Abstract
OBJECTIVE
Insulin plays an important role in the hypothalamic control of energy balance, especially by reducing food intake. Emerging data point to a pivotal role of reactive oxygen species (ROS) in energy homeostasis regulation, but their involvement in the anorexigenic effect of insulin is unknown. Furthermore, ROS signal derived from NADPH oxidase activation is required for physiological insulin effects in peripheral cells. In this study, we investigated the involvement of hypothalamic ROS and NADPH oxidase in the feeding behavior regulation by insulin.
RESEARCH DESIGN AND METHODS
We first measured hypothalamic ROS levels and food intake after acute intracerebroventricular injection of insulin. Second, effect of pretreatment with a ROS scavenger or an NADPH oxidase inhibitor was evaluated. Third, we examined the consequences of two nutritional conditions of central insulin unresponsiveness (fasting or short-term high-fat diet) on the ability of insulin to modify ROS level and food intake.
RESULTS
In normal chow-fed mice, insulin inhibited food intake. At the same dose, insulin rapidly and transiently increased hypothalamic ROS levels by 36%. The pharmacological suppression of this insulin-stimulated ROS elevation, either by antioxidant or by an NADPH oxidase inhibitor, abolished the anorexigenic effect of insulin. Finally, in fasted and short-term high-fat diet–fed mice, insulin did not promote elevation of ROS level and food intake inhibition, likely because of an increase in hypothalamic diet-induced antioxidant defense systems.
CONCLUSIONS
A hypothalamic ROS increase through NADPH oxidase is required for the anorexigenic effect of insulin.
The hypothalamus is a cerebral area involved in the regulation of energy homeostasis. In this context, insulin plays a pivotal role. By acting in the hypothalamus, insulin reduces food intake and body weight (1,2), activates sympathetic nerve outflow to the brown adipose tissue (3), and suppresses hepatic endogenous glucose production (4,5). In a situation of excess nutrient intake, the hypothalamus rapidly becomes resistant to insulin before obesity and diabetes onset (6,7). Moreover, inactivation of the neuronal insulin receptor leads to the development of diet-induced obesity with an increase in body fat, mild insulin resistance, and elevated plasma insulin levels (8). Therefore, it is of prime importance to elucidate cellular mechanisms by which insulin acts on hypothalamic cells to understand central early-onset diet-induced obesity and diabetes.
In recent years, emerging data point to a pivotal role of reactive oxygen species (ROS) in the energy homeostasis regulation by the hypothalamus. In response to an acute overload of nutrients, a subtle rise of the ROS concentration within this area is sufficient to reduce food intake (9) or stimulate the parasympathetic nervous system as well as pancreatic insulin secretion (10). Furthermore, the gut-derived hormone ghrelin exerts its central effect on feeding behavior by controlling hypothalamic ROS levels (11). However, the role of ROS in the anorexigenic effect of insulin and more generally in the brain insulin signaling remains unknown. In peripheral insulin-sensitive cells, ROS have been shown to control the crucial early steps in insulin signaling (12–14). Moreover, they contribute to propagation of the insulin cascade in these cells (15–17). Thus, transient bursts of small amounts of ROS triggered in response to insulin facilitate both the early and distal insulin signaling pathway. Reinforcing this concept, it has been recently shown that this transient insulin-induced ROS production is also required in neuronal cells for the enhancement of insulin receptor autophosphorylation, the first step in the insulin signaling pathway (18). These data lead us to hypothesize that insulin might trigger ROS elevation within the hypothalamus, which, in turn, would inhibit food intake.
In peripheral cells, it is well established that both the insulin-induced ROS rise and insulin cascade activation are dependent on NADPH oxidase activity (12–17,19). Blocking insulin-induced ROS production with an inhibitor of NADPH oxidase activity (diphenyleneidonium) dramatically reduces insulin signaling pathway activation and thus its physiological effects (15).
Despite the demonstration of the crucial role of ROS signaling and NADPH oxidase in peripheral insulin effects, their involvement in brain insulin signaling has not yet been investigated. Therefore, in this study we tested whether the anorexigenic insulin effect required a ROS-dependent signaling pathway within the hypothalamus. For this purpose, we measured hypothalamic ROS levels and food intake after intracerebroventricular insulin injection. The involvement of ROS and NADPH oxidase in insulin-induced food intake inhibition was evaluated by pretreatment with a ROS scavenger or an NADPH oxidase inhibitor. Because central insulin responsiveness is known to be altered by a short-term deficit (20) as well as an excess (7) of nutrient availability, we examined the consequence of 18-h fasting or 3 days' high-fat diet on insulin's ability to modify hypothalamic ROS levels and food intake.
RESEARCH DESIGN AND METHODS
All animal experiments were carried out in strict accordance with European Communities Council Directive 86/609/EEC and the Guide for the Care and Use of Laboratory Animals, edited by the National Research Council. For this study, we used 7- to 8-week-old male C57BL6/J mice (Harlan Laboratories). The animals were kept in a temperature-controlled room (22 ± 1°C) on a 12-h light/dark cycle with free access to food and water. Depending on the experiment being conducted, mice were fed ad libitum with standard diet (22.4% fat, 60.9% carbohydrate, and 16.7% protein, 2.9 kcal/g; R04T25; SAFE, Augy, France) or high-fat diet (42.5% fat, 42.5% carbohydrate, and 15% protein, 4.4 kcal/g; customized; SAFE). For the fasting group, food was removed just before dark onset (1900 h). For tissue collection, mice were killed by cervical dislocation.
Surgery.
Under anesthesia (0.4% isoflurane, 100% O2), mice underwent stereotaxic surgery to implant a chronic stainless steel cannula (Charles River). The third cerebral ventricle was targeted using the following coordinates from bregma: anterior-posterior, –0.825 mm; dorsal-ventral, –5 mm; and medial-lateral, 0 mm. The cannula was fixed to the skull using dental cement. Mice were finally housed individually and were allowed 1 week for recovery before the experiment.
Drug administration.
Injections into the third cerebral ventricle were performed in awake mice. The injections (1 μl) consisted of either 0.4 μU insulin (100 units/ml Actrapid recombinant human insulin; Novo Nordisk), 0.1 mmol/l Trolox (Calbiochem), 0.1 mmol/l diphenyleneiodonium (DPI; Sigma-Aldrich), or vehicle. Insulin, Trolox, and DPI were prepared as stock solutions in diluting medium for soluble insulin injection (Novo Nordisk), 1% ethanol, and 1% DMSO, respectively. For injections, all drugs and their respective vehicle were diluted in 0.9% NaCl. Injections were performed over 1 min. After the infusion, the guide cannula was kept in place for an additional 30 s to allow the drugs to diffuse away from the cannula tip.
Food intake measurement.
Food was removed 1 h before intracerebroventricular injection. Insulin was injected 4 h before dark onset (1500). Intracerebroventricular injections of Trolox or DPI were performed 30 min prior to the 0.4 μU insulin injection. Just before the beginning of the dark period (1900), food (10 g) was presented to the mice. Then, 12 and 24 h later, food intake and body weight were measured.
Blood glucose measurement.
Blood samples were collected from the tail 2 h after 4 μU insulin intracerebroventricular injection and used for measurements of blood glucose. It was directly measured on a glucose analyzer (Accu-Chek Active meter).
ROS detection.
The dye 2′,7′-dichlorofluorescein diacetate (H2DCFDA; Molecular Probes) was used to monitor intracellular change in H2O2 levels. After intracerebroventricular injections, hypothalami were dissected and immediately frozen in liquid nitrogen. After rapid thawing, tissues were homogenized (20 strokes) with a Dounce homogenizer and a B-type pestle in 250 μl of a ROS buffer (150 mmol/l KCl, 20 mmol/l Tris, 0.5 mmol/l EDTA, 1 mmol/l MgCl2, 5 mmol/l glucose, and 0.5 mmol/l octanoïc acid, pH 7.4). Homogenates were exposed to 16 μmol/l H2DCFDA and were incubated at 37°C for 30 min under agitation. Reaction was stopped with 125 μl of 70% ethanol plus 125 μl of 0.1 N HCl. Homogenates were then centrifuged at 3,000g for 15 min at 4°C. Supernatants were collected, neutralized with 175 μl of 1 mol/l NaHCO3, and centrifuged at 6,000g for 15 min at 4°C. As previously described (9,10), ROS level was evaluated by measuring fluorescence intensity with a microplate reader (Victor; Wallac). Intensity of fluorescence was expressed as arbitrary units per microgram of proteins.
Reduced glutathione assay.
Dissected hypothalami were immediately frozen in liquid nitrogen. Tissues were homogenized (20 strokes) with a Dounce homogenizer and a B-type pestle in a lysis saline solution (150 mmol/l KCl, and 3 mmol/l EDTA, pH 7.4). Homogenates (50 μl) were mixed with 450 μl of 5% metaphosphoric acid. Samples were then centrifuged at 1,500g for 10 min at 4°C. Supernatants were collected and used to detect reduced glutathione (GSH). Glutathione assay was performed by reverse-phase high-performance liquid chromatography as previously described (21). The results are expressed as ficomoles per microgram tissular protein.
Protein assay.
Protein concentration of samples was determined using the DC protein assay kit (Bio-Rad) according to the manufacturer's instructions.
Statistical analysis.
Data are the means ± SE. Depending on the experiment being conducted, data were compared by unpaired Student's t test, the Mann-Whitney U test, or one-way ANOVA. Differences among groups were considered significant at P < 0.05.
RESULTS
Third-ventricle insulin injection decreases food intake.
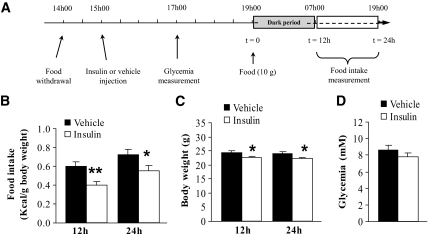
To study the role of hypothalamic insulin signaling in food intake regulation, we first aimed to select the best paradigm of acute insulin injection (Fig. 1A). We chose a third-ventricle injection because acute insulin administration into the lateral ventricle was without effect on food intake in mice (22). In comparison to vehicle-injected animals, insulin significantly reduced food intake 12 and 24 h after food presentation (Fig. 1B). This represented a 40% decrease in daily food intake. Concurrently to this food intake inhibition, insulin also significantly reduced body weight at 12 and 24 h (Fig. 1C). To ensure that intracerebroventricularly injected insulin did not indirectly affect food intake through diffusion out of the brain, we injected a higher dose of insulin and measured glycemia. No change in plasma glucose was observed in this condition, showing that the insulin effect on food intake only depends on its effect on the brain (Fig. 1D).
FIG. 1.
Acute insulin injection into the third ventricle reduces food intake at 12 and 24 h with no effect on plasma glucose. A: Schematic representation of experimental procedures for food intake measurement. B: Mice were injected into the third ventricle with insulin (0.4 μU) or vehicle. Food intake was assessed by weighing residual chow 12 and 24 h after food presentation (n = 9–10 mice per group). C: Body weight was also measured at these time points (n = 9–10 mice per group). D: Mice were injected into the third ventricle with a higher dose of insulin (4 μU) or vehicle. Plasma glucose was measured 2 h after injection (n = 4 mice per group). Data are the means ± SE. *P < 0.05, **P < 0.01 vs. vehicle-injected group.
Insulin induces a transient hypothalamic ROS increase, which is required for food intake inhibition.
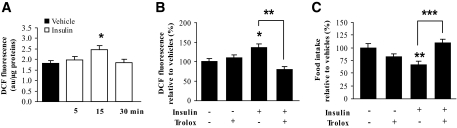
To determine whether insulin could induce ROS increase in the hypothalamus, insulin was injected into the third ventricle at a dose that inhibits food intake. Mice were killed 5, 15, and 30 min later, and the ROS level was evaluated using the fluorescent redox-sensitive dye H2DCFDA. Figure 2A shows that insulin induced a significant increase (+36%) in the ROS level 15 min postinjection. This insulin-induced ROS increase was transient because no modification of fluorescence intensity was observed at either 5 or 30 min postinjection.
FIG. 2.
Insulin induces a transient hypothalamic ROS increase, which is required for food intake inhibition. A: Insulin effect on ROS production. After intracerebroventricular injection of insulin (0.4 μU) or vehicle, ROS levels were assessed by oxidation of H2DCFDA probe in the hypothalamus at different times (n = 5 mice per group). Dichlorofluorescein (DCF) fluorescence is expressed in arbitrary units per microgram of protein. B and C: Effect of Trolox on insulin-induced ROS production and food intake inhibition. Trolox (0.1 mmol/l) was intracerebroventricularly injected 30 min before insulin (0.4 μU). ROS levels were assessed in the hypothalamus 15 min after injections (n = 5 mice per group) (B). Food intake was assessed 12 h after food presentation (n = 5–10 mice per group) (C). Data are the means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle- or insulin-injected group.
We then designed experiments to examine the physiological relevance of this insulin-induced hypothalamic ROS elevation. For this purpose, we assessed food intake in response to acute intracerebroventricular insulin injection in combination with an intracerebroventricular pretreatment of Trolox, a ROS scavenger. We first verified that Trolox alone did not induce significant change in basal ROS levels and food intake, and then we investigated the ability of Trolox to inhibit insulin-induced ROS production. As illustrated in Fig. 2B, Trolox delivery 30 min prior to insulin completely prevented insulin-stimulated ROS increase. We then measured the effect of Trolox pretreatment on food intake response. As for the ROS level, intracerebroventricular Trolox injection completely suppressed insulin-induced food intake inhibition without significantly modifying basal food intake (Fig. 2C). Therefore, these results indicate that the transient insulin-induced ROS elevation in the hypothalamus is required for food intake inhibition.
NADPH oxidase is required for insulin-induced ROS elevation and food intake inhibition.
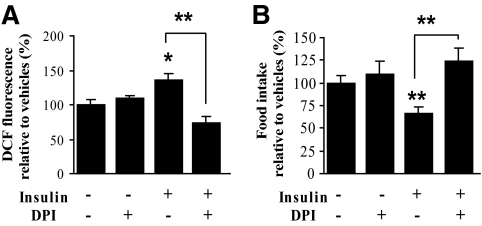
In peripheral tissues, ROS produced by membrane-bound NADPH oxidase are involved in the insulin signaling cascade. We questioned whether, as in the periphery, NADPH oxidase was involved in hypothalamic insulin ROS signaling to regulate food intake. For this purpose, we assessed food intake in response to acute intracerebroventricular insulin injection in combination with prior intracerebroventricular administration of DPI, a NADPH oxidase inhibitor. To test the ability of DPI to inhibit the ROS level increase, we first measured the hypothalamic ROS level 15 min after insulin injection with DPI pretreatment. DPI delivery was effective in fully preventing insulin-stimulated ROS elevation (Fig. 3A). In this condition, DPI completely restored basal food intake in insulin-injected mice (Fig. 3B). DPI treatment alone did not significantly modify basal ROS levels as well as food intake. Therefore, these results indicate that NADPH oxidase–dependent ROS increase within the hypothalamus is required for the central effect of insulin on food intake inhibition.
FIG. 3.
NADPH oxidase is required for the insulin-induced hypothalamic ROS increase and food intake inhibition. Effect of DPI on insulin-induced ROS level change and food intake inhibition is shown. DPI (0.1 mmol/l) was intracerebroventricularly injected 30 min before insulin (0.4 μU). ROS levels were assessed in the hypothalamus 15 min after injection (n = 10 animals per group) (A). Food intake was assessed 12 h after food presentation (n = 5–10 animals per group) (B). Data are the means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle- or insulin-injected group. DCF, dichlorofluorescein.
Impairment of insulin-induced food intake inhibition is associated with a lack of hypothalamic ROS level increase.
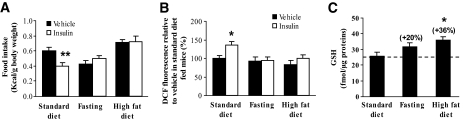
We then tested whether a modification of insulin effect on food intake was systematically associated with a change of ROS level within the hypothalamus. For this purpose, we chose two physiological situations that produce central insulin unresponsiveness: 18-h fasting (energy deficiency) (20) and 3 days' high-fat diet (energy excess) (7). Figure 4A shows that these two conditions effectively suppressed insulin-induced food intake inhibition. According to our hypothesis, insulin did not promote elevation of ROS level within the hypothalamus 15 min after intracerebroventricular injection (Fig. 4B).
FIG. 4.
Impairment of insulin-induced food intake inhibition is associated with a lack of ROS level elevation within the hypothalamus. Effect of 18 h fasting or 3 days' high-fat diet on insulin-induced food intake regulation and ROS production, and on glutathione levels. A and B: Mice were injected with insulin (0.4 μU) or vehicle. Food intake (n = 8–12 animals per group) (A) and ROS level (n = 5–10 per group) (B) were assessed 12 h and 15 min after insulin injection, respectively. *P < 0.05, **P < 0.01 vs. vehicle-injected mice fed standard diet. C: Hypothalamic GSH level was measured in standard diet–fed, fasted, and high-fat diet–fed mice (n = 5–10 per group). *P < 0.05 vs. mice fed with standard diet.
Because cellular ROS levels result from the balance between their production and removal by various antioxidants, we quantified hypothalamic glutathione, which is considered as a major cellular ROS scavenging system (23). Figure 4C shows that hypothalamic GSH levels were increased by 18-h fasting (+20%) and 3 days' high-fat diet (+36%, P < 0.05). Therefore, impairment of insulin-induced inhibition of food intake was associated with a lack of ROS increase in the hypothalamus, likely because of higher GSH level. These results strengthen the crucial role of hypothalamic ROS signaling in the anorexigenic effect of insulin.
DISCUSSION
In the current study, we describe the involvement of an NADPH oxidase–dependent ROS signaling pathway in the anorexigenic insulin effect. We demonstrate for the first time the physiological relevance of this concept in conscious mice.
Insulin was administrated into the third ventricle at a dose (2.4 nmol/l) that is consistent with brain insulin receptor affinity (24,25) and that produces maximal food intake inhibition in mice (2) and unpublished results). At this physiological concentration, insulin induced a transient ROS level elevation within the hypothalamus (Fig. 2). The current data demonstrate for the first time in vivo that insulin can effectively induce a change in ROS levels in the central nervous system. Despite a relatively low magnitude (+36%), this ROS level increase is similar to that observed in the hypothalamus after glucose, lipid, or ghrelin stimulation (9–11). These subtle ROS changes play a key role in the control of the autonomous nervous system and food intake. We show that this insulin-induced ROS increase is required for food intake inhibition, thus demonstrating its physiological relevance. First, the suppression of insulin-stimulated ROS production using pharmacological tools prevented insulin inhibition of food intake. Second, in two nutritional conditions of central insulin unresponsiveness (i.e., 18-h fasting or 3 days' high-fat diet), impairment of the insulin effect on food intake was associated with a lack of insulin-stimulated ROS elevation within the hypothalamus. These data strongly support the notion that cellular ROS constitute a pivotal second messenger in hypothalamic signaling pathways involved in energy homeostasis regulation, especially by controlling nutrient sensing (9,10) and hormonal signaling (11).
The involvement of an oxidant signal in the action of insulin has been suggested for decades, mainly with the observation that oxidants, including hydrogen peroxide, mimic insulin's effects on glucose transport and lipogenesis in adipose cells (26). A few years later, insulin itself was shown to elicit the generation of hydrogen peroxide in adipocytes (27). More recently, several elegant studies carried out by Mahadev and colleagues (13–16) clearly demonstrate that a physiological transient insulin-induced ROS elevation mediates the increase of glucose uptake through GLUT4 translocation to the plasma membrane in adipocytes. Finally, creating mildly and transiently oxidative intracellular conditions has been reported to enhance insulin receptor activation, suggesting that optimal insulin responsiveness involved a process of “redox priming” of the β-subunit (17,28). Our results reinforce these previous data. Altogether, they strongly support the idea that a ROS increase induced by insulin 1) forms an integral part of insulin signaling pathway in the central nervous system as in the periphery and 2) is relevant in whole-body energy homeostasis.
The observed insulin-induced ROS increase peaks and dissipates in a few minutes. Nevertheless, and in agreement with the study by Brown et al. (2), feeding behavior is significantly inhibited for several hours after acute intracerebroventricular insulin injection (Fig. 1). In adipocytes, the early insulin-stimulated production of ROS is required for insulin to elicit its late cellular responses, especially by activating the insulin receptor substrate (IRS)/phosphoinositide (PI)-3 kinase signaling pathway (13–16,18,29). Within the hypothalamus, both insulin-induced ROS and IRS/PI 3-kinase signaling pathways are essential for the long-term inhibitory insulin effect on food intake (Fig. 2) (30). Reciprocally, diet-induced impairment of insulin effect on food intake is associated with an alteration of these two insulin-activated signaling pathways (Fig. 4A and B) (7,31). The time course of insulin-induced ROS level increase is similar to that observed for insulin-induced PI 3-kinase activation in the hypothalamus, with activation a few minutes after stimulation, persisting for 10–15 min, and gradually declining over 30–60 min (32). Altogether, these data strongly suggest that the early insulin-induced ROS increase that we measured within the hypothalamus is a pivotal component of insulin-induced IRS/PI 3-kinase signaling pathway activation, leading to a long-term decrease in food intake, likely through modulation of the expression of genes such as neuropeptide Y (33). Actually, hypothalamic neuropeptide Y and pro-opio melanocortin C are well known to play a key role in food intake regulation and to be down- or upregulated by hormones such as leptin, ghrelin, or insulin (2,11,33,34). Because insulin-induced PI 3-kinase activation occurs in these neuronal subpopulations (32), one can speculate that the insulin-induced ROS increase occurs at least in one of these, as has been recently demonstrated for ghrelin (11).
That insulin induces a rapid and transient increase in ROS level within the hypothalamus led us to investigate the involvement of NADPH oxidase. Indeed, this enzyme can be rapidly activated by insulin, leading to a rapid and transient ROS increase in peripheral cells (14,15). In our experimental conditions and similarly to Mahadev et al. (15), pretreatment with a NADPH oxidase inhibitor (DPI) completely abolished 1) the rapid and transient insulin-induced ROS increase within the hypothalamus and 2) the inhibitory insulin effect on food intake (Fig. 3). We can thus conclude that NADPH oxidase plays a critical role in the insulin signaling pathway in the brain as in the periphery. There is now compelling evidence showing the presence of NADPH oxidase homologues in rodent or human brain tissue (35–38). Within the hypothalamus, immunoreactivity for NADPH oxidase subunits, such as p47phox and gp91phox, was found in arcuate, ventromedial, and paraventricular nuclei, which are well known to be involved in energy homeostasis regulation (36). Moreover, increasing data demonstrate the importance of redox signaling derived from NADPH oxidase in normal central nervous system processes, such as long-termpotentiation and hippocampal-dependent memory or the regulation of the cardiovascular system (38–42). Further in vivo studies would permit us to confirm that an acute insulin-induced NADPH oxidase activation mediates other hypothalamic insulin effects involved in energy homeostasis.
After fasting or short-term high-fat feeding, the brain became less responsive to insulin, which resulted in a loss of its effects on food intake (Fig. 4A) (7,20). In this context, insulin-induced hypothalamic ROS elevation was suppressed (Fig. 4B). To explain this result, we explored the involvement of antioxidant defense systems. Indeed, the rise in free fatty acid availability during fasting (because of lipolysis) and short-term high-fat feeding (because of diet) likely induced an increase in hypothalamic lipid oxidation (9,11,43), a mechanism known to produce ROS (44). In the physiological range, excess ROS is scavenged at the cellular level by stimulating the synthesis of antioxidant defense systems (44), as already demonstrated within the hypothalamus of food-deprived animals (9,45). We reported that hypothalamic GSH level is effectively higher after high-fat feeding and to a lesser measure after fasting (Fig. 4C), with a concomitant hypothalamic normal ROS level. These data strongly suggest that 1) high-fat diet induced excessive hypothalamic ROS production, which had been well regulated by the cellular scavenging system, and 2) this increased antioxidant defense system could consequently overquench ROS produced under insulin stimulation. Such a cellular mechanism has been already proposed to explain the impaired peripheral insulin sensitivity observed during physiological conditions such as pregnancy (46). In animal models of long-term high-fat diet–induced obesity (47,48) associated with hypothalamic insulin resistance (47), oxidative stress appears in the brain (48). This cellular stress has been proposed to be a critical mechanism underlying development of insulin resistance in obesity (49,50). We thus hypothesized that 1) the early diet-induced central insulin resistance is a consequence of an adaptive mechanism to avoid cytotoxic ROS production, although 2) in the long-term, the resistance might be caused by an overwhelming of antioxidant defense systems, and thus oxidative stress (44).
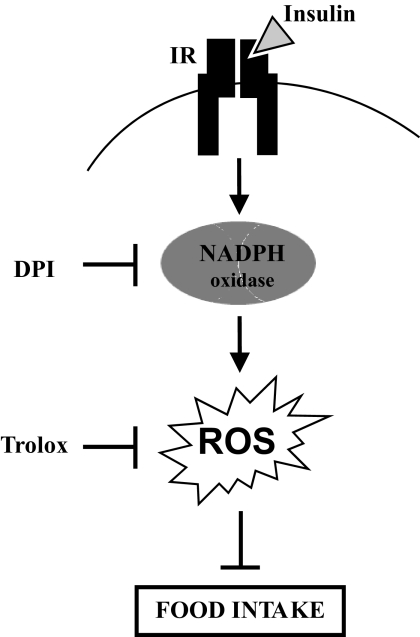
In conclusion, we report that insulin injected into the third ventricle promoted a subtle and transient increase in ROS level within the hypothalamus. This mechanism is required for insulin food intake inhibition and involves NADPH oxidase (Fig. 5). We thus suggest that 1) an NADPH oxidase–dependent ROS-sensitive signaling pathway is implied in hypothalamic insulin action, and 2) diet-induced impairment of hypothalamic ROS homeostasis may contribute to cerebral insulin resistance.
FIG. 5.
Proposed mechanism of the involvement of a hypothalamic NADPH oxidase–dependent ROS signaling pathway in the anorexigenic effect of insulin. Insulin binds to its receptor leading to NADPH oxidase activation and generation of ROS. This ultimately leads to inhibition of food intake, likely though modulation of gene expression. IR, insulin receptor.
Acknowledgments
This work was funded in part by Agence Nationale de la Recherche Grant ANR-05-PNRA-004 and a grant from the Institut Benjamin Delessert. T.J. received a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche and from the Fondation pour la Recherche Medicale.
No potential conflicts of interest relevant to this article were reported.
We are indebted to M. Nibbelink for her technical help. We thank M. Rigoulet for helpful discussions and insightful comments.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Woods SC, Lotter EC, McKay LD, et al. : Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979; 29: 503– 505 [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Clegg DJ, Benoit SC, et al. : Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav 2006; 89: 687– 691 [DOI] [PubMed] [Google Scholar]
- 3.Rahmouni K, Morgan DA, Liu X, et al. : Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 2004; 114: 652– 658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obici S, Zhang BB, Karkanias G, et al. : Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002; 8: 1376– 1382 [DOI] [PubMed] [Google Scholar]
- 5.Koch L, Wunderlich T, Seibler J, et al. : Hypothalamic central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 2008; 118: 2132– 2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg DJ, Benoit SC, Reed JA, et al. : Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 2005; 288: R981– R986 [DOI] [PubMed] [Google Scholar]
- 7.Ono H, Pocai A, Wang Y, et al. : Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest 2008; 118: 2959– 2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brüning JC, Gautam D, Burks DJ, et al. : Role of brain receptor in control of body weight and reproduction. Science 2000; 289: 2122– 2125 [DOI] [PubMed] [Google Scholar]
- 9.Benani A, Troy S, Carmona CM, et al. : Role for mitochondrial reactive oxygen species in brain lipid sensing. Diabetes 2007; 56: 152– 160 [DOI] [PubMed] [Google Scholar]
- 10.Leloup C, Magnan C, Benani A, et al. : Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes 2006; 55: 2084– 2090 [DOI] [PubMed] [Google Scholar]
- 11.Andrews ZB, Liu ZW, Walllingford N, et al. : UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 2008; 454: 846– 851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein BJ, Mahadev K, Wu X: Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 2005; 54: 311– 321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein BJ, Mahadev K, Wu X, et al. : Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 2005; 7: 1021– 1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahadev K, Zilbering A, Zhu Li, et al. : Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J Biol Chem 2001; 276: 21938– 21942 [DOI] [PubMed] [Google Scholar]
- 15.Mahadev K, Wu X, Zilbering A, et al. : Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem 2001; 276: 48662– 48669 [DOI] [PubMed] [Google Scholar]
- 16.Mahadev K, Wu X, Motoshima H, et al. : Integration of multiple downstream signals determines the net effect of insulin on MAP kinase vs. PI 3′-kinase activation: potential role of insulin-stimulated H2O2. Cell Signal 2004; 16: 323– 331 [DOI] [PubMed] [Google Scholar]
- 17.Mahadev K, Motoshima H, Wu X, et al. : The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 2004; 24: 1844– 1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storozhevykh TP, Senilova YE, Persiyantseva NA, et al. : Mitochondrial respiratory chain is involved in insulin-stimulated hydrogen peroxide production and plays an integral role in insulin receptor autophosphorylation in neurons. BMC Neurosci 2007; 8: 84– 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo JH, Ahn Y, Lee SR, et al. : The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell 2005; 16: 348– 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plata-Salaman CR, Oomura Y, Shimizu N: Dependence of food intake on acute and chronic ventricular administration of insulin. Physiol Behav 1986; 37: 717– 734 [DOI] [PubMed] [Google Scholar]
- 21.Galinier A, Carriere A, Fernandez Y, et al. : Site specific changes of redox metabolism in adipose tissue of obese Zucker rats. FEBS Lett 2006; 580: 6391– 6398 [DOI] [PubMed] [Google Scholar]
- 22.Woods SC, Chavez M, Park CR, et al. : The evaluation of insulin as a metabolic signal influencing behavior via the brain. Neurosci Biobehav Rev 1996; 20: 139– 144 [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Fang YZ, Yang S, et al. : Glutathione metabolism and its implications for health. J Nutr 2004; 134: 489– 492 [DOI] [PubMed] [Google Scholar]
- 24.Masters BA, Shemer J, Judkins JH, et al. : Insulin receptors and insulin action in dissociated brain cells. Brain Res 1987; 417: 247– 256 [DOI] [PubMed] [Google Scholar]
- 25.Schulingkamp RJ, Pagano TC, Hung D, et al. : Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Rev 2000; 24: 855– 872 [DOI] [PubMed] [Google Scholar]
- 26.Czech MP, Lawrence JC, Jr, Lynn WS: Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc Natl Acad Sci 1974; 71: 4173– 4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May JM, de Haën C: Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem 1979; 254: 9017– 9021 [PubMed] [Google Scholar]
- 28.Schmid E, El Benna J, Galter D, et al. : Redox priming of the insulin receptor β-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB 1998; 12: 863– 870 [DOI] [PubMed] [Google Scholar]
- 29.Leslie NR: The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 2006; 8: 1765– 1775 [DOI] [PubMed] [Google Scholar]
- 30.Niswender KD, Morrison CD, Clegg DJ, et al. : Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus. Diabetes 2003; 52: 227– 231 [DOI] [PubMed] [Google Scholar]
- 31.Clodfeldder-Miller B, De Sarno P, Zmijewska AA, et al. : Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J Biol Chem 2005; 280: 39723– 39731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu WA, Kaelin CB, Takeda K, et al. : PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 2005; 115: 951– 958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MS, Pak YK, Jang PG, et al. : Role of hypothalamic Foxo 1 in the regulation of food intake and energy homeostasis. Nature Neurosci 2006; 9: 901– 906 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MW, Woods SC, Porte D, et al. : Central nervous system control of food intake. Nature 2000; 404: 661– 671 [DOI] [PubMed] [Google Scholar]
- 35.Serrano F, Kolluri N, Wientjes FB, et al. : NAD(P)H oxidase immunoreactivity in the mouse brain. Brain Res 2003; 988: 193– 198 [DOI] [PubMed] [Google Scholar]
- 36.Kim MJ, Shin KS, Chung YB, et al. : Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res 2005; 1040: 178– 186 [DOI] [PubMed] [Google Scholar]
- 37.Vallet P, Charnay Y, Steger K, et al. : Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 2005; 132: 233– 238 [DOI] [PubMed] [Google Scholar]
- 38.Cheng G, Cao Z, Xu X, et al. : Homologs of gp91Phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001; 269: 131– 140 [DOI] [PubMed] [Google Scholar]
- 39.Tejada-Simon MV, Serrano F, Villasana LE, et al. : Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci 2005; 29: 97– 106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishida KT, Klann E: Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal 2007; 9: 233– 244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmermann MC, Davisson RL: Redox signaling in central neural regulation of cardiovascular function. Biophys Mol Biol 2004; 84: 125– 149 [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann MC, Dunlay RP, Lazartigues E, et al. : Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 2004; 84: 532– 539 [DOI] [PubMed] [Google Scholar]
- 43.Pocai A, Lam TKT, Obici S, et al. : Restauration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 2006; 116: 1081– 1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valko M, Leibfritz D, Moncol J, et al. : Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44– 84 [DOI] [PubMed] [Google Scholar]
- 45.Kuhla B, Kuhla S, Rudolph PE, et al. : Proteomics analysis of hypothalamic response to energy restriction in dairy cows. Proteomics 2007; 7: 3602– 3617 [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Scholl TO, Leskiw MJ, et al. : Association of glutathione peroxidase activity with insulin resistance and dietary fat intake during normal pregnancy. J Clin Endocrinol Metab 2003; 88: 5963– 5968 [DOI] [PubMed] [Google Scholar]
- 47.Posey K, Clegg DJ, Printz RL, et al. : Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 2009; 296: E1003– E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Dong F, Ren J, et al. : High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol 2005; 191: 318– 325 [DOI] [PubMed] [Google Scholar]
- 49.Houstis N, Rosen ED, Lander ES: Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006; 440: 944– 948 [DOI] [PubMed] [Google Scholar]
- 50.Urakawa H, Katsuki A, Sumida Y, et al. : Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab 2003; 88: 4673– 4676 [DOI] [PubMed] [Google Scholar]