and VH3 families that recognize a different epitopic region are also produced. In this respect, the TG2 serologically negative type 1 diabetic patients do not correspond to patients with celiac disease because very few VH5 antibodies were selected. On the other hand, the remarkable intestinal antibody response to TG2 revealed by the whole antibody libraries from the TG2− type 1 diabetic patients, although with a preferential use of the VH3 family, again suggests a relationship with celiac disease. Moreover, these antibodies, along with the VH1 and VH4 antibodies, were shown to recognize the endomysial TG2 according to the EMA pattern that is typical for the VH5 antibodies. The interpretation that this antibody production might be gluten dependent is strengthened by the number of antibodies to gliadin that were isolated from all of the type 1 diabetic patient libraries but not from the control subjects without celiac disease. We cannot predict whether type 1 diabetic patients with a normal serum level of anti-TG2 antibodies who show a strong anti-TG2 antibody intestinal response either are in a very early stage of celiac disease or will never progress toward the disease. In addition, we do not know whether a possible shift in the VH3-based antibody response to a VH5 response may be a significant step in the progression of the illness. Antigen spreading by both antibody and T-cell epitopes is a well-known feature of autoimmune diabetes, and we wonder whether this is a characteristic of the anti-TG2 antibody response as well. If the results that have been reported here are confirmed, the cloning of intestinal antibodies could be considered a useful tool for investigating the immunological background of both type 1 diabetes and celiac disease. In a preliminary study (28), we found that consumption of a gluten-free diet was beneficial for type 1 diabetic patients and was paralleled by a change in the profile of the intestinal antibody response. In this respect, the present study provides a more comprehensive interpretation of the possible immunotoxic effect of gluten.
Intestinal anti-TG2 antibodies have also been noted in the intestines of NOD mice (29). In NOD mice, the presence of the antibodies in the gut is accompanied by high antibody levels in the serum, but their presence is not dependent on the presence of gluten in the diet. The same could apply to type 1 diabetic patients who do not show the same special use of the VH genes. We have not investigated other autoimmune conditions, but we can exclude the notion that the finding of the intestinal anti-TG2 autoantibodies is simply related to HLA status or is merely a consequence of intestinal inflammation.
It is difficult to predict which functional effect could be exerted by the anti-TG2 antibodies present in the gut of type 1 diabetic patients. Studies conducted on antibodies derived from patients with celiac disease suggest that they at least partly inhibit enzymatic activity (30); furthermore, it was recently found that they favor proliferation in the epithelial compartment (31).
In conclusion, we have shown that the majority of patients with type 1 diabetes have anti-TG2 antibodies in their intestinal mucosa. The special use of the VH5 genes in type 1 diabetic patients with serum positivity for anti-endomysium suggests a gluten-dependent phenomenon analogous to what has been found in celiac disease. These patients belong to the spectrum of gluten sensitivity that ranges from an abnormal immune response to frank villous atrophy. More difficult is the interpretation of the finding of the intestinal anti-TG2 antibodies, not necessarily using the VH5 genes, in those with an absence of a high titer of these antibodies in the serum; in these patients, the relationship with gluten is uncertain. It could be an expression of autoimmunity, analogous to what has been observed in NOD mice, and be included in the more general derangement of the intestinal mucosal immunity observed in type 1 diabetes. In addition, the raised density of γδ+ IELs that has been observed in type 1 diabetes, irrespective of the presence of high serum titers of anti-TG2 antibodies, could be a feature of autoimmunity that has also been observed in other autoimmune conditions (32,33). Current studies with the aim of defining the molecular basis of the antibody response to TG2 will help to interpret the observations reported here and, in particular, to understand to what extent the presence of these antibodies and, in general, the derangement of the mucosal immunity is related to dietary gluten in type 1 diabetes.
Acknowledgments
This work was supported by Fondazione Cariplo, Compagnia Sanpaolo, and the Italian Ministry of University and Research (project “Intestino e autoimmunità”).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mallone R, van Ender P: T cells in the pathogenesis of type 1 diabetes. Curr Diab Rep 2008; 8: 101– 106 [DOI] [PubMed] [Google Scholar]
- 2.Savilahti E, Ormala T, Sukkonen T, Sandini-Pohjavouri U, Kantele JM, Arato A, Ilonen J, Åkerblom HK: Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) shows signs of immune activation. Clin Exp Immunol 1999; 116: 70– 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E: Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 2003; 52: 2287– 2295 [DOI] [PubMed] [Google Scholar]
- 4.Carratù R, Secondulfo M, De Magistris L, Iafusco D, Urio A, Cardone MG, Pontoni G, Cartenì M, Prisco F: Altered intestinal permeability to mannitol in diabetes mellitus type 1. J Pediatr Gastroenterol Nutr 1999; 28: 264– 269 [DOI] [PubMed] [Google Scholar]
- 5.Auricchio R, Paparo F, Maglio M, Franzese A, Lombardi F, Valerio G, Nardone G, Percopo S, Greco L, Troncone R: In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 2004; 53: 1680– 1683 [DOI] [PubMed] [Google Scholar]
- 6.Maki M, Hallstrom O, Huupponen T, Vesikari T, Visakorpi JK: Increased prevalence of coeliac disease in diabetes. Arch Dis Child 1984; 59: 739– 742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savilahti E, Simell O, Koskimies S, Rilva A, Akerblom HK: Coeliac disease in insulin dependent diabetes mellitus. J Pediatr 1986; 108: 690– 693 [DOI] [PubMed] [Google Scholar]
- 8.Sategna-Guidetti C, Grossa S, Pulitano R, Benaduce E, Dani FCarta Q: Celiac disease and insulin dependent diabetes mellitus: screening in adult population. Dig Dis Sci 1994; 39: 1633– 1637 [DOI] [PubMed] [Google Scholar]
- 9.Sollid LM: Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2: 647– 655 [DOI] [PubMed] [Google Scholar]
- 10.Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A: Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol 2001; 166: 4170– 4176 [DOI] [PubMed] [Google Scholar]
- 11.Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, Fesus L, Maki M: In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004; 53: 641– 648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troncone R, Franzese A, Mazzarella G, Paparo F, Auricchio R, Coto I, Mayer M, Greco L: Gluten sensitivity in a subset of children with insulin dependent diabetes mellitus. Am J Gastroenterol 2003; 98: 590– 595 [DOI] [PubMed] [Google Scholar]
- 13.Troncone R, Paparo F, Mazzarella G, Maglio M, Iovine G, Mayer M, Greco L, Auricchio S: The spectrum of gluten sensitivity. In Coeliac disease Auricchio S, Greco L, Maiuri L, Troncone R: Eds. Naples, Jean Gilder Editions, 2000, p. 151– 155 [Google Scholar]
- 14.Maki M, Huopponen T, Holm K, Hallstrom O: Seroconversion of reticulin autoantibodies predicts coeliac disease in insulin dependent diabetes mellitus. Gut 1995; 36: 239– 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmi TT, Collin P, Jarvinen O, Haimila K, Partanen J, Laurila K, Korponay-Szabo IR, Huhtala H, Reunala T, Maki M, Kaukinen K: Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Aliment Pharmacol Ther 2006; 24: 541– 552 [DOI] [PubMed] [Google Scholar]
- 16.Oberhuber G, Granditsch G, Vogelsang H: The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11: 1185– 1194 [DOI] [PubMed] [Google Scholar]
- 17.Paparo F, Petrone E, Tosco A, Maglio M, Borrelli M, Salvati VM, Miele E, Greco L, Auricchio S, Troncone R: Clinical, HLA, and small bowel immunohistochemical features of children with positive serum anti-endomysium antibodies and architecturally normal small intestinal mucosa. Am J Gastroenterol 2005; 100: 2294– 2298 [DOI] [PubMed] [Google Scholar]
- 18.Sblattero D, Florian F, Azioni E, Ziberna F, Tommasini A, Not T, Ventura A, Bradbury A, Marzari R: One-step cloning of anti tissue transglutaminase scFv from subjects with celiac disease. J Autoimmun 2004; 22: 65– 72 [DOI] [PubMed] [Google Scholar]
- 19.Sblattero D, Bradbury A: A definitive set of oligonucleotide primers for amplifying human V regions. Immunotechnology 1998; 3: 271– 278 [DOI] [PubMed] [Google Scholar]
- 20.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G: By-passing immunization: human antibodies from V-gene libraries displayed on phage. J Mol Biol 1991; 222: 581– 597 [DOI] [PubMed] [Google Scholar]
- 21.Silano M, De Vincenzi M: Bioactive antinutritional peptides derived from cereal prolamins: a review. Nahrung 1999; 43: 175– 184 [DOI] [PubMed] [Google Scholar]
- 22.Sblattero D, Berti I, Trevisiol C, Marzari R, Tommasini A, Bradbury A, Fasano A, Ventura A, Not T: Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol 2000; 95: 1253– 1257 [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson IM, Williams SC, Corbett SJ, Cox JPL, Winter G: V BASE Sequence Directory. Cambridge, U.K., Medical Research Council Centre for Protein Engineering, 1996 [Google Scholar]
- 24.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D: Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997; 3: 797– 801 [DOI] [PubMed] [Google Scholar]
- 25.Tosco A, Maglio M, Paparo F, Rapacciuolo L, Sannino A, Miele E, Barone MV, Auricchio R, Troncone R: Immunoglobulin A anti-tissue transglutaminase antibody deposits in the small intestinal mucosa of children with no villous atrophy. J Pediatr Gastroenterol Nutr 2008; 47: 293– 298 [DOI] [PubMed] [Google Scholar]
- 26.Picarelli A, Sabbatella L, Di Tola M, Di Cello T, Vetrano S, Anania MC: Antiendomysial antibody detection in fecal supernatants: in vivo proof that small bowel mucosa is the site of antiendomysial antibody production. Am J Gastroenterol 2002; 97: 95– 98 [DOI] [PubMed] [Google Scholar]
- 27.Mawhinney H, Love AH: Anti-reticulin antibody in jejunal juice in coeliac disease. Clin Exp Immunol 1975; 21: 394– 398 [PMC free article] [PubMed] [Google Scholar]
- 28.Sblattero D, Ventura A, Tommasini A, Cattin L, Martelossi S, Florian F, Marzari R, Bradbury A, Not T: Cryptic gluten intolerance in type 1 diabetes: identifying suitable candidates for a gluten free diet. Gut 2006; 55: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sblattero D, Maurano F, Mazzarella G, Rossi M, Auricchio S, Florian F, Ziberna F, Tommasini A, Not T, Ventura A, Bradbury A, Marzari R, Troncone R: Characterization of the anti-tissue transglutaminase antibody response in nonobese diabetic mice. J Immunol 2005; 174: 5830– 5836 [DOI] [PubMed] [Google Scholar]
- 30.Esposito C, Paparo F, Caputo I, Rossi M, Maglio M, Sblattero D, Not T, Porta R, Auricchio S, Marzari R, Troncone R: Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut 2002; 5: 177– 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C: Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology 2007; 132: 1245– 1253 [DOI] [PubMed] [Google Scholar]
- 32.Valentino R, Savastano S, Maglio M, Paparo F, Ferrara F, Dorato M, Lombardi G, Troncone R: Markers of potential coeliac disease in patients with Hashimoto's thyroiditis. Eur J Endocrinol 2002; 146: 479– 483 [DOI] [PubMed] [Google Scholar]
- 33.Iltanen S, Holm K, Partanen J, Laippala P, Mäki M: Increased density of jejunal γδ+ T cells in patients having normal mucosa—marker of operative autoimmune mechanisms? Autoimmunity 1999; 29: 179– 187 [DOI] [PubMed] [Google Scholar]