Abstract
OBJECTIVE
We sought to establish the extent and mechanisms by which sitagliptin and metformin singly and in combination modify islet disease progression in human islet amyloid polypeptide transgenic (HIP) rats, a model for type 2 diabetes.
RESEARCH DESIGN AND METHODS
HIP rats were treated with sitagliptin, metformin, sitagliptin plus metformin, or no drug as controls for 12 weeks. Fasting blood glucose, insulin sensitivity, and β-cell mass, function, and turnover were measured in each group.
RESULTS
Sitagliptin plus metformin had synergistic effects to preserve β-cell mass in HIP rats. Metformin more than sitagliptin inhibited β-cell apoptosis. Metformin enhanced hepatic insulin sensitivity; sitagliptin enhanced extrahepatic insulin sensitivity with a synergistic effect in combination. β-Cell function was partially preserved by sitagliptin plus metformin. However, sitagliptin treatment was associated with increased pancreatic ductal turnover, ductal metaplasia, and, in one rat, pancreatitis.
CONCLUSIONS
The combination of metformin and sitagliptin had synergistic actions to preserve β-cell mass and function and enhance insulin sensitivity in the HIP rat model of type 2 diabetes. However, adverse actions of sitagliptin treatment on exocrine pancreas raise concerns that require further evaluation.
The prevalence of type 2 diabetes and the associated morbidity and mortality are increasing (1). There is therefore interest in strategies to slow or prevent the development of type 2 diabetes. Although insulin resistance secondary to lifestyle changes likely contributes to the increased prevalence, most insulin-resistant individuals increase insulin secretion and remain nondiabetic (2). In contrast, in those genetically vulnerable to develop type 2 diabetes, β-cell function fails to appropriately adapt to insulin resistance, leading to hyperglycemia (3,4).
Prospective studies in humans have reported a progressive decline in β-cell function preceding development of type 2 diabetes (5,6). Autopsy studies reveal that the islet in type 2 diabetes is characterized by a ∼60% deficit in β-cells and islet amyloid derived from islet amyloid polypeptide (IAPP), a 37–amino acid peptide cosecreted with insulin by β-cells (7). The cause of the defect in β-cell mass in type 2 diabetes remains unresolved but is likely attributable, at least in part, to endoplasmic reticulum stress–induced β-cell apoptosis, noted both at autopsy and in isolated islets from people with type 2 diabetes (8,9). Based on these observations, it is apparent that to favorably modify disease progression in type 2 diabetes, preservation of β-cell mass and function in the setting of insulin resistance is required.
Our primary objective in the current study was to test the hypothesis that the combination of two potentially synergistic therapies, the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin and hepatic insulin sensitizer metformin, modify progression of islet dysfunction and loss of β-cell mass in type 2 diabetes. Because it is not possible to evaluate β-cell mass or turnover in vivo in humans, we undertook these studies in the human IAPP transgenic (HIP) rat because it approximates the islet and metabolic phenotype of type 2 diabetes in humans (10–12).
Metformin has previously been shown to delay onset of type 2 diabetes (13). Glucagon-like peptide 1 (GLP-1) has reversed loss of β-cell mass in some murine models of diabetes by both increasing new β-cell formation and decreasing β-cell apoptosis (14–16). The DPP-4 inhibitor sitagliptin increases GLP-1 concentrations (17) and modestly lowers glucose levels when used alone in type 2 diabetes (18,19) with an additive effect in combination with metformin (20,21).
Therefore, we sought to address the following questions. First, do metformin or sitagliptin individually or in combination favorably modify disease progression (reducing β-cell loss and dysfunction) at the level of the islet in the HIP rat model of type 2 diabetes? Second, is any protection of β-cell mass accomplished by decreased β-cell apoptosis and/or increased β-cell formation? Third, what are the respective actions of these drugs on insulin sensitivity and secretion singly, and in combination, in this model of type 2 diabetes? Unexpectedly, we encountered marked ductal metaplasia in 25% of high-fat diet–fed HIP rats treated with sitagliptin and severe hemorrhagic pancreatitis in one sitagliptin-treated animal. Because those findings have potentially important clinical implications, we evaluated the exocrine effects of sitagliptin. These latter studies provided some insights into the reported association of GLP-1 mimetic therapy by exenatide (22) or liraglutide (23) and pancreatitis, and they provide some cautions about the potential long-term effects of GLP-1 mimetic therapy, including DPP-4 inhibition in diabetes.
RESEARCH DESIGN AND METHODS
A total of 40 Sprague-Dawley rats (wild type; n = 7) and rats expressing human IAPP (HIP rats; n = 33) were used in the current study. Generation of HIP rats has been described in detail previously (11). Rats were bred and housed individually throughout the study at the University of California Los Angeles animal housing facility and subjected to standard 12-h light/dark cycle. The University of California Los Angeles institutional animal care and use committee approved all surgical and experimental procedures. To establish the actions of sitagliptin and metformin singly and in combination on islet protection, 2-month-old wild-type and HIP rats were fed high-fat diet ad libitum for 12 weeks (60% fat, 20% protein, and 20% carbohydrates; no. D12492; Research Diets, New Brunswick, NJ) and randomly assigned into five independent treatment groups: wild-type rats (no drug treatment, n = 7), HIP rats (no drug treatment, n = 8), HIP rats given sitagliptin (200 mg · kg body wt−1 · day−1 sitagliptin, n = 8), HIP rats given metformin (200 mg · kg body wt−1 · day−1 metformin, n = 9), and HIP rats given sitagliptin plus metformin (200 mg · kg body wt−1 · day−1 sitagliptin and metformin, n = 8). Sitagliptin was provided by Merck Research (Rahway, NJ), and metformin was purchased from Toronto Research Chemicals (Toronto, Canada). Compounds were administered after premixing with high-fat diet, which was performed by Research Diets. After 12 weeks of diet/drug treatment, animals were anesthetized with isoflourane (2.5%) by inhalation until effect (Isoflourane Vapor 19.1; Summit Anesthesia, Portland, OR). Indwelling catheters were then inserted into the right internal jugular vein and left carotid artery for subsequent in vivo metabolic studies, as previously described (24). All catheters were filled with 100 units/ml heparin/saline solution, exteriorized to the back of the neck, and encased in the infusion harness (Instech, Plymouth Meeting, PA).
Hyperglycemic clamp and arginine bolus injection.
To assess glucose- and arginine-stimulated insulin secretion, wild-type rats (n = 6), HIP rats (n = 8), HIP rats given sitagliptin (n = 8), HIP rats given metformin (n = 6), and HIP rats given sitagliptin plus metformin (n = 6) underwent a hyperglycemic clamp followed by an arginine bolus injection, as previously described (10). In brief, after a 30-min equilibration period (−30 to 0 min), plasma samples were taken for measurements of baseline fasting glucose and insulin. Thereafter, animals received an intravenous glucose bolus (375 mg/kg) followed by a variable 50% (wt/vol) glucose infusion to clamp arterial glucose at ∼250 mg/dl (0–70 min). At t = 60 min, rats received a bolus injection of l-arginine solution (1 mmol/kg; Sigma, St. Louis, MO). Arterial blood samples (50 μl) were taken at baseline (−30 and 0 min), at 1 and 5 min, and every 15 min thereafter during the clamp for immediate determination of plasma glucose and subsequent analysis for insulin.
Hyperinsulinemic-euglycemic clamp and 3H-glucose infusion.
To assess insulin sensitivity and glucose turnover, wild-type rats (n = 5), HIP rats (n = 6), HIP rats given sitagliptin (n = 7), HIP rats given metformin (n = 6), and HIP rats given sitagliptin plus metformin (n = 6) underwent a hyperinsulinemic-euglycemic clamp with concomitant infusion of [3-3H]glucose to assess glucose turnover, as previously described (10). Briefly, rats received primed (3 μCi) continuous (0.05 μCi/min) infusion of [3-3H]glucose (Perkin Elmer, Boston, MA) for a 90-min basal period that increased to 0.2 μCi/min for 120 min throughout the hyperinsulinemic-euglycemic clamp, which was achieved by constant infusion of regular human insulin (Novolin; Novo Nordisk, Princeton, NJ) at 4 mU · kg−1 · min−1, variable glucose (50% wt/vol) infusion, and somatostatin infusion (10 μg · kg−1 · min−1; Bachem, CA) to inhibit endogenous insulin secretion. Plasma glucose levels were determined every 10 min, and additional blood samples (∼100 μl) were collected at baseline (−30 min) and at the end of the clamp for determination of plasma insulin. Blood samples (∼150 μl) for determination of tracer-specific activity were drawn at fasting (from – 40 to 0 min) and during insulin infusion.
Endocrine pancreas histology.
Rats were killed by 120 mg/kg i.v. sodium pentobarbital. The pancreas was then rapidly removed from killed rats and fixed in 4% paraformaldehyde overnight at 4°C. Paraffin-embedded pancreatic sections were stained first for hematoxylin/eosin and insulin (guinea pig anti-insulin, 1:100; Zymed, Carlsbad, CA). The β-cell mass was measured by first quantifying the pancreatic fractional area positive for insulin and multiplying this by the pancreatic weight. In addition, sections were costained by immunofluorescence for insulin (guinea pig anti-insulin, 1:100; Zymed, Carlsbad, CA) and terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL) method (Roche Diagnostics, Mannheim, Germany) for quantification of β-cell apoptosis, and they were costained for insulin (guinea pig anti-insulin, 1:100; Zymed) and Ki-67 (mouse anti–Ki-67, 1:50; Dako, Carpinteria, CA) for determination of β-cell replication. All β-cells per pancreatic section (∼2,500 cells per section) were examined in detail and counted at ×200 magnification (×20 objective, ×10 ocular) for the total number of TUNEL- and Ki-67–positive β-cells. The frequency of TUNEL and Ki-67 was presented as the percentage of total β-cells per section. Fluorescent slides were analyzed and imaged using a Leica microscope (Leica Microsystems, Wetzlar, Germany) and images acquired using OpenLab microscope software (Improvision) and analyzed using ImagePro Plus software.
Exocrine pancreas histology.
Pancreas sections were deparaffinized in xylene and rehydrated in ethanol gradient, and pancreatic sections were stained in Harris hematoxylin solution (HHS16; Sigma) and eosin Y solution (HT110132; Sigma). For immunofluorescence, antigen retrieval was performed via microwave heating in citrate buffer (H-3300; Vector, Burlingame, CA) except for TUNEL staining, which used proteinase-K digestion (V302B; Promega, Madison, WI) at 37°C for 15 min. Slides were blocked in Tris-buffered saline (3% BSA, 0.2% TX-100, and 2% donkey serum) for 1 h. The following primary antibodies were used for 12-h incubation: ductal cell marker cytokeratin (mouse anti-pancytokeratin, 1:50; Sigma), marker of cell fibrosis fibrinectin (rabbit anti-fibrinectin, 1:500; Sigma), replication marker Ki-67 (mouse anti–Ki-67, 1:50; Dako), apoptosis marker (TUNEL method; Roche Diagnostics), marker of T-cell infiltration (rabbit anti-CD3; Abcam, Cambridge, MA), marker of macrophage infiltration (rabbit anti–CD-11C; Abcam), GLP-1 receptor (rabbit anti–GLP-1 receptor, 1:100; Novus Biologicals, Littleton, CO), pancreatic and duodenal homeobox 1 (PDX-1) (rabbit anti–PDX-1, 1:1,000; Millipore, St. Louis, MO), and insulin (guinea pig anti-insulin, 1:100; Zymed). Secondary antibodies labeled with Cy3 and fluorescein isothiocyanate were obtained from Jackson Laboratories (West Grove, PA) and used at dilutions of 1:200 for 1-h incubation. To determine ductal cell replication and apoptosis, in each pancreatic section we quantified the total number of Ki-67–, TUNEL-, and cytokeratin-positive cells (∼1,000 cytokeratin-positive cells per section were counted). The frequency of ductal cell replication and apoptosis in each animal was presented as a total number of TUNEL- or Ki-67–positive cells per total number of cytokeratin-positive cells.
Analytical procedures.
Plasma glucose concentrations were measured by the glucose oxidase method (Glucose Analyzer 2; Beckman, Fullerton, CA). Plasma insulin was measured using competitive colorimetric enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH). Plasma glucose specific activity, hepatic glucose production, and glucose disposal was calculated as previously described in detail (10). Disposition index was calculated as the product of first-phase insulin secretion during the hyperglycemic clamp (expressed as pmol/l · min) and insulin sensitivity determined by mean glucose infusion rates (expressed in mg · kg−1 · min−1) required to maintain euglycemia during the hyperinsulinemic-euglycemic clamp.
Statistical analysis.
Statistical analysis was performed using ANOVA with Fisher's post hoc test where appropriate. Regression analysis was performed using Statistica (version 6; Statsoft, Tulsa, OK). Data in graphs and tables are the means ± SE. Findings were assumed to be statistically significant at P < 0.05.
RESULTS
Blood glucose concentrations, body weight, and food intake.
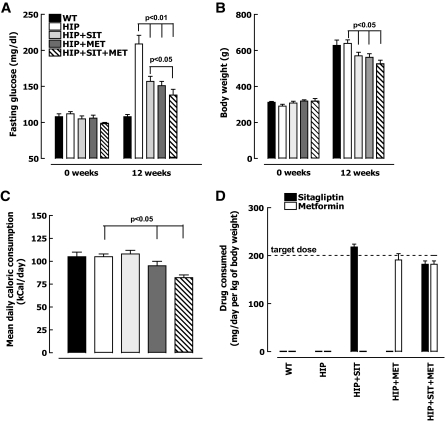
Prior to initiation of high-fat diet, blood glucose was comparable (105 ± 4 mg/dl) in wild-type and HIP rats (Fig. 1A). After 12 weeks of high-fat diet, plasma glucose increased to 209 ± 12 mg/dl in HIP rats but was unchanged (108 ± 3 mg/dl) in wild-type rats. Both metformin and sitagliptin alone had a comparable effect on restraining this increase in blood glucose concentration in HIP rats (increased to 154 ± 7 vs. 209 ± 12 mg/dl, P < 0.05), whereas the combination of sitagliptin and metformin had a synergistic effect (increased to 138 ± 8 vs. 209 ± 12 mg/dl, P < 0.01). Weight gain on the high-fat diet was comparable in wild-type (from 312 ± 5 to 628 ± 30 g) (Fig. 1B) and untreated HIP rats (from 291 ± 10 to 639 ± 14 g) (Fig. 1B) but was ∼10% less in either sitagliptin- or metformin-treated HIP rats (P < 0.05) (Fig. 1B) and ∼15% less in HIP rats treated with combination therapy (P < 0.05) (Fig. 1B). Food intake was decreased in HIP rats treated with metformin (∼10%, P < 0.05 vs. HIP) (Fig. 1C) and with both metformin and sitagliptin (∼20%, P < 0.05 vs. HIP) (Fig. 1C).
FIG. 1.
Fasting plasma glucose (A), body weight (B), mean daily food intake (C), and mean daily drug consumption (D) after 12-week treatment with 60% high-fat chow diet in wild-type (WT) rats (n = 7), HIP rats (n = 8), HIP rats treated with sitagliptin (HIP+SIT; n = 8), HIP rats treated with metformin (HIP+MET; n = 9), and HIP rats treated with combination therapy (HIP+SIT+MET; n = 8). Data are means ± SE.
β-Cell mass, replication, and apoptosis.
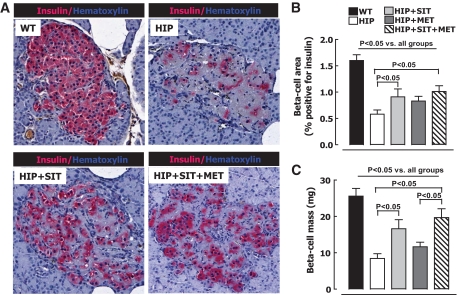
β-Cell mass was ∼70% decreased in untreated HIP versus wild-type rats on high-fat diet (8.4 ± 1.3 vs. 25.6 ± 2.1 mg, P < 0.05) (Fig. 2) as a consequence of increased β-cell apoptosis, as previously reported (12). Sitagliptin therapy alone led to preservation of β-cell mass compared with untreated HIP rats (8.4 ± 1.3 vs. 16.6 ± 2.5 mg, P < 0.01) (Fig. 2). In HIP rats treated with metformin alone, β-cell mass was not significantly different from untreated HIP rats (8.4 ± 1.3 vs. 11.6 ± 1.3 mg, P = 0.24 for HIP rats vs. HIP rats given metformin) (Fig. 2), but those treated with combination therapy of sitagliptin and metformin had even better preservation of β-cell mass than those treated with sitagliptin alone (8.4 ± 1.3 vs. 19.7 ± 2.4 mg, P < 0.001 for HIP rats vs. HIP rats given sitagliptin plus metformin) (Fig. 2).
FIG. 2.
A: Typical islets from wild-type (WT) rats, HIP rats, HIP rats treated with sitagliptin (HIP+SIT), and HIP rats treated with sitagliptin and metformin (HIP+SIT+MET) stained for insulin (pink) and hematoxylin (blue). β-Cell area (B) and mean β-cell mass (C) after 12-week treatment with 60% high-fat diet in wild-type rats (n = 7), HIP rats (n = 8), HIP rats treated with sitagliptin (HIP+SIT; n = 8), HIP rats treated with metformin (HIP+MET; n = 9), and HIP rats treated with combination therapy (HIP+SIT+MET; n = 8). Data are the means ± SE. (A high-quality digital representation of this figure is available in the online issue.)
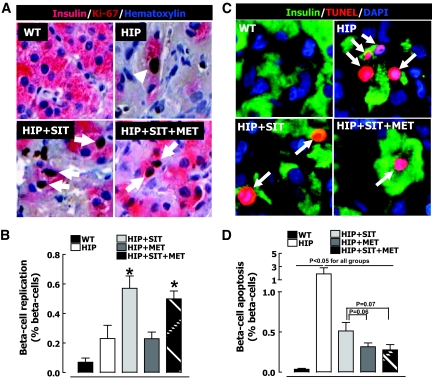
The frequency of β-cell replication quantified by Ki-67 was increased by sitagliptin alone (0.2 ± 0.1 vs. 0.6 ± 0.1% for HIP rats vs. HIP rats given sitagliptin, P < 0.05) (Fig. 3A and B) and by combination therapy (0.2 ± 0.1 vs. 0.5 ± 0.1% for HIP rats vs. HIP rats given sitagliptin plus metformin, P < 0.05) (Fig. 3A and B). In contrast, metformin alone had no discernable effect on β-cell replication. Sitagliptin treatment alone decreased the frequency of β-cell apoptosis in HIP rats by ∼55% (P < 0.05) (Fig. 3C and D). Metformin treatment alone was even more effective at decreasing β-cell apoptosis in HIP rats (by ∼75%, P < 0.05) (Fig. 3C and D), whereas sitagliptin and metformin in combination had an action to suppress β-cell apoptosis that was comparable to that of metformin alone (Fig. 3C and D).
FIG. 3.
A: Examples of islets stained for insulin (pink) and replication marker Ki-67 (brown) and nuclear stain hematoxylin (blue) imaged at 20×. B: Frequency of β-cell replication in wild-type (WT) rats (n = 7), HIP rats (n = 8), HIP rats treated with sitagliptin (HIP+SIT; n = 8), HIP rats treated with metformin (HIP+MET; n = 9), and HIP rats treated with combination therapy (HIP+SIT+MET; n = 8). C: Examples of islets stained for insulin (green) and apoptosis marker (TUNEL; red) and nuclear stain (DAPI; blue) imaged at ×20. D: Frequency of β-cell apoptosis in wild-type rats (n = 7), HIP rats (n = 8), HIP rats treated with sitagliptin (n = 8), HIP rats treated with metformin (n = 9), and HIP rats treated with combination therapy (n = 8). Data are means ± SE. *P < 0.05 vs. wild type, HIP, and HIP plus metformin. Arrows indicate examples of insulin-positive Ki-67 and TUNEL-positive cells. (A high-quality digital representation of this figure is available in the online issue.)
Insulin sensitivity.
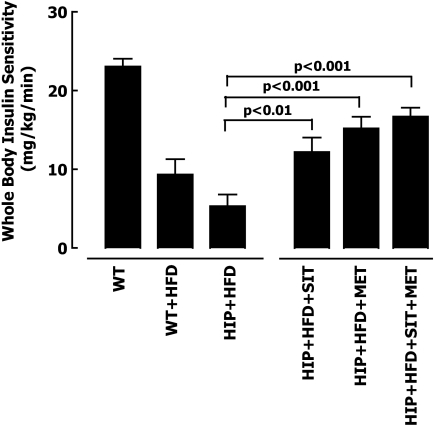
The impact of metformin and/or sitagliptin on insulin sensitivity was evaluated in HIP rats by hyperinsulinemic-euglycemic clamp and the isotope dilution technique. As expected, the high-fat diet induced insulin resistance in both wild-type and HIP rats (Fig. 4). Insulin sensitivity, assessed by the mean glucose infusion rates during the hyperinsulinemic period, was enhanced by either metformin (14.2 ± 1.4 vs. 5.3 ± 1.5 mg · kg−1 · min−1, P < 0.001) (Fig. 4 and supplemental Table 1, which is available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0058/DC1) or sitagliptin (11.4 ± 1.7 vs. 5.3 ± 1.5 mg · kg−1 · min−1, P < 0.01) therapy alone compared with high-fat diet HIP rats, and in combination they had a slight additive effect (15.6 ± 1.1 vs. 5.3 ± 1.5 mg · kg−1 · min−1, P < 0.001). In the fasting state, isotopically measured hepatic glucose release was approximately twofold greater in HIP versus wild-type rats (9.7 ± 1.4 vs. 5.1 ± 1.3 mg · kg−1 · min−1, P < 0.05) (supplemental Table 1). In contrast, metformin alone or in combination with sitagliptin led to a ∼40% suppression of fasting hepatic glucose release in HIP rats, whereas sitagliptin alone had no measurable effect on hepatic glucose release in the fasting state in HIP rats (supplemental Table 1). With insulin stimulation during the hyperinsulinemic-euglycemic clamp, hepatic glucose release in HIP rats was suppressed minimally compared with wild-type rats (7 vs. 100% for HIP vs. wild-type, P < 0.05) (supplemental Table 1), confirming marked hepatic insulin resistance. Metformin alone or in combination with sitagliptin partially restored hepatic insulin sensitivity in HIP rats, as indicated by ∼60% suppression of hepatic glucose release during the hyperinsulinemic clamp (supplemental Table 1). Insulin-stimulated glucose disposal tended to be ∼30% higher in all three drug-treated groups compared with nontreated HIP rats. The slightly decreased weight gain in the metformin- and sitagliptin-treated HIP rats may have contributed to the increased insulin sensitivity with each of these therapies.
FIG. 4.
Mean glucose infusion rates during the hyperinsulinemic-euglycemic clamp after 12-week treatment with 60% high-fat diet (HFD) in wild-type (WT) rats (n = 5), HIP rats (n = 6), HIP rats treated with sitagliptin (HIP+SIT; n = 7), HIP rats treated with metformin (HIP+MET; n = 6), and HIP rats treated with combination therapy (HIP+SIT+MET; n = 6). Data are means ± SE.
β-Cell function.
Glucose-mediated insulin secretion (examined by hyperglycemic clamp) was markedly attenuated in HIP compared with wild-type rats on a high-fat diet (648 ± 141 vs. 5,423 ± 480 pmol/l · min, P < 0.05) (Fig. 5A). There was no significant enhancement of first-phase (Fig. 5A) or second-phase (data not shown) glucose-mediated insulin secretion in HIP rats treated with sitagliptin or metformin alone when considered independently of insulin sensitivity. However, taking insulin sensitivity into account in the calculated disposition index (Fig. 5B), glucose-mediated insulin secretion in HIP rats was comparably enhanced by either metformin or sitagliptin alone, and in combination these drugs had a synergistic action to further enhance the disposition index (P < 0.05 for HIP rats vs. HIP rats given sitagliptin plus metformin) (Fig. 5B). Glucose-potentiated arginine-stimulated insulin secretion was also markedly attenuated in HIP versus wild-type rats (3,077 ± 528 vs. 8,809 ± 1,179 pmol/l, P < 0.05) (Fig. 5C), and again there was no appreciable benefit from either sitagliptin or metformin independently or in combination on this metric (Fig. 5C), which is generally considered a surrogate of β-cell mass (25). It is therefore of interest to note that the glucose-potentiated arginine-elicited first-phase insulin response did not reflect β-cell mass (Fig. 5D) in metformin- and/or sitagliptin-treated HIP rats.
FIG. 5.
Mean first-phase insulin response during the hyperglycemic clamp (A), mean disposition index (B), mean first-phase insulin response to arginine (C), and the relationship between β-cell mass and first-phase insulin response to arginine (D) after 12-week treatment with 60% high-fat diet in wild-type (WT) rats (n = 6), HIP rats (n = 8), HIP rats treated with sitagliptin (HIP+SIT; n = 8), HIP rats treated with metformin (HIP+MET; n = 6), and HIP rats treated with combination therapy (HIP+SIT+MET; n = 6). Data are means ± SE.
Pancreatitis in an HIP rat treated with sitagliptin.
The focus on the exocrine actions of sitagliptin arose as a consequence of an unexpected observation of marked necrotizing pancreatitis in one of eight rats treated with sitagliptin (Fig. 6 and supplemental Figs. 1 and 2). The region of pancreatitis was apparent as a mass of ∼2 cm and histologically characterized by hemorrhagic necrosis, fibrosis, inflammatory cell infiltrate, and areas of ductal metaplasia (supplemental Figs. 1 and 2) (26–30). Although pancreatitis was present in 1 of 8 HIP rats treated with sitagliptin, it was not detected in any of the 17 HIP rats not treated with sitagliptin (Table 1) or any of the 89 HIP rat pancreases reported previously (7,10,12). Given this unexpected finding, we evaluated all the pancreases from this study for ductal metaplasia and increased ductal turnover, characteristics frequently present in pancreatitis in humans (29–31).
FIG. 6.
Necrotizing pancreatitis in a HIP rat treated with sitagliptin for 12 weeks. A: Representative image at ×2 magnification of the exocrine pancreas stained for hematoxylin and eosin from an HIP rat treated with sitagliptin for 12 weeks with necrotizing pancreatitis. Note partially preserved lobular configuration of the exocrine pancreas; however, note the significant loss of acinar cell density and the widening of the septae (arrow) as well as a complete loss of acinar cells in some areas (circle). B: Representative image at ×4 magnification. At this higher magnification, septal fibrosis and inflammation (arrows) are better appreciated as well as partial and complete loss of acinar cells (circle). C: Representative image at ×20 magnification. At this magnification, acinar cell injury and angulated tubular ductal structures within the acini are clearly seen (circle). Note the extensive septal inflammation and fibrosis (*). D: Representative image at ×40 magnification. At this higher magnification, angulated tubular ductal structures and surrounding tissue fibrosis are better appreciated. (A high-quality digital representation of this figure is available in the online issue.)
TABLE 1.
Incidence of pancreatitis, ductal metaplasia, and increased ductal turnover by group
| Wild type | HIP | HIP + SIT | HIP + MET | HIP + SIT + MET | |
|---|---|---|---|---|---|
| Number studied | 7 | 8 | 8 | 9 | 8 |
| Pancreatitis | 0 | 0 | 1 | 0 | 0 |
| Ductal metaplasia | 0 | 0 | 2 | 0 | 1 |
| Increased ductal proliferation* | — | 4 | 8 | 2 | 1 |
Data are n.
*Increase in ductal cell proliferation was defined as an increase in ductal proliferation 3 SDs above the mean of wild-type rats. HIP + MET, HIP rats treated with metformin; HIP + SIT, HIP rats treated with sitagliptin; HIP + SIT + MET, HIP rats treated with combination therapy.
Ductal metaplasia.
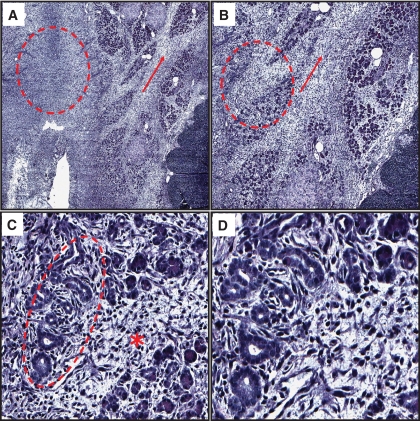
Ductal metaplasia was present in a total of three HIP rats treated with sitagliptin (Table 1). One of the three sitagliptin-treated HIP rats with ductal metaplasia also displayed marked pancreatitis (Table 1). These regions of ductal metaplasia were located both separate from and adjacent to islets of Langerhans (Fig. 7 and supplemental Fig. 3) and consisted of angulated tubular structures, interspersed fibrosis, and inflammatory cells. In some areas these were adjacent to atrophic acinar cells (Fig. 7 and supplemental Fig. 3). Ductal metaplasia was immunoreactive for cytokeratin and Ki-67 (Fig. 8A), indicating a high rate of cell turnover. Furthermore, metaplastic areas included numerous fibroblasts (by morphology and fibrinectin immunoreactivity) (Fig. 8B) and were absent of PDX-1 expression (Fig. 8C).
FIG. 7.
Extensive ductal metaplasia in HIP rats treated with sitagliptin for 12 weeks. A and B: Representative images at ×10 magnification of ductal cell metaplasia observed in a rat treated with sitagliptin for 12 weeks. Metaplastic regions consisted of angulated tubular structures, interspersed fibrosis, and inflammatory cells and were located both adjacent to islets of Langerhans (*) as well as separated from islets (circle). C: Representative image at ×20 magnification. At this higher magnification, an apparent transition from intact acinar cells to damaged/atrophic acinar cells to angulated tubular ductal structures is seen. D: Representative image at ×40 magnification. At this magnification the presence of extensive angulated tubular ductal structures and surrounding tissue fibrosis within the metaplastic region is better appreciated. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 8.
Extensive ductal metaplasia in sitagliptin-treated HIP rats is characterized by increased ductal cell turnover, tissue fibrosis, and absence of PDX-1 expression. A: Representative image at ×20 magnification of ductal cell metaplasia in an HIP rat treated with sitagliptin for 12 weeks stained for ductal cell marker (cytokeratin; green), replication marker (Ki-67; red), and nuclear stain (DAPI; blue). The extent of ductal cell replication within metaplasia is highlighted by coexpression of cytokeratin and Ki-67 immunoreactivity shown in the insert. B: The same area of ductal metaplasia was stained for ductal cell marker (cytokeratin; green), fibroblast marker (fibrinectin; red), and nuclear stain (DAPI; blue). C: The same area of ductal cell metaplasia stained for ductal marker (cytokeratin; green), PDX-1 (red), and insulin (blue). (A high-quality digital representation of this figure is available in the online issue.)
Ductal cell turnover.
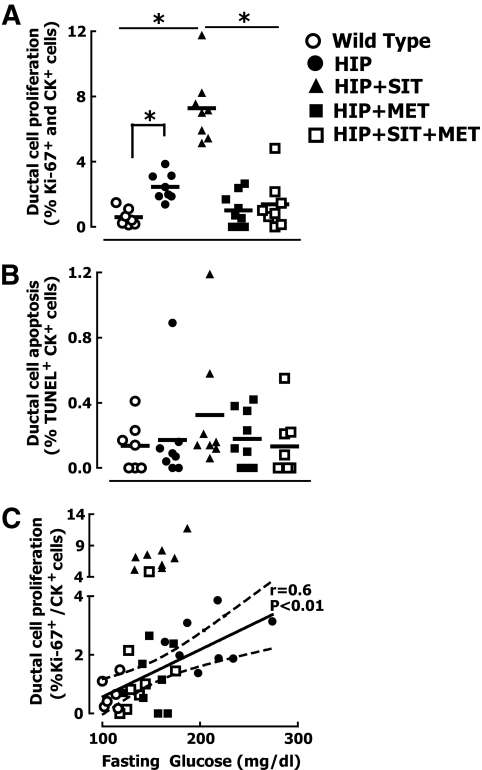
Ductal replication quantified by Ki-67 immunoreactivity was increased fourfold in untreated diabetic HIP rats versus wild-type controls (0.6 ± 0.2 vs. 2.5 ± 0.3%, P < 0.05) (Figs. 9 and 10A). Sitagliptin treatment led to an additional three-fold increase in the frequency of ductal cell replication versus untreated HIP rats (2.5 ± 0.3 vs. 7.3 ± 0.7%, P < 0.05) (Figs. 9 and 10 and supplemental Fig. 4) and a 12-fold increase compared with wild-type rats. Intriguingly, metformin treatment abrogated the effects of sitagliptin on ductal cell proliferation (7.3 ± 0.7 vs. 1.4 ± 0.6%, P < 0.05 for HIP rats given sitagliptin vs. HIP rats given sitagliptin plus metformin) (Figs. 9 and 10A). The frequency of ductal replication was positively correlated with fasting blood glucose concentration, with an apparent continuum between wild-type and HIP rats (Fig. 10C), with the sitagliptin-only–treated group displaced to a higher slope (Fig. 10C). Addition of metformin to sitagliptin restored the frequency of ductal replication to the same relationship with fasting glucose concentrations observed in rats not exposed to sitagliptin (Fig. 10C).
FIG. 9.
Ductal cell replication is increased in HIP rats treated with sitagliptin for 12 weeks. Representative images at ×20 magnification of exocrine ducts stained for cytokeratin (green), replication marker Ki-67 (red), and nuclear stain (DAPI; blue) in wild-type control rats, diabetic HIP rats, HIP rats treated with sitagliptin, or HIP rats treated with combination therapy. *Examples from sitagliptin-treated rats represent metaplasia and pancreatitis–free areas of the exocrine pancreas. Arrows indicate cytokeratin/Ki-67–positive cells. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 10.
Increased ductal cell turnover in HIP rats treated with sitagliptin. Quantification of ductal cell replication (A) and apoptosis (B) in wild-type rats, HIP rats, and HIP rats treated with either sitagliptin (HIP+SIT), metformin (HIP+MET), or combination therapy of sitagliptin and metformin (HIP+SIT+MET) for 12 weeks. C: Regression analysis of the relationships between ductal cell proliferation versus fasting plasma glucose. Note that ductal cell replication in sitagliptin-treated rats was quantified only in metaplasia and pancreatitis–free areas of the exocrine pancreas. *P < 0.05.
GLP-1 receptor, PDX-1, and insulin expression in exocrine ducts.
As previously reported (32), GLP-1 receptors were expressed in pancreatic ducts, but with no differences between treatment groups (supplemental Fig. 5). PDX-1– and insulin-positive ductal cells were observed after sitagliptin treatment in HIP rats (supplemental Fig. 6A). Although most β-cells were present within well-defined islets, occasional individual β-cells were present scattered in the exocrine pancreas. These scattered β-cells were approximately sixfold more abundant in sitagliptin-treated compared with untreated HIP rats (P < 0.05) (supplemental Fig. 6B). Interestingly, the number of scattered β-cells was also increased in metformin-treated animals, but not in animals that received combination therapy of sitagliptin plus metformin.
DISCUSSION
Our primary objective was to establish whether metformin or sitagliptin alone and in combination favorably modified disease progression in the HIP rat model of type 2 diabetes. Although loss of β-cell mass in the HIP rat was slowed by this combination therapy, unexpected adverse actions on the exocrine pancreas were also observed.
Metformin has been shown to delay type 2 diabetes onset in humans (13). Because enhanced insulin sensitivity through lifestyle changes also delays diabetes (13), at least part of the protective effect of metformin may be mediated by metformin's actions to enhance hepatic insulin sensitivity through its actions on AMP-activated kinase (33). Metformin decreased β-cell apoptosis in isolated human islets from patients with type 2 diabetes (34). In the current study, metformin was more effective than sitagliptin in reducing β-cell apoptosis in the high-fat diet –fed HIP rat. Although sitagliptin alone also suppressed β-cell apoptosis, there was no added benefit of sitagliptin on metformin-mediated suppression of β-cell apoptosis. Sitagliptin enhanced β-cell replication in HIP rats, consistent with prior studies of GLP-1– and GLP-1 mimetic–induced β-cell replication in a variety of murine models (14,15,32). The benefits of sitagliptin and/or metformin on β-cell mass and function reported here may have been mediated by either direct effects of the drugs on β-cells or indirectly by their actions to lower blood glucose. Hyperglycemia can contribute to both loss of β-cell mass by increasing β-cell apoptosis and/or loss of β-cell function (35). The current study was designed to examine effects of sitagliptin and metformin treatment in an in vivo model of type 2 diabetes, with the advantage of best approaching actions in humans with type 2 diabetes, but with the limitation of precluding distinction between direct and indirect effects of drugs on β-cell mass and function.
GLP-1–mediated increased β-cell replication has to be interpreted with caution. Juvenile rodents, in common with juvenile humans, have a period of postnatal expansion of β-cell numbers mediated by β-cell replication (36,37). Such studies (including this one) in relatively young rodents have exposed β-cells to increased GLP-1 when they remain replication competent. Recent studies have demonstrated that the capacity for new β-cell formation through β-cell replication is attenuated in adult rodents after epigenetic modifications of β-cells, and thus, not surprisingly, older rodents do not exhibit the same GLP-1–mediated β-cell replication as that observed in juvenile rodents (38,39). It is perhaps not surprising that under conditions of increased GLP-1 secretion (post–gastric bypass) in humans, despite earlier predictions (40), neither β-cell replication nor the fractional area of pancreas occupied by β-cells was increased (41). Likewise, long-standing exposure of nonhuman primates to the GLP-1 mimetic exenitide was reported to not increase β-cell mass (42). Because the incremental effect of sitagliptin on metformin to preserve β-cell mass in the current study appeared to be mediated through its action to foster β-cell replication, it is possible that no such added benefit would be present in humans.
The unexpected finding of hemorrhagic pancreatitis in one of the sitagliptin-treated rats prompted further analysis of the exocrine pancreas in this study. We report increased ductal proliferation in all sitagliptin-treated rats that were not also treated with metformin. We also noted ductal metaplasia in three sitagliptin-treated rats, one of which was also treated with metformin. Increased ductal proliferation and ductal metaplasia are well-recognized components of pancreatitis in humans (29–31), and they therefore offer a plausible mechanism for the GLP-1–induced pancreatitis reported in humans treated with the GLP-1 mimetics exenatide or liraglutide (22,23). Ductal GLP-1 receptor expression was not altered in any of the treatment groups. It cannot be assumed that the actions of sitagliptin to induce exocrine (or endocrine) pancreatic changes are mediated through GLP-1 because other regulatory peptides are also degraded by DPP-4 (43). Furthermore, we cannot rule out direct actions of sitagliptin on the exocrine pancreas. However, because pancreatitis has also been reported in humans treated with GLP-1 agonists (22,23), it seems likely that the exocrine effects of sitagliptin treatment reported here are a consequence of increased GLP-1 concentrations.
Perhaps of most concern, increased ductal cell turnover and ductal metaplasia are also well-characterized risk factors for pancreatic ductal cancer (31,44,45), as is pancreatitis (46). As yet, no increase in pancreatic cancer has been reported in patients treated with GLP-1 mimetics or DPP-4 inhibitors. However, these drugs have only been available for a relatively short period of time. Any influence that GLP-1–based therapy might have to increase the incidence of pancreatic cancer through chronically increased ductal cell turnover would be expected to take several years. The incidence of colon carcinoma associated with chronic epithelial replication and regeneration in the setting of inflammation in ulcerative colitis starts to increase 8–10 years after disease onset (47).
The current study may also shed some light on the increased incidence of pancreatitis and pancreatic cancer in patients with diabetes. Although pancreatitis or pancreatic cancer can lead to diabetes (48), epidemiological studies imply that the converse may also be true (49–52). Exocrine pancreatic insufficiency and pancreatitis are common in both autoimmune-mediated type 1 and type 2 diabetes (48,53). In the current study, we noted increased ductal turnover in the HIP rat related to plasma glucose concentrations (Fig. 10C). This implies that hyperglycemia per se may be sufficient to induce increased ductal cell turnover. While controversial, it has been proposed that there may be attempted β-cell regeneration in diabetes from progenitor cells that are proximate to, or within, pancreatic ducts (54).
Moreover, it has been proposed that GLP-1–based therapy enhances β-cell formation by increasing β-cell transdifferentiation from these putative duct-related stem cells (14,32). The action of sitagliptin treatment alone to increase ductal replication, apparently still in a glucose-sensitive manner but at a higher set point, is consistent with a complimentary interaction between glucose and GLP-1 concentrations to activate ductal cell proliferation. The observed PDX-1– and insulin-positive ductal cells in sitagliptin-treated HIP rats support this postulate.
An intriguing finding in the current study is the fact that addition of metformin to sitagliptin prevented the sitagliptin-mediated increase in ductal replication. Because metformin therapy has been shown to increase GLP-1 levels in some studies (55), the action to counter sitagliptin-mediated increased ductal replication is presumably independent of GLP-1. It is possible that the effect was mediated indirectly through metabolic actions of metformin to enhance insulin sensitivity or decrease blood glucose concentrations (56). Alternatively, metformin might act directly on ductal cells to suppress proliferation. Antiproliferative effects of metformin have been reported in prostate cancer cell lines and explanted prostate cells in mice (57). Recent epidemiological studies have revealed that metformin therapy is associated with a reduced incidence of cancer, including pancreatic cancer (58). The latter might be a consequence of the metabolic actions and/or the direct antiproliferative effects of metformin.
It is unknown whether sitagliptin actions on ductal turnover and/or induction of ductal metaplasia observed in the HIP rat extends to humans. It is plausible that these effects are restricted to the rat. It will be important to address this in pancreata, when available, from humans with type 2 diabetes who have been treated with GLP-1 mimetic therapy. Because the action of sitagliptin to increase ductal turnover was dependent on hyperglycemia, GLP-1 mimetic treatment on the exocrine pancreas in nondiabetic animal models, as used in classical toxicology screening studies, would presumably miss this effect and its potential long-term adverse consequences.
In summary, sitagliptin, and metformin, had synergistic effects on preserving β-cell mass in the HIP rat model of type 2 diabetes. Metformin was most effective at suppressing β-cell apoptosis. Sitagliptin fostered increased β-cell replication, but this is likely of limited benefit in adult humans. Of concern, we noted pancreatitis in one, ductal metaplasia in three and increased ductal turnover in all sitagliptin-treated HIP rats. Because the apparent adverse effects of GLP-1 mimetic therapy are at least to some extent offset by concurrent use of metformin, it is perhaps judicious to use GLP-1 mimetic therapy (including DPP-4 inhibiters) only in addition to metformin until potential long-term adverse effects of GLP-1–based therapy on exocrine pancreas can be ruled out in humans with diabetes.
Supplementary Material
Acknowledgments
Studies were supported by grants from the National Institutes of Health (NIH) (DK59579) and the Larry Hillblom Foundation (to P.C.B.). A.V.M. was supported by the NIH Ruth L. Kirschstein National Research Service Award.
Studies were also supported by the Merck Research Foundation (to P.C.B.). No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We are grateful for the support and excellent suggestions of our colleagues at the Larry Hillblom Islet Research Center, Anil Bhushan and Senta Georgia, and are also grateful to Matthias Hebrok from the University of California San Francisco.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.King H, Aubert RE, Herman WH: Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414– 1431 [DOI] [PubMed] [Google Scholar]
- 2.Polonsky KS, Given BD, Van Cauter E: Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest 1988; 81: 442– 448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E: Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med 1988; 318: 1231– 1239 [DOI] [PubMed] [Google Scholar]
- 4.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT: Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967; 46: 323– 335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA: Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006; 55: 1074– 1079 [DOI] [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 8.Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC: High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 2007; 56: 2016– 2027 [DOI] [PubMed] [Google Scholar]
- 9.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ: Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007; 50: 752– 763 [DOI] [PubMed] [Google Scholar]
- 10.Matveyenko AV, Butler PC: Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 2006; 55: 2106– 2114 [DOI] [PubMed] [Google Scholar]
- 11.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC: Diabetes due to a progressive defectin beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat): a new model for type 2 diabetes. Diabetes 2004; 53: 1509– 1516 [DOI] [PubMed] [Google Scholar]
- 12.Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC: Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes 2009; 58: 906– 916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu G, Stoffers DA, Habener JF, Bonner-Weir S: Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999; 48: 2270– 2276 [DOI] [PubMed] [Google Scholar]
- 15.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M: Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 2001; 50: 2237– 2243 [DOI] [PubMed] [Google Scholar]
- 16.Hui H, Nourparvar A, Zhao X, Perfetti R: Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 2003; 144: 1444– 1455 [DOI] [PubMed] [Google Scholar]
- 17.Deacon CF, Holst JJ: Dipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspective. Biochem Biophys Res Commun 2002; 294: 1– 4 [DOI] [PubMed] [Google Scholar]
- 18.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE: Sitagliptin Study 021 Group Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29: 2632– 2637 [DOI] [PubMed] [Google Scholar]
- 19.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H: Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49: 2564– 2571 [DOI] [PubMed] [Google Scholar]
- 20.Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP: Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9: 186– 193 [DOI] [PubMed] [Google Scholar]
- 21.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G: Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638– 2643 [DOI] [PubMed] [Google Scholar]
- 22.Cure P, Pileggi A, Alejandro R: Exenatide and rare adverse events. N Engl J Med 2008; 358: 1969– 1970[discussion 1971–1962] [DOI] [PubMed] [Google Scholar]
- 23.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B: Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473– 481 [DOI] [PubMed] [Google Scholar]
- 24.Matveyenko AV, Veldhuis JD, Butler PC: Measurement of pulsatile insulin secretion in the rat: direct sampling from the hepatic portal vein. Am J Physiol Endocrinol Metab 2008; 295: E569– E574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson RP: Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes 2007; 56: 2420– 2424 [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi Y, Inaba M, Kusafuka K, Okazaki K, Ikehara S: Establishment of animal models for three types of pancreatitis and analyses of regeneration mechanisms. Pancreas 2006; 33: 371– 381 [DOI] [PubMed] [Google Scholar]
- 27.Emmrich J, Weber I, Nausch M, Sparmann G, Koch K, Seyfarth M, Lohr M, Liebe S: Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion 1998; 59: 192– 198 [DOI] [PubMed] [Google Scholar]
- 28.Kloppel G: Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 2007; 20( Suppl. 1): S113– S131 [DOI] [PubMed] [Google Scholar]
- 29.Willemer S, Adler G: Histochemical and ultrastructural characteristics of tubular complexes in human acute pancreatitis. Dig Dis Sci 1989; 34: 46– 55 [DOI] [PubMed] [Google Scholar]
- 30.Bockman DE, Boydston WR, Anderson MC: Origin of tubular complexes in human chronic pancreatitis. Am J Surg 1982; 144: 243– 249 [DOI] [PubMed] [Google Scholar]
- 31.Parsa I, Longnecker DS, Scarpelli DG, Pour P, Reddy JK, Lefkowitz M: Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res 1985; 45: 1285– 1290 [PubMed] [Google Scholar]
- 32.Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S: GLP-1/exendin-4 facilitates beta-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract 2006; 73: 107– 110 [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE: Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167– 1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S: Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab 2004; 89: 5535– 5541 [DOI] [PubMed] [Google Scholar]
- 35.Poitout V, Robertson RP: Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008; 29: 351– 366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC: Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008; 57: 1584– 1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA: Very slow turnover of beta-cells in aged adult mice. Diabetes 2005; 54: 2557– 2567 [DOI] [PubMed] [Google Scholar]
- 38.Tschen S, Dhawan S, Gurlo T, Bhushan A: Age-dependent decline in beta cell proliferation restricts the capacity of beta cell regeneration in mice. Diabetes 2009; February19[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rankin MM, Kushner JA: Adaptive beta cell proliferation is severely restricted with advanced age. Diabetes 2009; March5[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV: Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353: 249– 254 [DOI] [PubMed] [Google Scholar]
- 41.Meier JJ, Butler AE, Galasso R, Butler PC: Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 2006; 29: 1554– 1559 [DOI] [PubMed] [Google Scholar]
- 42.Carpenter T, Trautmann ME, Baron AD: Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353: 2192– 2194[author reply 2192–2194] [DOI] [PubMed] [Google Scholar]
- 43.Hegen M, Kameoka J, Dong RP, Morimoto C, Schlossman SF: Structure of CD26 (dipeptidyl peptidase IV) and function in human T cell activation. Adv Exp Med Biol 1997; 421: 109– 116 [DOI] [PubMed] [Google Scholar]
- 44.Wagner M, Greten FR, Weber CK, Koschnick S, Mattfeldt T, Deppert W, Kern H, Adler G, Schmid RM: A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev 2001; 15: 286– 293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner M, Weber CK, Bressau F, Greten FR, Stagge V, Ebert M, Leach SD, Adler G, Schmid RM: Transgenic overexpression of amphiregulin induces a mitogenic response selectively in pancreatic duct cells. Gastroenterology 2002; 122: 1898– 1912 [DOI] [PubMed] [Google Scholar]
- 46.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andren-Sandberg A, Domellof L: Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993; 328: 1433– 1437 [DOI] [PubMed] [Google Scholar]
- 47.Eaden JA, Abrams KR, Mayberry JF: The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001; 48: 526– 535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meisterfeld R, Ehehalt F, Saeger HD, Solimena M: Pancreatic disorders and diabetes mellitus. Exp Clin Endocrinol Diabetes 2008; 116( Suppl. 1): S7– S12 [DOI] [PubMed] [Google Scholar]
- 49.Everhart J, Wright D: Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA 1995; 273: 1605– 1609 [PubMed] [Google Scholar]
- 50.Michaud DS, Wolpin B, Giovannucci E, Liu S, Cochrane B, Manson JE, Pollak MN, Ma J, Fuchs CS: Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2007; 16: 2101– 2109 [DOI] [PubMed] [Google Scholar]
- 51.Ishikawa O, Ohhigashi H, Wada A, Tateishi R, Ishiguro S, Okano Y, Sasaki Y, Imaoka S, Koyama H, Iwanaga T: Morphologic characteristics of pancreatic carcinoma with diabetes mellitus. Cancer 1989; 64: 1107– 1112 [DOI] [PubMed] [Google Scholar]
- 52.Cuzick J, Babiker AG: Pancreatic cancer, alcohol, diabetes mellitus and gall-bladder disease. Int J Cancer 1989; 43: 415– 421 [DOI] [PubMed] [Google Scholar]
- 53.Lankisch PG, Manthey G, Otto J, Koop H, Talaulicar M, Willms B, Creutzfeldt W: Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion 1982; 25: 211– 216 [DOI] [PubMed] [Google Scholar]
- 54.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A: The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes 2004; 5( Suppl. 2): 16– 22 [DOI] [PubMed] [Google Scholar]
- 55.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM: Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care 2001; 24: 489– 494 [DOI] [PubMed] [Google Scholar]
- 56.Bailey CJ, Turner RC: Metformin. N Engl J Med 1996; 334: 574– 579 [DOI] [PubMed] [Google Scholar]
- 57.Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F: The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27: 3576– 3586 [DOI] [PubMed] [Google Scholar]
- 58.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD: Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304– 1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.